
From Mesenchymal Stromal Stem Cells to Insulin-Producing Cells: Challenges in Translation for a Clinical Application
*Corresponding Author(s):
Mohamed A GhoneimUrology And Nephrology Center, Mansoura University, Egypt
Tel:+20 502202671,
Fax:+20 502202677
Email:ghoneimma@yahoo.com
Abstract
Cell transplantation for the control of diabetes may be carried out within a scaffold or an encapsulation device. The use of Mesenchymal Stromal Cells (MSCs) as a source for Insulin-Producing Cells (IIPCs) provides a chance for an autologous application. Hence, the use of encapsulation for immune isolation may not be necessary. Differentiated Bone Marrow-Derived (BM-MSCs) from Sprague Dawley (SD) rats were used to treat chemically-induced diabetes in an in bred group. IPCs were loaded onto a PLG scaffold and transplanted subcutaneously. Out of 9 surviving rats, only 4 became euglycemic. It was postulated that this modest outcome may be due to lack of sufficient immediate oxygenation. An intense foreign body tissue reaction induced by the scaffold material could be an additional detrimental factor to the transplanted cells. For a successful transplant, the scaffold material should not only be biodegradable and biocompatible, but should also induce a minimal foreign body tissue reaction. Furthermore, the blood supply to the transplanted cell should be adequate. This could be achieved in a 2-staged procedure, by preparing a vascularized bed before transplantation. A one-stage procedure is also possible provided that a drug delivery component is incorporated to allow immediate oxygenation, promote early vascularization and provide suitable growth factors.
Keywords
Cell transplantation; Insulin-producing cells; Mesenchymal stromal stem cells
INTRODUCTION
We have provided evidence that mesenchymal (stromal) stem cells obtained from Human Bone Marrow (HBM-MSCs) or from Human Adipose Tissue (HAT-MSCs) can be differentiated to form insulin-producing cells (IPCs), albeit at modest proportions [1]. Transplantation of these cells under the renal capsule of chemically- induced diabetic nude mice resulted in euglycemia [2]. We have also demonstrated that the transplanted cells underwent further differentiation in vivo to reach a peak of ~ 18%, four weeks after transplantation [3]. Encouraged by these outcomes, we have tried to apply such an approach in larger animals (dogs). This would provide an opportunity to estimate the required number of IPCs / Kg body weight for diabetic control, and also permit the evaluation of the functional longevity of these cells. A chance is also provided to test the subcutaneous compartment as a potential site for cell transplantation. In this study, IPCs from human origin were utilized. Their transplantation in dogs within an immuno-isolation device was imperative. To this end, we have used a macro-encapsulation devise (TheraCyte. Inc., Irvine, CA, USA). Such a device can be easily removed to examine their contents at pre-determined periods. Following clinical induction of diabetes, 5 x 106 IPCs / Kg body weight were loaded into two capsules and transplanted under the rectus sheath. Euglycemia could be achieved in 4 out of 6 dogs during an observation period of 6 months. Thereafter, there was a gradual and progressive decline in the control of diabetes [4]. At six months, histologic examination of a retrieved capsule revealed that the capsule was encased in a significant amount of fibrous tissue. This could interfere with free diffusion of glucose into and/or release of insulin out of the capsule. Furthermore, it may interfere with the blood supply and affect the metabolic demands of the transplanted cells.
The above observations clearly point out several problems with the currently available macro-encapsulation devices. Transplanted cells need immediate oxygenation, early vascularization without induction of a foreign body tissue reaction that would ultimately lead to peri-capsular fibrosis. Kevin D’Amor presented the results of the first in-human clinical evaluation of embryonic stem cells-derived islet replacement for type I diabetes (Clinical Trials. gov.: NCT 02239354). It seems that they have also encountered problems with encapsulation. It was reported that the trial was paused to improve the Encaptra device (ViaCyte, Inc. San Diego, CA, USA) [5]. In a second attempt, they will encapsulate their cells into an open device, to allow in growth of new blood vessels, and administer anti-inflammatory agents as well as a standard immunosuppressive regimen.
As an alternative for encapsulation, scaffolds can be used for cell transplantation, particularly if the donor cell were autologous. Herein, we present an initial experience with using scaffolds to treat chemically-induced diabetes in Sprague Dawley rats, using scaffolds loaded with adipose tissue derived insulin producing cells.
MATERIAL AND METHODS
The Experimental animals
Adult, inbred, male Sprague Dawley (SD) rats (12 weeks old), 150-180 g served as both donors and recipients. The experimental animals were delivered from the Animal Research Facility of Urology and Nephrology Center, Mansoura University and housed in individual cages, kept in a 12-hours light-dark cycle and supplied with food and water ad libitum. The experiment was performed under a protocol approved by the guidelines of the Institutional Animal Care and Use Committee.
Isolation and expansion of rat BM-MSCs
Rat bone marrow stem cells were isolated from the long bones of six adult Sprague Dawley rats according to the method previously described [6]. Cells were cultured at a density of 1×106/mL in 25 cm2 cell culture flasks coated with 0.1% gelatin in water solution (STEMCELL Technologies, Vancouver, Canada) and fed with DMEM containing 10% Fetal Bovine Serum (FBS) (Sigma-Aldrich, USA), GlutaMAX 100X (1 ml/L) (Gibco, USA) and penicillin-streptomycin solution (100 U/mL) (Sigma-Aldrich). The non-adherent populations were discarded. Adherent spindle-shaped mesenchymal stem cells (MSCs) were fed every 2-3 days with DMEM supplemented with 10 % FBS. Cells were detached at 80% confluence, with 0.25% trypsin solution (Sigma-Aldrich, USA) containing 1 mmol/L EDTA (Sigma-Aldrich). This step was repeated for three passages.
Differentiation of rat BM-MSCs into IPCs
Differentiation was carried out according to a protocol reported previously by Tayaramma et al. with some modifications [7]. Initially, the cells were cultured in serum free DMEM supplemented with 1% dimethyl sulfoxide (DMSO) for one day (Sigma-Aldrich). The medium was then replaced with serum-free DMEM containing 100 ng/mL activin-A (R&D systems Inc. Minneapolis, USA), 3 μM CHIR99021 (Sigma-Aldrich) and 100 nM Wortmannin (ENZO Life Sciences Inc., NY, USA) for 2 days. The medium was then replaced with serum-free DMEM supplemented with 100 ng/mL active in-A and 3 μM CHIR99021 for 2 more days. Thereafter, the cells were cultured in serum-free DMEM with 55 nM Trichostatin-A (TSA, Sigma-Aldrich) for 3 days. Finally, the cells were cultured for additional 7 days in high glucose (25 μM) medium containing DMEM: DMED/F12 (Sigma-Aldrich) at a ratio of 1:1. This mixture was supplemented with 10% FBS (Sigma-Aldrich), 10 μM Glucagon like peptide-1 (GLP-1, Sigma-Aldrich), 10 nM Nicotinamide (Sigma-Aldrich) and 10 ng/ml PRDX6 (Sigma-Aldrich).
Characterization of differentiated BM-MSCs
Immunolabelling
Anti-insulin Antibody (1:200) (mouse monoclonal, Novus Biologicals, Littleton, CO, USA), anti-C-peptide antibody (1:100) (rabbit polyclonal; Cell Signaling Technology) was used as the primary antibodies. Anti-mouse immunoglobulin G (IgG; H + L) Alexa Fluor 488 conjugate (1:200) and anti-rabbit (IgG; H + L) Alexa Fluor 555 conjugate (1:100) (Cell Signaling Technology) were utilized as the secondary antibodies.
Immunocytochemistry
Differentiated rat BM-MSCs were cultured on 2-chamber slides (Nunc, Rochester, NY, USA), and fixed with 4% paraformaldehyde for 10 minutes at room temperature. Cells were then permeabilized by methanol (100%) for 10 minutes and blocked with goat serum (5%) at RT for one hour, followed by overnight incubation at 4oC with the primary antibodies. Thereafter, the cells were washed with PBS and incubated for 2 hours at RT with the secondary antibodies. Nuclei were counterstained by DAPI (Invitrogen, UK). For negative controls; the primary antibody was omitted. Leica TCS SP8 microscope (Leica Microsystems, Mannheim, Germany) was used for capturing confocal images.
Induction of diabetes
Diabetes was chemically-induced in rats using a single intraperitoneal injection of 45 mg/kg body weight of Streptozotocin (STZ) (Sigma Aldrich, St. Louis, Missouri USA). Animals with 2 consecutive blood glucose readings higher than 300 mg/dl were considered diabetic.
Cell transplantation
For transplantation, 7 x 106 differentiated cells were used for each animal. All the surgical procedures were carried out under general anesthesia. Scaffolds (1X1 cm2) were sterilized in 70% ethanol for 60minutes then washed by rinsing 5 times in sterile Phosphate Buffer Saline (PBS). A total of 7 x 106 cells in 100ul of media were mixed with 10 ul of fibrinogen and 10ul of thrombin were loaded onto the top of each scaffold and incubated for 60 minutes. The cell loaded-scaffolds were transplanted under the dorsal skin of 10 animals.
Follow-up
The animals were followed up for a period of 12 weeks. Fasting blood sugar was determined every 2 weeks. At the end of the observation period, a blood sugar curve was carried out. Then the animals were sacrificed and their pancreata were removed. Samples were sent for histopathologic examination.
Immunofluorescent and histological studies
The harvested scaffolds were processed according to the manufacturer's instructions (10% formalin, alcohol gradients, xylene gradients, and finally paraffin embedding). Sections were taken at a thickness of 3 µm and mounted on positive-charged coated slides (Citoglas, Citotest Labware Manufacturing Co., Haimen, China). The slides were then deparaffinized using xylene and a decreasing ethanol gradient. Antigens were unmasked by boiling of the slides in 10 mM sodium citrate buffer (pH 6.0) for 30 min and a sub boiling temperature was maintained for 10 min. The sections were blocked with 5% normal goat serum and then incubated overnight with the primary antibody at 4oC. Thereafter, the slides were washed 3 times in PBS and incubated with the secondary antibody for 2 hr. at RT. Nuclei were using 4’,6-diamidino-2-phenylindole. Image J software 1.51h (developed by NIH) was used to determine the proportion of insulin-producing cells within the graft. To this end, 10 fields were randomly selected for cell counting which was carried out by 2 independent histopathologists. The results from all fields were calculated and expressed as the mean proportion insulin-positive cells among total transplanted cells. In all the above studies, confocal digital images were captured using a Leica TCS SP8 microscope (Leica Microsystems, Mannheim, Germany). For immunolabeling of the native pancreas, the primary antibody was mouse monoclonal anti-insulin (Novus Biologicals) and the secondary antibody was the Power-Stain Version 1.0 Poly HRP DAB Kit for mouse (Genemed Biotechnologies).
RESULTS
The differentiated BM-MSCs
At the ends of differentiation, immunofluorescent labeling demonstrated that approximately 0.5% of cells were positive for insulin and c-peptide (Figure 1).
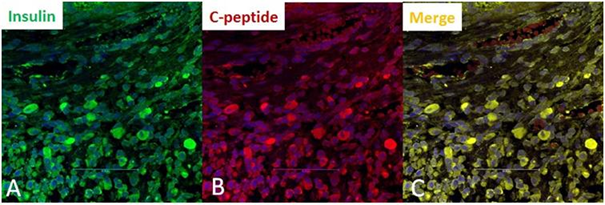 Figure 1: Immunocytochemistry of rat bone marrow-derived mesenchymal stem cells, at the end of in vitro differentiation. Evidence of successful differentiation was seen in only 0.5% of cells. A) Cells with insulin-positive granules (green); B) Cells with c-peptid-positive granules (red); C) Co-expression of insulin and c-peptide within the same cells (yellow).
Figure 1: Immunocytochemistry of rat bone marrow-derived mesenchymal stem cells, at the end of in vitro differentiation. Evidence of successful differentiation was seen in only 0.5% of cells. A) Cells with insulin-positive granules (green); B) Cells with c-peptid-positive granules (red); C) Co-expression of insulin and c-peptide within the same cells (yellow).
Outcomes of the vivo transplantation
Fasting blood sugar
After induction of diabetes, the fasting blood sugar increased in all animals reaching values of more than 400 mg/dL. One rat died 2 days after the surgical procedure. Out of the remaining 9 rats, the fasting blood sugar became normalized in 4 animals, 2 weeks after transplantation. These animals remained euglycemic throughout the observation period. The other 5 rats remained hyperglycemic. Differences in fasting blood sugar levels among the 2 groups were statistically significant at all time points post-transplant (Figure 2).
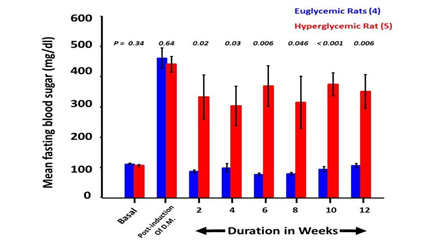 Figure 2: Fasting blood sugar values.
Figure 2: Fasting blood sugar values.
Glucose tolerance curves
After the intravenous injection of glucose, the blood sugar levels increased sharply to reach a maximum after 30 minutes. This was followed by a gradual decline to reach basal values after 180 minutes in the 4 cured rats. Glucose levels among the 5 uncured animals remained high throughout the experiment (Figure 3).
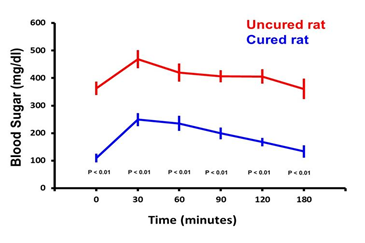 Figure 3: Glucose tolerance curves.
Figure 3: Glucose tolerance curves.
At the end of the observation period, glucose tolerance curves were carried out for cured as well as the diabetic animals. After administration of glucose, the blood sugar levels increased sharply to reach a maximum after 30min. Thereafter, the curve for the cured rats, (in blue), showed a gradual decline to reach basal values after 180min. The glucose levels among the 5 uncontrolled rats remained high throughout the experiment (in red).
Immunofluorescence studies of the retrieved scaffolds
This study revealed insulin-positive cells among all the removed scaffolds. Their proportion relative to the total number of transplanted cells was more than 10% among the euglycemic animals and less than 5% in the uncontrolled ones. Furthermore, glucagon and somatostatin were only expressed among the cured animals (Figure 4).
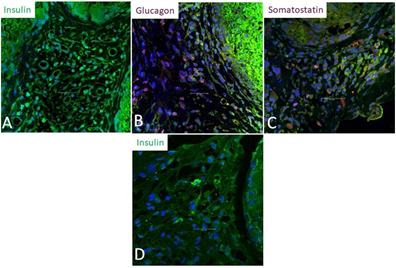 Figure 4: Immunofluorescence studies of the retrieved scaffolds:
Figure 4: Immunofluorescence studies of the retrieved scaffolds:
A) Insulin-positive cells (green) among one of the cured rats. Their proportion was 12.5% out of all transplanted cells.
B) Glucagon-positive cells (red). These were seen only among the scaffolds of cured rats.
C) Somatostatin positive cells (red). These were seen only among the scaffolds of cured rats.
D) Insulin-positive cells (green) among one of the uncontrolled rats. Their proportion was 1.5% out of all transplanted cells.
Histology of the native pancreata
The removed pancreata from all animals, showed very few atrophic islets. This confirms that the observed cure in some animals was due to the transplanted cells and not due to regeneration of the native pancreata (Figure 5).
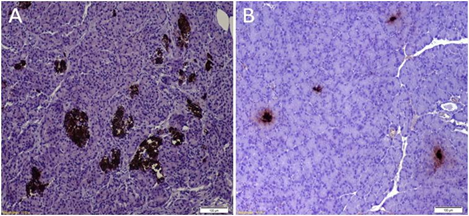 Figure 5: Pancreatic Histology:
Figure 5: Pancreatic Histology:
A) Histology of a pancreas from a normal rat: Normal and healthy islets which were insulin-positive.
B) Histology of a pancreas from a cured rat: The islets were small and atrophic.
DISCUSSION
The use of MSCs as a source of IPCs provides a chance for autologous application. Hence, the need for loading the cells within an encapsulation device may not be necessary. Scaffolds are, in effect, an open system that allows free permeation of oxygen and nutrients. They also allow a 3-D arrangement of the loaded cells, provided that its design has a suitable configuration.
In this contribution, differentiated BM-8MSCs from SD rats were used to treat chemically induced diabetes in an inbred group. IPCs were loaded on a PLG scaffold and were transplanted in a pocket under the dorsal skin of the diabetic animals. During an observation period of 12 weeks, glucose levels became normalized in only 4 of the surviving rats. Histology of the retrieved scaffolds from the cured animals revealed viable insulin and c-peptide positive cells. On the contrary, few cells were seen within the scaffolds retrieved from the uncontrolled ones. We have postulated that this may be due to insufficient immediate oxygenation after cell transplantation in the poorly vascularized subcutaneous tissue. It was also suggested that an intense foreign body tissue reaction, provoked by the scaffold material, can be detrimental to the newly transplanted cells [8]. In a thorough review, Mitrousis et al, outlined the necessary factors for a successful cell transplantation [9]. The biomaterial of the scaffold is critical. While it should be biodegradable and biocompatible, it should also induce a minimal foreign body tissue reaction. Currently, we are testing several polymers for their impact on cell viability, replication ability and any induced a foreign body reaction following their subcutaneous transplantation.
Subcutaneous cell transplantation is very attractive [10]. Its simple access via a minimal invasive procedure is possible. The monitoring of the grafted tissue is feasible. However, its blood supply is meager. A 2-staged procedure is suggested by Pepper and colleagues [11]. In the first stage, plastic tubes are inserted under the skin to induce formation of new blood vessels. Cell transplantation into the pre-vascularized bed is carried out in a second stage. A one-stage procedure may also be possible provided that a drug delivery system is incorporated. This should allow immediate oxygenation at physiological levels, elements that promote early vascularization and provide suitable growth factors(s), notably extendin-4. Testing these different approaches is currently underway in our research laboratory.
Many research centers and innovative companies are competing to win the race to treat diabetes, particularly type 1, by cell therapy. It is still a long way but hopefully not too long.
REFERENCES
- Gabr M, Zakaria M, Refaie A, Abdel-Rahman E, Reda A, et al. (2017) From human mesenchymal stem cells to insulin-producing cell: comparison between bone marrow and adipose tissue-derived cells. BioMed Res Int 2015: 3854232.
- Gabr M, Zakaria M, Refaie A, Ismail A, Abou-El-Mahasen M,et al. (2013) Insulin-producing cells from human bone marrow mesenchymal stem cells control streptozotocin-induced diabetes in nude mice. Cell Transplant 22: 133-145.
- Gabr M, Zakaria M, Refaie A, Khater S, Ashamallah S, et al. (2015) Differentiation of human bone marrow mesenchymal stem cells into insulin-producing cells: evidence for further maturation in vivo. BioMed Res Int 2015: 575837.
- Gabr M, Zakaria M, Refaie A, Ismail A, Khater S, et al. (2018) Insulin-producing cells from adult human bone marrow mesenchymal stromal cells could control chemically induced diabetes in dogs: a preliminary study. Cell Transplant 27: 937-947.
- Odorico J, Markmann J, Melton D, Greenstein J, Hwa A, et al. (2018) Report of the key opinion leaders meeting on stem cell-derived beta cells. Transplantation 102: 1223-1229.
- Gabr M, Sobh M, Zakaria M, Refaie A, Ghoneim M (2008) Transplantation of insulin-producing clusters derived from adult bone marrow stem cells to treat diabetes in rats. Exp Clin Transplant 6: 236-243.
- Tayaramma T, Ma B, Rohde M, Mayer H (2006) Chromatin?remodeling factors allow differentiation of bone marrow cells into insulin?producing cells. Stem Cells 24: 2858-2867.
- Asawa Y, Sakamoto T, Komura M, Watanabe M, Nishizawa S, et al. (2012) Early stage foreign body reaction against biodegradable polymer scaffolds affects tissue regeneration during the autologous transplantation of tissue-engineered cartilage in the canine model. Cell Transplant 21: 1431-1442.
- Mitrousis N, Fokina A, Shoichet MS (2018) Biomaterials for cell transplantation. Nat Rev Mater 3: 441-456.
- Bertuzzi F, De Carlis L (2018) Subcutaneous islet allotransplantation without immunosuppression therapy: The dream of the diabetologists and of their patients. Transplantation 102: 351-352.
- Pepper A, Gala-Lopez B, Pawlick R, Merani S, Kin, et al. (2015) A prevascularized subcutaneous device-less site for islet and cellular transplantation. Nat Biotechnol 33: 518-523.
Citation: Refaie AF, Gabr MM, El-Halawani SM, KhaterSM, Ashamallah S, et al. (2020) From Mesenchymal Stromal Stem cells to Insulin-Producing Cells: Challenges in Translation for a Clinical Application. J Stem Cell Res Dev Ther 6: 027.
Copyright: © 2020 Ayman F Refaie, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.

