
Hydrophilic polymer coating improved airway injury and reduce mucous adhesion in silicone airway stents
*Corresponding Author(s):
Roy Joseph ChoInterventional Pulmonary, Department Of Pulmonary, Allergy, Critical Care And Sleep Medicine, University Of Minnesota, Minneapolis MN, United States
Email:Choxx548@umn.edu
Abstract
Background
Airway stenting allows restoration of airway patency however associated airway injury and mucostasis can incur significant comorbidity and mortality. We aim to compare a coated stent vs non-coated stent in both degree of airway injury and mucostasis.
Methods
We modified commercially available silicone stents with a hydrophilic polymer from Toray Industries. We conducted an in vivo survival study in six mainstem airways of three pigs to compare the degree of airway injury, mucostasis, and stent occlusion between coated vs control stented airways.
Results
We implanted a total of six 14x15mm silicone stents (one per mainstem bronchi) into three pigs. In all three airway sections, the degree of inflammation, submucosal gland hyperplasia, and fibrosis and granulation tissue formation was highest in the control vs coated stents. The control stent had higher tissue pathology scores in the distal and at stent sections than coated stents (62 vs 47 and 21 vs 14; respectively). There was a higher degree of mucostasis and percent occlusion in the control vs coated stents (3.59 and 5 vs 1.29 and 2.08; respectively).
Conclusions
This study demonstrates promising results in the reduced mucous adhesion within coated stents; moreover, demonstrates consistency in reduced airway injury compared to control stents. Future studies including larger number of subjects would be needed to corroborate our findings.
Keywords
Interventional pulmonary; Airway stent; Bronchial stenosis; Mucostasis, X-ray Photoelectron Spectroscopy (XPS); Time-of-Flight Secondary Ion Mass Spectroscopy (TOF-SIMS)
Highlight Box
- Key Findings: Coated stents demonstrated reduced airway injury and less mucous adhesion than control stents.
- What is known and what is new? Airway stenting can rapidly improve airway patency and associated symptomatology; however, airway injury and scarring and build-up of debris imposes substantial morbidity and mortality after stent placement. This study demonstrates that a hydrophilic polymer coated stent can improve airway injury and mucous adhesion in silicone stents placed in the airway.
- The implication of this findings is that our proprietary anti-mucous adhesion coating may help to improve post-stent complications. Larger randomized studies will be needed in human models to corroborate our findings.
Introduction
The airway stenting space in the 21st century has exponentially grown with the development of newer stent design, materials, and deployment devices [1]. One of the mainstays of treatment for symptomatic central airway obstruction is consideration of airway stenting; however, all stents incur airway injury and development of mucostasis [2-4]. Over the past four-years, our group has experimented with coating silicone stents with a hydrophilic polymer that has shown to resist mucous adhesion in vitro and has demonstrated promise in reducing airway injury in a randomized, single-blinded study in porcine models [5-7]. The objective of this study is to corroborate our previous findings; in addition, to investigate stent mucostasis and percent occlusion in coated vs control stents without routine therapeutic suctioning.
Methods
The study was conducted at the University of Minnesota’s Advanced Preclinical Imaging Center (APIC) and Interventional Pulmonary Airway Lab. The protocol was approved by the Institutional Animal Care and Use Committee of the University of Minnesota, Minneapolis MN (Protocol ID 2012-38723A).
Preparation and Surface Analysis of the Hydrophilic Coating on Silicone Stents
Toray’s proprietary hydrophilic polymer was applied to a Novatech tracheobronchial silicone stent from Boston Medical Products (Shrewsbury, MA, USA). The preparation technique was previously described by our group [5]. Small sections were cut and tested using X-ray Photoelectron Spectroscopy (XPS) and Time-of-Flight Secondary Ion Mass Spectroscopy (TOF-SIMS) to confirm existence of the hydrophilic polymer and coating homogeneity, respectively.
In vivo porcine model preparation and stent deployment
The study assessed the 8-week performance of coated and control (uncoated) stents in three Yorkshire farm pigs (body weight 45 kg). A total of two stents (coated and control) were implanted into each pig. We randomized the coated stent location to either left or right mainstem bronchus; whereby, the other bronchus would have the control stent implant. This model was selected on account of the similarity between porcine and adult human central bronchial diameters [8]. Routine therapeutic suctioning during the surveillance bronchoscopies was not performed unless it was life-threatening.
Inhalation and intravenous medications used for the induction and maintenance of general anesthesia followed our APIC laboratory and IUCAC guidelines. The stents were sterilized with ethylene oxide (ETO) using a low temperature recipe prior to implantation. Karl Storz (Tuttlingen, Germany) equipment including a 14-mm diameter adult rigid bronchoscope, rigid alligator forceps, and rigid peanut forceps were used for stent deployment and adjustment. The standard 14x40mm stents were cut to 15mm length to prevent inadvertent lobar obstruction and perceived morbidity during this 8-week study. The cut-end was intentionally placed in the proximal position of the mainstem bronchus. This was done because the proximal edge would be facing the distal trachea which would not be used for evaluation of this study.
The stent performance was assessed by means of flexible bronchoscopy (Tele-Pak, Karl Storz) on a weekly basis for a duration of 8-weeks after initial stent placement. The performance evaluation involved the examination of the stents to assess migration, granulation tissue overgrowth at both ends, and degree of mucostasis. Any mucous build-up was suctioned clear with a flexible bronchoscope.
At eight weeks from initial stent placement, the animal was euthanized, and the tracheobronchial tree was resected and placed in formalin for histological assessment (see Figure 1). Three formalin-fixed sections of upper airway tissue collected at the tracheal bifurcation from 3 animals were provided by the submitter. These tissues were trimmed to yield six sections of tissue per animal (Figures 1 & 2).
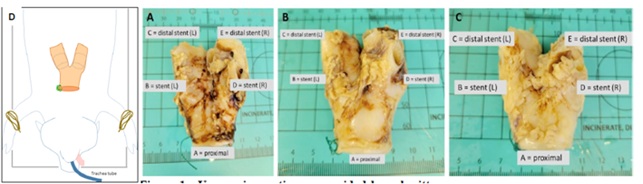 Figure 1: Intact upper airway tissue from the distal tracheal, the tracheal bifurcation, and the left and right main-stem bronchi (containing intraluminal stents). The individual images reflect animal #133 / 22-100-1 (A), #134 / 22-100-2 (B), and #135 / 22-100-3 (C). Each animal was trimmed to create 5 cassettes, labelled A (trachea), B (right bronchus at stent), C (right bronchus distal to stent), D (left bronchus at stent), and E (left bronchus distal to stent).
Figure 1: Intact upper airway tissue from the distal tracheal, the tracheal bifurcation, and the left and right main-stem bronchi (containing intraluminal stents). The individual images reflect animal #133 / 22-100-1 (A), #134 / 22-100-2 (B), and #135 / 22-100-3 (C). Each animal was trimmed to create 5 cassettes, labelled A (trachea), B (right bronchus at stent), C (right bronchus distal to stent), D (left bronchus at stent), and E (left bronchus distal to stent).
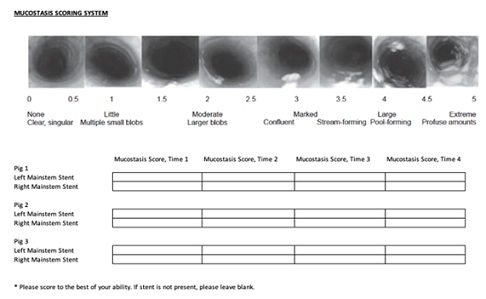 Figure 2: Gross Airway Inspection Scoring Sheet. Adapted and revised from Koblinger JK, Nicol K, McDonald K, Wasko A, Logie N, Weiss M, and Leguillette R. J Vet Intern Med. 2011;25(5):1118-26.
Figure 2: Gross Airway Inspection Scoring Sheet. Adapted and revised from Koblinger JK, Nicol K, McDonald K, Wasko A, Logie N, Weiss M, and Leguillette R. J Vet Intern Med. 2011;25(5):1118-26.
Assessment of Airway Injury
The insult to the airway was assessed using gross observation of mucostasis, granulation tissue formation and stent migration; mucous weight at termination of the study; and histological assessment of mucosal injury and application of an airway injury score adapted from Sinha R and colleagues [9]. One to three tissue sections were paraffin-embedded, sectioned, mounted onto slides, and stained with Hematoxylin and Eosin (H&E) and Periodic acid-Schiff (PAS) using standard techniques [10]. H&E and PAS-stained sections were evaluated using light microscopy according to STP guidelines [11]. H&E-stained sections were evaluated and scored using a modified version of a previously published algorithm. Grading scheme provided in Table 1.
|
Histologic Parameter |
Scoring Details |
|
Cilia Loss |
All three graded 0, 1, 2, 3, 4, as absent, <25%, 25-50%. 50-75%, and 100% |
|
Epithelial Flattening |
|
|
Ulceration |
|
|
Inflammation Severity |
Graded 0, 1, 2, and 3 as absent, mild, moderate, and severe |
|
Inflammation Depth |
Grade 0, 1, 2, and 3 as absent, affecting the mucosa, affecting submucosa, and mucosa, or affecting the mucosa, submucosa, and perichrondrium |
|
Epithelial Hyperplasia |
Graded 0, 1, 2, and3, as absent, mild, moderate, and severe |
|
Hemorrhage |
|
|
Submucosal Gland Hyperplasia |
|
|
Fibrosis/Granulation Tissue |
|
|
Goblet cells |
Estimated in increments of 20% on PAS stained slides |
Table 1: Summary of Histologic Injury and Repair Scoring Scheme for Airway Tissues.
Histological assessment and calculation of the airway injury score were performed along three sections of the airway that were in direct contact with the stent, this included proximal, at stent, and distal. The slides were randomized and then reviewed by a pathologist who was blinded to the stent identity and subsection classification. The subsections on each slide were semi-quantitatively assessed and graded for inflammation, granulation tissue, and extent of tissue damage (see Table 1). Absent and low grades for each variable were considered within normal physiological spectrum.
Stent Mucostasis Assessment
Mucostasis assessment was provided by using a mucostasis scoring system described by Koblinger JK and colleagues [12]. The bronchoscopist graded each stent based on this scoring system and was blinded to the identity of the porcine model and stent type (Figures 3 & 4).
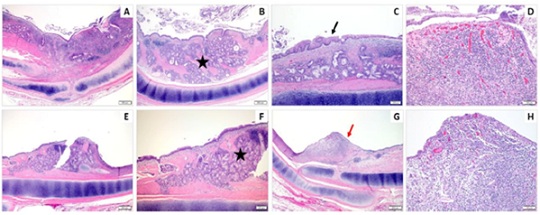 Figure 3: Composite of H&E-stained images from the “At Stent” site from the Coated Stents. In both representative animals, there is expansion of the mucosa and submucosa by mucus gland hyperplasia (black star, B&F), a mixture of epithelial hyperplasia with squamous metaplasia (black arrow, C), epithelial ulceration (red arrow, G), and an often marked inflammatory infiltrate with fibrosis and neovascularization (D&H). Magnification: 20X (A,E), 40X (B-C & F-G), and 100X (D&H).
Figure 3: Composite of H&E-stained images from the “At Stent” site from the Coated Stents. In both representative animals, there is expansion of the mucosa and submucosa by mucus gland hyperplasia (black star, B&F), a mixture of epithelial hyperplasia with squamous metaplasia (black arrow, C), epithelial ulceration (red arrow, G), and an often marked inflammatory infiltrate with fibrosis and neovascularization (D&H). Magnification: 20X (A,E), 40X (B-C & F-G), and 100X (D&H).
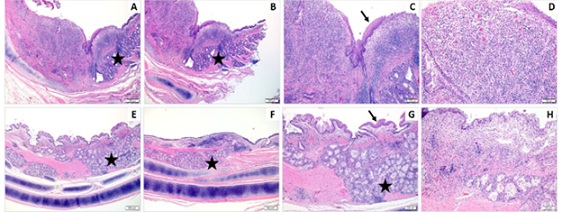 Figure 4: Composite of H&E-stained images from the “At Stent” site from the Control Stents. In both representative animals, there is expansion of the mucosa and submucosa by mucus gland hyperplasia (black star, B&F), a mixture of epithelial hyperplasia with squamous metaplasia (black arrow, C), epithelial ulceration (not shown), and an often marked inflammatory infiltrate with fibrosis and neovascularization (D&H). Magnification: 20X (A,E), 40X (B-C & F-G), and 100X (D&H).
Figure 4: Composite of H&E-stained images from the “At Stent” site from the Control Stents. In both representative animals, there is expansion of the mucosa and submucosa by mucus gland hyperplasia (black star, B&F), a mixture of epithelial hyperplasia with squamous metaplasia (black arrow, C), epithelial ulceration (not shown), and an often marked inflammatory infiltrate with fibrosis and neovascularization (D&H). Magnification: 20X (A,E), 40X (B-C & F-G), and 100X (D&H).
Statistical analysis
The null hypothesis regarding the performance (migration, granulation tissue formation, and mucous retention) in a porcine model was that the pathology score and mucous weight would be equal between the two stent types. Due to the low sample size of three pigs or six airways, descriptive statistics were used to analyze the data.
Results
All three pigs survived to the 8-week termination endpoint. All six 14x15mm silicone stents were successfully deployed without cardiopulmonary compromise. Gross comparison at the weekly surveillance bronchoscopies revealed that both stents remained intact at 8-weeks. One coated stent migrated out at the 6-week surveillance. Only one pig received therapeutic suctioning due to life threatening mucous plug.
Table 2, compares the marked difference in degree of inflammation severity, submucosal gland hyperplasia, and fibrosis and granulation tissue formation between coated and control stents along the three airway sections. In these airway sections, the degree of inflammation, submucosal gland hyperplasia, and fibrosis and granulation tissue formation was highest in the control vs coated stents.
|
|
Inflammation |
Hemorrhage |
Cilia Loss |
Epithelial Flattening |
Ulceration |
Epithelial Hyperplasia |
Submucosal Gland Hyperplasia |
Fibrosis and Granulation Tissue |
|
Coated-At Stent |
42 |
6 |
31 |
16 |
7 |
9 |
17 |
13 |
|
Coated-Distal |
18 |
2 |
7 |
7 |
0 |
6 |
5 |
0 |
|
Control-At Stent |
53 |
9 |
17 |
17 |
14 |
21 |
24 |
20 |
|
Control-Distal |
27 |
2 |
6 |
6 |
0 |
10 |
6 |
3 |
Table 2: Pathology Score for Individual Stented Airways and Summary. Individual pathology score for stented airway (Top) and Summary for coated vs non-coated stents are illustrated (Bottom).
Tables 3 illustrates the histopathological airway injury scores in all three pigs. Main sections including at-stent and distal-stent are illustrated. The control stent had higher tissue pathology in the distal and at stent sections than coated stents (62 and 21 vs 47 and 14); respectively.
|
CPSR# |
Animal# |
Side (stent type) |
Section |
Pathology Scores |
|
22-100-1 |
133 |
Left (coated) |
At Stent |
58 |
|
Distal |
23 |
|||
|
Right (control) |
At stent |
53 |
||
|
Distal |
18 |
|||
|
22-100-2 |
134 |
Left (control) |
At stent* |
62 |
|
Distal |
17 |
|||
|
Right (coated) |
At stent |
57 |
||
|
Distal |
17 |
|||
|
22-100-3 |
135 |
Left (control) |
At stent** |
70 |
|
Distal |
28 |
|||
|
Right (coated) |
At stent |
26 |
||
|
Distal |
2 |
|
Stent Type |
Section |
Pathology Score |
|
Coated |
Distal |
47 |
|
At Stent |
14 |
|
|
Control |
Distal |
62 |
|
At Stent |
21 |
Table 3: Pathology Score for Individual Stented Airways. Individual pathology score for stented airway (Top) and summary for coated vs control are illustrated (bottom).
* Significant proximal migration noted.
** No stent noted at the time of tissue collection.
Stent mucostasis and percent occlusion within the stent were assessed in all three pigs (Table 4 and Figure 5). There was a higher degree of mucostasis and % occlusion in the control vs coated stents (5 and 3.59 vs 2.08 and 1.29; respectively).
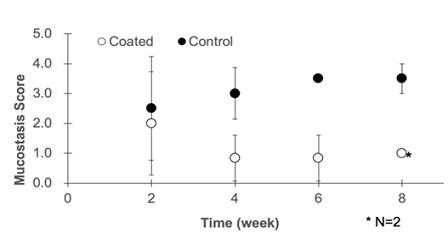 Figure 5: Quantification of Mucus Deposition within the Stent. Mucus was collected with a suction tube. The suction tube was flashed with 10mL of saline. The mucus and saline were collected into the trap, and dried under a heating condition
Figure 5: Quantification of Mucus Deposition within the Stent. Mucus was collected with a suction tube. The suction tube was flashed with 10mL of saline. The mucus and saline were collected into the trap, and dried under a heating condition
|
|
Week 2 |
Week 4 |
Week 6 |
Unplanned Event* |
Week 8 |
|||||||
|
ID and stent location |
Stent Type |
%Occlusion |
Mucostasis |
%Occlusion |
Mucostasis |
%Occlusion |
Mucostasis |
%Occlusion |
Mucostasis |
%Occlusion |
Mucostasis |
|
|
133-Left* |
Coated |
5 |
1 |
0 |
1 |
0 |
1 |
|
|
0 |
1 |
|
|
133-Right |
Control |
20 |
3.5 |
0 |
3.5 |
0 |
3.5 |
|
|
0 |
4 |
|
|
134-Left |
Control |
0 |
0.5 |
0 |
2 |
0 |
3.5 |
|
|
0 |
3.5 |
|
|
134-Right* |
Coated |
20 |
4 |
0 |
1.5 |
0 |
1 |
|
|
0 |
1 |
|
|
135-Left |
Control |
10 |
3.5 |
0 |
3.5 |
0 |
3.5 |
30 |
5 |
NA |
NA |
|
|
135-Right* |
Coated |
0 |
1 |
0 |
0 |
0 |
0 |
|
|
0 |
3 |
|
|
|
|
|||||||||||
|
|
|
%Occlusion |
Mucostasis |
|||||||||
|
Coated Total |
|
2.08 |
1.29 |
|||||||||
|
Control Total |
|
5 |
3.59 |
|||||||||
Table 4: Gross Inspection of Percent Occlusion and Mucostasis within the Implanted Stent over the 8-Week Study.
*Unplanned event occurred as #135 had worsening respiratory symptoms and required emergent bronchoscopy surveillance per protocol.
NA = Not applicable, stent was not available for measurements due to migration into trachea and subsequent removal.
Discussion
Adverse effects from airway injury as a response to a foreign body occurs through a locoregional inflammatory cascade that progresses to a chronic inflammatory phase characterized by the formation of granulation and fibrous tissue along a provisional matrix layer of the object [13-17]. If left unchecked, this could disrupt stent integrity causing migration and/or loss of stent patency, and infection leading to morbidly and possible mortality.
As reported in our previous work, hydrophilic surface modification has shown to be effective in preventing mucin adhesion onto silicone-based materials. We have shown a clear signal for reduced mucostasis and airway injury in a single pig model and reduced airway injury associated with the coated stent in our previous randomized animal study [6, 7]. The coated group had consistently lower histological airway injury profile and airway injury score compared to the control group. In this current study, we have continued to observe reduced airway injury associated with the coated stent compared to control. Additionally, we have demonstrated less mucous adhesion and percent occlusion associated with the coated stents during the 8-weeks observation without routine therapeutic suctioning.
In this study, we believe that bias was reduced by randomizing each stent to either left or right mainstem bronchus, randomizing the histological samples prior to the final assessment and blinding the pathologist to stent identity. However, there are several limitations to address. Because the weekly bronchoscopy evaluations represent a snapshot in time of the mucous within each stent, it is possible that small variations could occur on a day-to-day basis which would not be accounted for in our measurement for mucous adhesion. Like other studies evaluating degree of airway injury, there are no robust consistent scoring system to reflect injury related to an implantable device. The best injury score would encompass both depth of injury and type of cellular inflammation. In our previous study, the degree of mucostasis within the stent was determined by collecting the actual samples within the stent. This process was difficult and variable which produced large deviations in mucous weight. In this study, we used a modified mucous scoring system which may be a more sensitive method to determine mucostasis. We believe bias was reduced as the evaluator was blinded to animal and stent ID. Despite these limitations, the results of the current study demonstrate decreased airway injury, mucostasis, and %stent occlusion between the coated and control stents. Albeit the benefits for airway stenting in restoring airway patency and improving related symptoms, the challenge that remains is being able to overcome the device-host reactions (e.g. mucostasis and formation of granulation tissue) that result in overall airway injury. Our group has demonstrated continuing evidence using a hydrophilic polymer coating on silicone stents as one potential solution to mitigate these host-driven consequences which could disrupt the host-device inflammatory response and airway injury. The coated stents demonstrate promising characteristics in reducing mucin adhesion leading to lower mucostasis and percent stent occlusion; in addition, to decreasing the degree of airway injury along the contact between the stent and airway mucosa.
Conclusion
The coated stents continued to demonstrate lower airway pathology and airway injury compared to control stents. Moreover, the coated stents demonstrated less mucostasis and percent occlusion. Future animal studies incorporating larger sample size and longer survival duration will be needed to corroborate our findings.
Acknowledgements
We would like to thank the APIC and Dr Cho’s Airway Lab at the University of Minnesota in providing the necessary equipment and support to complete this study.
Foot Note
Conflict of Interest: Roy Cho is a consultant for Toray Industries.
Human/Animal Ethics Approval Declaration: This study confirmed and was approved by University of Minnesota’s IUCAC committee.
Data Availability Statement: Data is available upon request.
Contributions
- Lead, concept and study design: Roy Cho, Koji Kadowaki, Robroy MacIver, Kazuhiro Tanahashi
- Methods and data: Roy Cho, Kazuhiro Tanahashi, Davis Seelig, Ryan Hunter.
References
- Tian S, Huang H, Hu Z, Dong Y, Bai C (2022) A narrative review of progress in airway stents. J Thorac Dis 15: 1674-1683.
- Ost DE, Shah AM, Lei X, Godoy MCB, Jimenez CA, et al. (2012) Respiratory Infections Increase the Risk of Granulation Tissue Formation Following Airway Stenting in Patients with Malignant Airway Obstruction. CHEST 141: 1473-1481
- Folch E, Keyes C (2018) Airway stents. Ann Cardiothorac 7: 273-283.
- Lee HJ, Labaki W, Yu DH, Salwen B, Gilbert C, et al. (2017) Airway stent complications: the role of follow-up bronchoscopy as a surveillance method. J Thorac Dis 9: 4651-4659.
- Glumac D, Kadowaki K, Cho RJ, Peterson G, Hunter R, et al. (2022) An anti-fouling airway stent. DMD 1031: 1-5.
- Cho RJ, Kadowaki K, Seelig D, Glumac DE, Kent LA, et al. (2022) Implantation of a Hydrophilic Polymer Coated Silicone Airway Stent Improves Airway Injury and Mucostasis in a Porcine Model: A Pilot Study. Archives in Respiratory & Pulmonary Medicine 1: ARPM.MS.ID.000504.
- Cho RJ, Kadowaki K, Seelig D, Glumac DE, Kent LA, et al. (2023) To Compare the Effects of a Standard versus Hydrophilic Polymer Coated Airway Stent in a Porcine Model: A randomized, single-blinded study. Journal of Bronchology and Interventional Pulmonology, in press.
- Judge EP, Hughes JM, Egan JJ, Maguire M, Molloy EL, et al. (2014) Anatomy and bronchoscopy of the porcine lung. A model for translational respiratory medicine. Am J Respir Cell Mol Biol 51: 334-343
- Sinha R, Correia R, Gardner D, Grau-Roma L, de Brot S, et al. (2018) Mucosal injury following short-term tracheal intubation: A noval animal model and composite tracheal injury score. Laryngoscope Investigative Otolaryngology 3: 257-262.
- Tucker DK, Foley JF, Hayes-Bouknight SA, Fenton SE (2016) Preparation of high quality hematoxylin and eosin-stained sections from rodent mammary gland whole mounts for histopathologic review. Toxicol Pathol 44: 1059-1064.
- Crissman JW, Goodman DG, Hildebrandt PK, Maronpot RR, Prater DA, et al. (2004) Best practices guidelines: Toxicology histopathology. Toxicol Pathology 32: 126-131.
- Koblinger JK, Nicol K, McDonald K, Wasko A, Logie N, et al. (2011) J Vet Intern Med 25:1118-1126.
- Criner GJ, Eberhardt R, Fernandez-Bussy S, Gompelmann D, Maldonado F, et al. (2020) Interventional bronchoscopy. Am J Respir Crit Care Med 202: 29-50.
- Guibert N, Saka H, Dutau H (2020) Airway stenting: technological advancements and its role in interventional pulmonology. Respirology 25: 953-962.
- Murgu SD, Egressy K, Laxmanan B, Doblare G, Ortiz-Comino R, et al. (2016) Central airway obstruction. Chest 150: 426-441.
- McGrath DJ, Thiebes AL, Cornelissen CG, O'Brien B, Jockenhoevel S, et al. Evaluating the interaction of a tracheobronchial stent in an ovine in-vivo model. Biomech Model Mechanobiol 17: 499-516.
- Major MR, Wong VW, Nelson ER, Longaker MT, Gurtner GC (2015) The foreign body response. Plast Reconstr Surg 135: 1489-1498.
Citation: Cho RJ, Kadowaki K, Seelig D, Hunter RC, MacIver RH, et al. (2024) Hydrophilic polymer coating improved airway injury and reduce mucous adhesion in silicone airway stents. J Pulm Med Respir Res 10: 084.
Copyright: © 2024 Roy Joseph Cho, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.

