
Imbalanced Angiogenic Factors in Hemoptysis Vasculature from Bronchiectasis
*Corresponding Author(s):
Yoshiteru MorioCenter For Pulmonary Circulation And Hemoptysis, Department Of Respiratory Medicine, National Hospital Organization Tokyo National Hospital, Takeoka Kiyose-shi Tokyo, Japan
Tel:+ 81 424912111,
Email:morio.yoshiteru.pn@mail.hosp.go.jp
Abstract
Bronchiectasis (BE) often causes life-threatened hemoptysis. There raises a higher risk for relapsed hemoptysis when complicated with chronic pulmonary infection in BE patients. Hemoptysis may occur through rupture of aberrant vessels in bronchial artery-pulmonary artery shunt. Although imbalanced angiogenic factors may account for aberrant vessels responsible for hemoptysis, a precise mechanism of the aberrant vasculature formation is still unclear. We present a case report of a 72-year-old female with BE and repeated hemoptysis, who underwent surgical resection because of uncontrollable hemoptysis by Bronchial Artery Embolization (BAE). We performed immunohistochemical examination and observed imbalanced angiogenic factors in the aberrant vessels responsible for hemoptysis of the specimens from surgical resections.
Keywords
Angiopoietin-1; Angiogenesis; Bronchiectasis; Hemoptysis; Tie-2; Vascular endothelium growth factor
Introduction
Bronchiectasis (BE) is one of the most frequent causes of hemoptysis in pulmonary diseases [1]. Hemoptysis is a life-threatening symptom due to asphyxia and blood loss and then can also impair quality of life in patients [2-5]. While hemoptysis occurs substantially in 20-37% [6,7], there raises a higher risk for relapsed hemoptysis when complicated with chronic pulmonary infection in BE patients [8,9].
Candidates of therapeutic strategy for hemoptysis include hemostatic agents, bronchoscopy, Bronchial Artery Embolization (BAE) and surgery [2,4,5]. BAE, a minimally invasive procedure, is the first-line treatment for hemoptysis patients [5]. We have previously reported that BAE is efficacious for the control of hemoptysis in patients with Mycobacterium avium complex infection, chronic pulmonary aspergillosis and cryptogenic hemoptysis as well as in BE patients [1,10-12]. However, there remains to be concerned for recurrent hemoptysis in some patients after BAE.
Hemoptysis may occur through rupture of aberrant vessels in bronchial artery-pulmonary artery shunt. Life-threatened hemoptysis occurs presumably in 30-40% from systemic artery-pulmonary circulation shunts [13]. Aberrant vessels responsible for hemoptysis manifest often in BE patients with chronic pulmonary infection [8,9]. However, a precise mechanism of the aberrant vasculature formation is still unclear. While homeostasis of vasculature is regulated by angiogenic factors, including Vascular Endothelium Growth Factor (VEGF) and Angiopoietin-1 (Ang1), aberrant vasculature may occur in imbalance of angiogenic factors during inflammation [14-17].
We present a case report of a 72-year-old female with BE and repeated hemoptysis, who underwent surgical resection because of uncontrollable hemoptysis by BAE. We hypothesized that angiogenic factors may be imbalanced in the aberrant vessels responsible for hemoptysis and then performed immunohistochemical examination of angiogenic factors with the specimens from surgical resections.
Case Presentation
A 72-year-old female was referred to our hospital for repeated hemoptysis even though receiving continuous prescription of hemostatic agents. She formerly smoked 20 cigarettes per day for 43 years and had history of infant tuberculosis. She was received several prescriptions for hypertension and hyperlipidemia for 20 years, and underwent intracephalic aneurysm clip at age of 62 years. In recent 5 years, she was suffering from repeated hemoptysis.
A physical examination revealed middle-to-late inspiratory coarse crackles in the right lower lung field. Both chest X-ray and Computed Tomography (CT) revealed bronchiectatic changes and collapsed shrinkages in right lower lobe (Figure 1). Both contrast-enhanced CT and three-dimensional CT revealed convolution and aneurysm of bronchial artery (Figure 1). Bronchial arteriography demonstrated 7-mm dilated convolution and 20-mm sized pooling of contrast injection in the right lower lobe. Outflow from the vascular lesion into right lower pulmonary arteries was also stained during the early arterial phase, suggesting a presence of bronchial artery-pulmonary artery shunt (Figure 1). Bronchoscopic study was not performed because of the patient’s anxiety for airway bleeding.
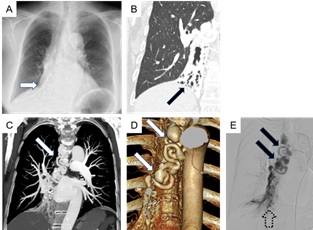 Figure 1: A, B: Chest X-ray and Computed Tomography (CT) revealed bronchiectatic changes and collapsed shrinkages in right lower lobe (arrows). C, D: Contrast-enhanced CT and three-dimensional CT revealed convolution and aneurysm of bronchial artery (arrows). E: Bronchial arteriography demonstrated 7-mm dilated convolution and 20-mm sized pooling of contrast injection in the right lower lobe (arrows). Outflow from the vascular lesion into right lower pulmonary arteries was also stained (dashed arrow).
Figure 1: A, B: Chest X-ray and Computed Tomography (CT) revealed bronchiectatic changes and collapsed shrinkages in right lower lobe (arrows). C, D: Contrast-enhanced CT and three-dimensional CT revealed convolution and aneurysm of bronchial artery (arrows). E: Bronchial arteriography demonstrated 7-mm dilated convolution and 20-mm sized pooling of contrast injection in the right lower lobe (arrows). Outflow from the vascular lesion into right lower pulmonary arteries was also stained (dashed arrow).
Since 20-mm sized aneurysm of bronchial artery elongated, the patient underwent both initial BAE at proximal aneurysm of bronchial artery and subsequent right posterolateral thoracotomy and then middle-lower lobectomy was taken. She was discharged home without any incident on postoperative day 14 and resolved her hemoptysis. No overt appearance of new aneurysm in bronchial artery has seen in follow-up CT 1 year later.
We performed immunohistochemical examination of angiogenic factors with specimens from surgical resections. We compared immunohistochemical stainings of VEGF, Ang1 and its receptor, Tie2, in pulmonary arteries between bleeding lesion adjacent to bronchial artery (i.e. pathological lesions) and non-bleeding lesion distant from bronchial artery (i.e. control lesions) in the specimens. The immunohistochemical staining was classified accordingly into following three grades. Grade 0: no staining; Grade 1: partial staining; Grade 2: whole staining of pulmonary arterial wall. In each tissue section, at least 50 consecutive pulmonary arteries were analyzed at X20 magnification. Predominant expressions of Ang1 and Tie2 were observed in pulmonary artery from non-bleeding lesion. In contrast, while expression of VEGF was augmented, those of Ang1 and Tie2 were attenuated in pulmonary artery from bleeding lesion (Figure 2). Collectively, these immunohistochemical findings suggested that imbalanced angiogenic factors emerged in the aberrant vessels responsible for hemoptysis.
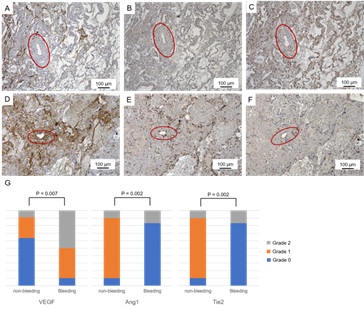 Figure 2: A-C: Representative immunohistochemical stainings of VEGF (A), Ang1 (B) and Tie2 (C) in pulmonary arteries (ovals) distant from bronchial artery in the specimens from surgical resections. D-F: Those of VEGF (D), Ang1 (E) and Tie2 (F) in pulmonary arteries (ovals) adjacent to bronchial artery in the specimens. G: Distributions of immunohistochemical staining grades for each angiogenic factor in pulmonary arteries between bleeding lesion adjacent to bronchial artery and non-bleeding lesion distant from bronchial artery in the specimens from surgical resections. P values were analyzed statistically by Fisher's exact test.
Figure 2: A-C: Representative immunohistochemical stainings of VEGF (A), Ang1 (B) and Tie2 (C) in pulmonary arteries (ovals) distant from bronchial artery in the specimens from surgical resections. D-F: Those of VEGF (D), Ang1 (E) and Tie2 (F) in pulmonary arteries (ovals) adjacent to bronchial artery in the specimens. G: Distributions of immunohistochemical staining grades for each angiogenic factor in pulmonary arteries between bleeding lesion adjacent to bronchial artery and non-bleeding lesion distant from bronchial artery in the specimens from surgical resections. P values were analyzed statistically by Fisher's exact test.
Discussion
BE is one of the most frequent cause of hemoptysis in pulmonary diseases [1]. While hemoptysis occurs substantially in 20-37% [6,7], there raises a higher risk for relapsed hemoptysis when complicated with chronic pulmonary infection in BE patients [9,10]. BAE is the first-line treatment among several candidates of therapeutic strategy for hemoptysis [5]. Despite long-term efficacy of BAE to control hemoptysis, there remains to be concerned for recurrent hemoptysis after BAE in some BE patients [1,3,4,18,19].
Hemoptysis through rupture of aberrant vessels may occur due to vulnerable vasculature, especially in adjacent pulmonary artery, against a higher blood pressure in bronchial artery-pulmonary artery shunt. Life-threatened hemoptysis occurs presumably in 30-40% from systemic artery-pulmonary circulation shunts [13]. However, a precise mechanism of the aberrant vasculature formation remains to be elucidated.
Homeostasis of vasculature is regulated by orchestration between angiogenic factors, including VEGF and Ang1.VEGF is not only essential for sprouting and migration of endotheliums during angiogenesis but also has important functions for maintenance of vascular system. Ang1 and Tie2 system has important functions in regulating vascular integrity and maintenance of quiescent endothelial state [20]. After VEGF-driven phase of active angiogenesis, Ang1 and Tie2 system contributes to vascular homeostasis by regulating endothelial barrier function [14].
Aberrant vasculature responsible for hemoptysis may occur in imbalance of angiogenic factors during inflammation from pulmonary aspergillosis, tuberculosis or BE [15-17]. We examined and compared immunohistochemical staining of VEGF, Ang1 and Tie2 in pulmonary arteries between bleeding lesion adjacent to bronchial artery and non-bleeding lesion distant from bronchial artery in the specimens from surgical resections. While expressions of Ang1 and Tie2 but not VEGF were observed predominantly in pulmonary artery from non-bleeding lesion, augmented expression of VEGF and attenuated expressions of Ang1 and Tie2 were observed in pulmonary artery from bleeding lesion. These immunohistochemical findings suggested that imbalanced angiogenic factors emerged in the aberrant vessels responsible for hemoptysis. Existences of imbalanced angiogenic factors in vascular malformation, including arteriovenous fistula, have been demonstrated in retina, dura and cerebral vessels [14,21,22]. Since there raises a possibility in contribution of imbalanced angiogenic factors to vascular malformation, we speculate that imbalanced angiogenic factors may play a role for hemoptysis vasculature from BE.
Conclusion
We present here a 72-year-old female with BE and repeated hemoptysis, who underwent surgical resection because of uncontrollable hemoptysis by BAE. Despite long-term efficacy of BAE to control hemoptysis, there remains to be concerned for recurrent hemoptysis after BAE in some BE patients. Although imbalanced angiogenic factors were observed in the aberrant vessels responsible for hemoptysis in the present case report, the precise mechanism of vasculature formation remains to be elucidated. Since such findings cannot be currently endorsed, future studies in this area are strongly encouraged.
Conflict of Interest
The authors have no conflict of interest to declare. We thank Asari I. for assistance with immunohistochemical examination in the present case report. No fund supports the present case report.
References
- Takeda K, Kawashima M, Masuda K, Kimura Y, Yamamoto S, et al. (2020) Long-term outcomes of bronchial artery embolization for patients with non-mycobacterial non-fungal infection bronchiectasis. Respiration 99: 961-969.
- American College of Radiology (2019) ACR appropriateness criteria hemoptysis. American College of Radiology, Virginia, United States.
- Lee MK, Kim SH, Yong SJ, Shin KC, Kim HS, et al. (2015) Moderate hemoptysis: Recurrent hemoptysis and mortality according to bronchial artery embolization. Clin Respir J 9: 53-64.
- Ishikawa H, Hara M, Ryuge M, Takafuji J, Youmoto M, et al. (2017) Efficacy and safety of super selective bronchial artery coil embolisation for haemoptysis: A single-centre retrospective observational study. BMJ Open 7: 014805.
- Panda A, Bhalla AS, Goyal A (2017) Bronchial artery embolization in hemoptysis: A systematic review. Diagn Interv Radiol 23: 307-317.
- Dimakou K, Triantafillidou C, Toumbis M, Tsikritsaki K, Malagari K, et al. (2016) Non CF-bronchiectasis: Aetiologic approach, clinical, radiological, microbiological and functional profile in 277 patients. Respir Med 116: 1-7.
- King P, Holdsworth S, Freezer N, Holmes P (2006) Bronchiectasis. Intern Med J 36: 729-737.
- Maleux G, Matton T, Laenen A, Bonne L, Cornelissen S, et al. (2018) Safety and efficacy of repeat embolization for recurrent hemoptysis: A 16-year retrospective study including 223 patients. J Vasc Interv Radiol 29: 502-509.
- Lee JH, Kwon SY, Yoon HI, Yoon CJ, Lee KW, et al. (2007) Haemoptysis due to chronic tuberculosis vs. bronchiectasis: Comparison of long-term outcome of arterial embolisation. Int J Tuberc Lung Dis 11: 781-787.
- Okuda K, Masuda K, Kawashima M, Ando T, Koyama K, et al. (2016) Bronchial artery embolization to control hemoptysis in patients with Mycobacterium avium complex. Respir Investig 54: 50-58.
- Ando T, Kawashima M, Masuda K, Takeda K, Okuda K, et al. (2019) Exacerbation of chronic pulmonary aspergillosis was associated with a high rebleeding rate after bronchial artery embolization. Respir Investig 57: 260-267.
- Ando T, Kawashima M, Masuda K, Takeda K, Okuda K, et al. (2017) Clinical and angiographic characteristics of 35 patients with cryptogenic hemoptysis. Chest 152: 1008-1014.
- Lu GD, Zu QQ, Liu XL, Wang B, Zhou CG, et al. (2016) Embolisation for life-threatening haemoptysis complicated by systemic artery-pulmonary circulation shunts. Int J Tuberc Lung Dis 20: 276-281.
- Eklund L, Kangas J, Saharinen P (2017) Angiopoietin-Tie signalling in the cardiovascular and lymphatic systems. Clin Sci (Lond) 131: 87-103.
- Inoue K, Matsuyama W, Hashiguchi T, Wakimoto J, Hirotsu Y, et al. (2001) Expression of vascular endothelial growth factor in pulmonary aspergilloma. Intern Med 40: 1195-1199.
- Corr P (2006) Management of severe hemoptysis from pulmonary aspergilloma using endovascular embolization. Cardiovasc Intervent Radiol 29: 807-810.
- Park HY, Hahm CR, Jeon K, Koh WJ, Suh GY, et al. (2012) Serum vascular endothelial growth factor and angiopoietin-2 are associated with the severity of systemic inflammation rather than the presence of hemoptysis in patients with inflammatory lung disease. Yonsei Med J 53: 369-376.
- Woo S, Yoon CJ, Chung JW, Kang SG, Jae HJ, et al. (2013) Bronchial artery embolization to control hemoptysis: Comparison of N-butyl-2-cyanoacrylate and polyvinyl alcohol particles. Radiology 269: 594-602.
- Chun JY, Belli AM (2010) Immediate and long-term outcomes of bronchial and non-bronchial systemic artery embolisation for the management of haemoptysis. Eur Radiol 20: 558-565.
- Farkas L, Gauldie J, Voelkel NF, Kolb M (2011) Pulmonary hypertension and idiopathic pulmonary fibrosis: A tale of angiogenesis, apoptosis, and growth factors. Am J Respir Cell Mol Biol 45: 1-15.
- Klisch J, Kubalek R, Scheufler KM, Zirrgiebe U, Drevs J, et al. (2015) Plasma vascular endothelial growth factor and serum soluble angiopoietin receptor sTIE-2 in patients with dural arteriovenous fistulas: A pilot study. Neuroradiology 47: 10-17.
- Zheng Q, Du J, Zhang Z, Xu J, Fu L, et al. (2013) Association study between of Tie2/angiopoietin-2 and VEGF/KDR pathway gene polymorphisms and vascular malformations. Gene 523: 195-198.
Citation: Enomoto Y, Takeda K, Kawashima M, Masuda K, Fukumoto K, et al. (2021) Imbalanced Angiogenic Factors in Hemoptysis Vasculature from Bronchiectasis. J Pulm Med Respir Res 7: 067.
Copyright: © 2021 Yu Enomoto, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.

