
Immunological Diagnosis of Entervirus 71 in Developing Countries
*Corresponding Author(s):
Chit Laa PohResearch Centre For Biomedical Sciences, Sunway University, Bandar Sunway, 47500 Subang Jaya, Selangor, Malaysia
Tel:+60 374918622,
Email:pohcl@sunway.edu.my
Abstract
Hand, Foot and Mouth Disease (HFMD) is a commonly occurring mild febrile disease in young children (<6 years of age). Clinical symptoms are high fever, rash, ulcers in the mouth and vesicles on the hands and feet. The two most common pathogens causing HFMD are Enterovirus (EV-A71) and Coxsackievirus (CV-A16). In recent years, large HFMD outbreaks have occurred in Asia and instead of manifesting itself as a mild disease; HFMD caused by EV71 has been increasingly associated with severe neurological disorders and high fatalities. More than 7 million cases of HFMD have been reported with over 2000 fatalities in China. Other Human Enteroviruses (HEVs) such as CV-A6, 8, A10, A16, Coxsackie B5, Echovirus 4, Echovirus 19 and Echovirus 30 have also been isolated from HFMD outbreaks but they have not been associated with fatal infections. Since there is no effective vaccine or antiviral for the treatment of EV-A71, surveillance of the pathogen in the community and social distancing by isolation of infected patients provide prospects for control of large outbreaks. The control measures are highly dependent on rapid identification of EV-A71 from clinical specimens. RT-PCR, real-time RT-PCR and Reversed Transcription Loop-Mediated Isothermal Amplification (RT-LAMP) are highly sensitive and specific in detecting EV-A71 but these molecular approaches require expensive equipment and molecular reagents, trained personnel and could not be readily adopted for use in rural and provincial hospitals in developing countries. This review provides an update of the immunoassays that have been developed for the rapid and accurate diagnosis of EV-A71 in developing countries.
Keywords
INTRODUCTION
BACKGROUND
HFMD is commonly regarded as a mild febrile disease in infants and young children less than 6 years of age. Children usually develop high fever, sore throat and rash on the hands and feet. Blisters can develop in the mouth which tends to lead to ulcers (herpangina). The most common pathogens isolated from HFMD infections are EV-A71 and Coxsackievirus CA-16 [4]. Instead of causing a mild disease, HFMD caused by EV-A71 has been increasingly associated with severe neurological disorders and high fatalities in recent outbreaks in Asia [5]. The surveillance registry of China reported 7,200,092 probable cases of HFMD, with 2457 deaths from 2008-2012, but only 267,942 cases (3.7%) were laboratory confirmed [6]. Besides EV-A71 and CV-A16, other HEVs such as Coxsackievirus types A 8, A 10 and A12, Coxsackie B5, Echovirus type 4, Echovirus 19 and Echovirus 30 were reported to be isolated as viral pathogens in HFMD outbreaks [7]. However, the other Enteroviruses have not been commonly associated with fatal infections. Thus, there is a need to identify the viral pathogen(s) in every major HFMD outbreak that had high fatalities.
EpidemiologyAlthough EV-A71 was first isolated from a child in California, USA, in 1969 [8], there is phylogenetic evidence to show that it was present in the Netherlands as early as 1963 [9]. Subsequently, smaller outbreaks with neurological infections caused by EV-A71 were reported in Australia, Japan, Sweden and the USA [10]. High fatalities were reported for two large outbreaks caused by EV-A71 in Bulgaria in 1975 and in Hungary three years later. There were 44 fatalities amongst 451 children presenting with non-specific febrile illness or neurological disease in Bulgaria [11] and 47 deaths amongst 1550 children (826 aseptic meningitis and 724 encephalitis) in Hungary [12]. Smaller outbreaks and sporadic clusters had occurred in Hong Kong in 1985 and in Australia in 1986 before another large outbreak involving 2618 HFMD cases and 34 deaths were reported for Sarawak in 1997 [13]. Taiwan reported the largest HFMD outbreak in 1998 involving 1.5 million cases with 78 deaths [14]. In 2000, a large HFMD outbreak occurred in Singapore involving 3790 patients and there were 5 deaths, 3 due to HFMD and 2 to non-HFMD [15]. Since then, HFMD is recognised as an endemic mild disease in both Malaysia and Singapore. China was the next country to report a large HFMD outbreak involving 490,000 infections with 126 deaths in 2008. Since 2009, the number of HFMD infections in China had steadily increased and there were 2,819,581 HFMD cases being reported with 394 deaths in 2014. Vietnam reported 4265 HFMD cases with two deaths in 2015 [16]. Outside the Asia Pacific region, smaller outbreaks or sporadic infections with no fatality or low fatalities have been reported in Europe [17].
The EV-A71 genome is about 7.4 K band has a 5’ Non-Translated Region (5’NTR), a long Open Reading Frame (ORF) and a short 3’NTR followed by a polyadenylated (poly A) tail. The 5’NTR contains an Internal Ribosome Entry Site (IRES) which allows viral protein translation in a cap-independent manner [18]. The ORF is translated into a single large polyprotein of approximately 2100 amino acids, which is divided into three regions (P1-P3). The polyprotein undergoes a series of processing events, culminating in the maturation cleavage of the polyprotein, giving rise to structural and non-structural viral proteins [19]. The four structural proteins, VP1, VP2, VP3 and VP4, are encoded by the P1 region, which constitutes the virus capsid. Proteins derived from the non-structural P2 (2Apro, 2B, 2BC, and 2CATPase) and P3 (3A, 3AB, 3B, 3Cpro, 3CDpro, and 3Dpol) regions are most directly involved in virus replication [20] (Figure 1).
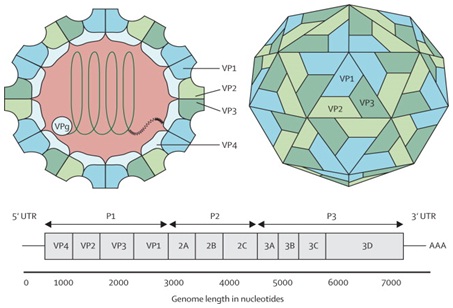
The capsid consists of 60 protomers, each consisting of four polypeptides that comprise the structural proteins: VP1, VP2, VP3, and VP4 and are encoded by the P1 region of the genome. The P2 and P3 regions encode for seven non-structural proteins: 2A-2C and 3A-3D (the EV-A71 genome is represented by the green line, followed by poly-A residues at the 3’UTR). Reproduced from viral zone, with permission from Swiss Institute of Bioinformatics.
Three genotypes (genogroups) of EV-A71 were established based on the phylogenetic analysis of the VP1 gene. They are designated as A, B and C and each genotype differ from each other by at least 15% at the nucleotide level [21]. Genotype A consists of only one member and is represented by the prototype BrCr strain which was not reported outside of the USA until 2008 when it was isolated from five children presenting with HFMD from the Anhui province in China. The B genotype group can be further subdivided into 5 sub-genotypes (B1-B5) and were predominant in Malaysia and Singapore. The C genotype group is represented by 5 sub-genotypes. Since 2000, the sub-genotype C4 was the predominant sub-genotype circulating in China. There were reports of shifts of sub-genotype dominance in Taiwan and Japan, from B-sub-genotypes to C-sub-genotypes [22,23]. In more recent years, three new genotypes were discovered; one in India (designated as genotype D), one in Central Africa (designated as genotype E) and another from Madagascar (designated as genotype F) by sequencing the VP1 and VP2 regions of clinical isolates [24].
The frequency and size of HFMD outbreaks in Asia present a challenging public health issue. Most countries in Asia have made HFMD a notifiable disease and outbreak control measures are targeted at interrupting person to person transmission or removing EV-A71 from contaminated surfaces of inanimate objects. The usual route of transmission is through the oral-faecal route and distancing measures were effective in reducing the rate of transmission [25]. Since there is neither a vaccine to prevent nor antivirals to treat HFMD due to EV-A71, empirical supportive treatment using fluid replacement and pain relieving is the current option [26]. Expensive treatment involving administration of Intravenous Immunoglobulins (IVIG) appears to be useful for severe disease in some studies but due to the lack of randomized, placebo-controlled phase 2 trials, there is no clear evidence to support the claim that it is an effective treatment strategy [27].
CLINICAL CASE REPORT
She had been regularly attending the antenatal consultations with no risk factors identified. Her prenatal laboratory results were unremarkable except for GBS-unknown. She had three normal obstetric ultrasounds (one of each trimester); her blood type was A+. Pregnancy was uneventful with no history of vomiting, blood loss or abdominal trauma.
On admission at the delivery unit, the obstetric ultrasound revealed no fetal movements with the presence of heart beat. The Cardiotocograph (CTG) was not tranquilizing as it showed prolonged deceleration and reduced variability with pathological trace that suggested a sinusoidal pattern and, as a result, an emergent caesarean section was performed (Figure 1).A baby boy was born weighing 2610g. The newborn had a circular of the umbilical cord around the arms. On examination at birth, he was markedly pale and hypotonic with respiratory depression. Orothracheal intubation and connection to mechanical ventilation was immediately performed. He responded well and was extubated 4 minutes after and transferred to the neonatal unit with oxygen directly to his face, for further evaluation and management. The Apgar score was 5/8/8.
Initial blood gas from the umbilical cord revealed pH 7.27, pCO2 50.6 mm Hg, Hemoglobin 4.4, g/dL, bicarbonate 21.9 mmol/L and lactates 5.8 mmol/L. Laboratory exams revealed 4.0 g/dL of hemoglobin, white blood cell count of 47.700/10 EXP 9/L with 22.7% neutrophils (10.800), platelets count 183.000/10 EXP 9/L, DHL 680 UI/L, CK 190 UI/L. Further laboratory evaluation was unchanged (bilirubin, cardiac enzymes and C reactive protein). Coombs test and viral serology for Parvovirus B19 and Cytomegalovirus were negative. Hemoglobin electrophoresis showed a presence of 5% fetal hemoglobin on mother’s blood. Kleihauer-Betke test was performed, since it is a more specific exam and quantifies the amount of blood transfusion. It revealed 17.8% of fetal red cells in maternal circulation, which corresponds to a volume of approximately 890 mL of fetal blood based on the formula: (% of fetal cells determined by Kleihauer-Betke test/100) X 5000 mL = volume of FMH (in mL) [3] and also according to the fact that 1% of fetal erythrocytes in maternal circulation is equivalent to a fetal hemorrhage of 50mL [4].
Two red blood cell transfusions were made and at 12 hours of life his hemoglobin was 13.3 g/dL, white blood cells count of 10.100/uL (Neutrophils: 64.4%), platelets count of 219.000/uL and erythroblasts 87/100 leucocytes.
The outcome was favorable with hemodynamic and respiratory stability and absence of abnormal movements. Cranial ultrasonography showed, in the 3rd day of life, frontal bilateral parenchymal hyperechogenicity, was not present on 11th day of life as the ultrasounds were made by two different physicians. The authors admit that the hyperechogenicity have not been valorized by the second physician.
Follow-up at 2 and 4 months revealed a normal physical and neurological examination.
Diagnosis of Enterovirus 71
Molecular techniques such as real-time qRT-PCR has the advantage of speed and accuracy of diagnosing EV-A71 and other Enteroviruses [30] but the requirement for a sophisticated real-time PCR instrument, expensive reagents and the need to sequence the RT-PCR products tend to hinder its diagnostic applications in massive outbreak situations. Rapid identification of EV-A71 in rural clinics and hospitals is needed to identify patients who require hospitalizations. An improvement in molecular diagnosis of EV-A71 based on the Reverse Transcription Loop-Mediated Isothermal Amplification (RT-LAMP) assay has received good evaluation in terms of its sensitivity and specificity [31]. Although the requirement for an expensive real-time PCR can be dispensed with, there is still the need for an expensive and specific commercial loop amp RNA amplification kit and a fluorescent detection reagent [32] which may prohibit laboratories from the rural hospitals from the developing countries to adopt a molecular approach for the rapid diagnosis of EV-A71 from large outbreaks in the field. It has been developed for research and has not been commercialised.
Immunoassays
The commercial immunological assays relied mainly on the use of purified whole virions and the epitope present can be cross-reactive due to the high genome homology shared by some Enterovirus serotypes such as CV-A16 and human Echovirus 6 with EV-A71. Pozzetto et al., reported that IgM positive anti-Enterovirus antibodies were not serotype specific [37]. An immunodominant VP1 linear epitope bearing the core sequence LEGTTNPNG was identified by Foo et al. The GST-fusion protein carrying the epitope was showing significant immunoreactivity in the Western blot assay but was non-reactive with anti-EV-A71 IgG in ELISA [38]. Routsias et al., designed Enterovirus serotype specific synthetic peptides spanning the amino-terminal 1-15 residues of VP1 of the 10 most common Enterovirus serotypes reported to the Centers for Disease Control and Prevention in USA from 1970 to 2008. They showed that majority of the IgM positive sera were reactive with a single serotype specific peptide, thus establishing the homotypic nature of the peptide-ELISA assay. The specificity of peptide recognition was assessed in competitive inhibition studies. Homologous peptides were able to inhibit the binding of IgM to their target antigens from 67-95%, thus supporting specific peptide recognition [39]. Alignment analysis of amino acid sequences of EV-A71 showed that amino acid residues from 6-43 was highly conserved among all genotypes and sub-genotypes of EV-A71 and are different from strains of CV-A16 and Echovirus 6. When a VP1 truncated protein carrying the N-terminal antigenic epitope expressed as a GST-VP16-43 fusion protein was used in an indirect ELISA assay to capture anti-EV-A71 IgM in human sera, it showed a sensitivity of 77.8% and 100% specificity for early diagnosis of EV-A71 [40]. The sensitivity of this EV-A71-specific assay could perhaps be further improved by employing a direct IgM capture format. The authors speculated that the lower sensitivity could be due to the presence of specific IgG in the test sera which competed with the EV-A71specific IgM for antigen binding. A cross-neutralizing epitope within residues 136-150 of VP2 which was highly conserved among EV-A71 genotypes and sub-genotypes was identified to be a good surrogate biomarker in potency testing of EV-A71 vaccine candidates. A synthetic peptide VP2-28 which corresponded to amino residues 136-150 of VP2 was employed to develop an epitope specific sandwich enzyme-linked Immunosorbent assay (Q-ELISA). However, the VP2-28 peptide specific Q-ELISA was found to recognize neutralizing antisera only from rabbits and was non-reactive with antisera from mice and rats immunized with formalin-inactivated whole EV-A71 virion. It is unknown whether the synthetic peptide VP2-28 will be able to elicit neutralizing antibodies in humans and this need to be assessed before the Q-ELISA can be further developed for immunoassays with human sera [41].
He et al., developed an epitope blocking ELISA (EB-ELISA) which was able to detect specific serum antibodies to purified EV-A71 virus and differentiate it from serum antibodies to other Enterovirussubtypes such as CVA4, CVA6, CVA10 and CV-A16. In EB-ELISA, antibodies from human sera specific for EV-A71 could be detected by the ability to block the binding of a specific Mab IC6 to the target epitope present in the EV-A71 virion. The EB-ELISA was found to be more sensitive than the virus neutralization and the Immunofluorescent test (IFA). It has a specificity of 100% in detection of EV-A71 viruses from 100 samples of human sera with positive neutralization titer [42]. Five monoclonal antibodies which specifically reacted with EV-A71 and did not cross-react with CV-A16 or Echovirus type 6 (ECHO6) were produced by Xu et al. When the five monoclonal antibodies were evaluated in a Capture ELISA assay format, they were highly specific for EV-A71 particles. The binding specificities of four of the monoclonal antibodies varied with different EV-A71 sub genotypes but not for MAb 27. A linear epitope DVIESSIGDSVSRAL located at the N-terminus (aa 6-20) of EV-A71 VP1 was identified to be highly conserved in all EV-A71 sub genotypes by peptide ELISA [43]. Thus, MAb 27 may have specific applications in diagnosis of EV-A71 in Capture-ELISA assays but will need further confirmation using clinical specimens.
Conclusion
Competing Interests
REFERENCES
- http://www.picornaviridae.com/Enterovirus/Enterovirus.htm
- Rotbart HA, O'Connell JF, McKinlay MA (1998) Treatment of human Enterovirus infections. Antiviral Res 38: 1-14.
- Ooi MH, Wong SC, Podin Y, Akin W, del Sel S, et al. (2007) Human Enterovirus 71 disease in Sarawak, Malaysia: a prospective clinical, virological, and molecular epidemiological study. Clin Infect Dis 44: 646-656.
- McMinn PC (2002) An overview of the evolution of Enterovirus 71 and its clinical and public health significance. FEMS Microbiol Rev 26: 91-107.
- World Health Organization (2011) A guide to clinical management and public health response for hand, foot and mouth disease. World Health Organization, Geneva, Switzerland.
- Xing W, Liao Q, Viboud C, Zhang J, Sun J, et al. (2014) Hand, foot, and mouth disease in China, 2008-12: an epidemiological study. Lancet Infect Dis 14: 308-318.
- Solomon T, Lewthwaite P, Perera D, Cardosa MJ, McMinn P, et al. (2010) Virology, epidemiology, pathogenesis, and control of Enterovirus 71. Lancet Infect Dis 10: 778-790.
- Schmidt NJ, Lennette EH, Ho HH (1974) An apparently new Enterovirus isolated from patients with disease of the central nervous system. J Infect Dis 129: 304-309.
- van der Sanden S, Koopmans M, Uslu G, van der Avoort H; Dutch Working Group for Clinical Virology (2009) Epidemiology of Enterovirus 71 in the Netherlands, 1963 to 2008. J Clin Microbiol 47: 2826-2833.
- Gilbert GL, Dickson KE, Waters MJ, Kennett ML, Land SA, et al. (1988) Outbreak of Enterovirus 71 infection in Victoria, Australia, with a high incidence of neurologic involvement. Pediatr Infect Dis J 7: 484-488.
- Chumakov M, Voroshilova M, Shindarov L, Lavrova I, Gracheva L, et al. (1979) Enterovirus 71 isolated from cases of epidemic poliomyelitis-like disease in Bulgaria. Arch Virol 60: 329-340.
- Nagy G, Takátsy S, Kukán E, Mihály I, Dömök I (1982) Virological diagnosis of Enterovirus type 71 infections: experiences gained during an epidemic of acute CNS diseases in Hungary in 1978. Arch Virol 71: 217-227.
- Lum LC, Wong KT, Lam SK, Chua KB, Goh AY, et al. (1998) Fatal Enterovirus 71 encephalomyelitis. J Pediatr 133: 795-798.
- Ho M, Chen ER, Hsu KH, Twu SJ, Chen KT, et al. (1999) An epidemic of Enterovirus 71 infection in Taiwan. Taiwan Enterovirus Epidemic Working Group. N Engl J Med 341: 929-935.
- Chong CY, Chan KP, Shah VA, Ng WY, Lau G, et al. (2003) Hand, foot and mouth disease in Singapore: a comparison of fatal and non-fatal cases. Acta Paediatr 92: 1163-1169.
- World Health Organization (2015) Hand, Foot, and Mouth Disease surveillance summary. World Health Organization, Geneva, Switzerland.
- Hassel C, Mirand A, Lukashev A, TerletskaiaLadwig E, Farkas A, et al. (2015) Transmission patterns of human Enterovirus 71 to, from and among European countries, 2003 to 2013. Euro Surveill 20: 30005.
- Brown B, Oberste MS, Maher K, Pallansch MA (2003) Complete genomic sequencing shows that polioviruses and members of human Enterovirus species C are closely related in the noncapsid coding region. J Virol 77: 8973-8984.
- Belsham GJ, Sonenberg N (1996) RNA-protein interactions in regulation of picornavirus RNA translation. Microbiol Rev 60: 499-511.
- UniProtKB - Q66478 (POLG_HE71B), UniProt.
- Brown BA, Oberste MS, Alexander JP Jr, Kennett ML, Pallansch MA (1999) Molecular epidemiology and evolution of Enterovirus 71 strains isolated from 1970 to 1998. J Virol 73: 9969-9975.
- Huang SW, Hsu YW, Smith DJ, Kiang D, Tsai HP, et al. (2009) Reemergence of Enterovirus 71 in 2008 in taiwan: dynamics of genetic and antigenic evolution from 1998 to 2008. J Clin Microbiol 47: 3653-3662.
- Iwai M, Masaki A, Hasegawa S, Obara M, Horimoto E, et al. (2009) Genetic changes of Coxsackievirus A16 and Enterovirus 71 isolated from hand, foot, and mouth disease patients in Toyama, Japan between 1981 and 2007. Jpn J Infect Dis 62: 254-259.
- Bessaud M, Razafindratsimandresy R, Nougairède A, Joffret ML, Deshpande JM, et al. (2014) Molecular comparison and evolutionary analyses of VP1 nucleotide sequences of new African human Enterovirus 71 isolates reveal a wide genetic diversity. PLoS One 9: 90624.
- Ang LW, Koh BK, Chan KP, Chua LT, James L, et al. (2009) Epidemiology and control of hand, foot and mouth disease in Singapore, 2001-2007. Ann Acad Med Singapore 38: 106-112.
- Ooi MH, Wong SC, Mohan A, Podin Y, Perera D, et al. (2009) Identification and validation of clinical predictors for the risk of neurological involvement in children with hand, foot, and mouth disease in Sarawak. BMC Infectious Diseases 9: 3.
- Zhang Q, MacDonald NE, Smith JC, Cai K, Yu Het al. (2014) Severe Enterovirus type 71 nervous system infections in children in the Shanghai region of China: clinical manifestations and implications for prevention and care. Pediatr Infect Dis J 33: 482-487.
- Lim KA, Benyesh-Melnick M (1960) Typing of viruses by combinations of antiserum pools. Application to typing of Enteroviruses (Coxsackie and ECHO). J Immunol 84: 309-317.
- Hsueh C, Jung SM, Shih SR, Kuo TT, Shieh WJ, et al. (2000) Acute encephalomyelitis during an outbreak of Enterovirus type 71 infection in Taiwan: report of an autopsy case with pathologic, immunofluorescence, and molecular studies. Mod Pathol 13: 1200-1205.
- Tan EL, Yong LL, Quak SH, Yeo WC, Chow VT, et al. (2008) Rapid detection of Enterovirus 71 by real-time TaqMan RT-PCR. J Clin Virol 42: 203-206.
- Nie K, Qi SX, Zhang Y, Luo L, Xie Y, et al. (2012) Evaluation of a direct reverse transcription loop-mediated isothermal amplification method without RNA extraction for the detection of human Enterovirus 71 subgenotype C4 in nasopharyngeal swab specimens. PLoS One 7: 52486.
- Zhang H, Nie K, Liu Y, Luo L, Huang W et al. (2012) Evaluation of Reverse Transcription Loop-Mediated Isothermal Amplification assays for Rapid Detection of Human Enterovirus 71 and Coxsackievirus A16 in Clinical Samples. Advances in Infectious Diseases 2: 110-118.
- Tsao KC, Chan EC, Chang LY, Chang PY, Huang CG, et al. (2002) Responses of IgM for Enterovirus 71 infection. J Med Virol 68: 574-580.
- Xu F, Yan Q, Wang H, Niu J, Li L, et al. (2010) Performance of detecting IgM antibodies against Enterovirus 71 for early diagnosis. PLoS One 5: 11388.
- Yu N, Guo M, He SJ, Pan YX, Chen XX, et al. (2012) Evaluation of human Enterovirus 71 and Coxsackievirus A16 specific immunoglobulin M antibodies for diagnosis of hand-foot-and-mouth disease. Virol J 9: 12.
- Huang KY, Yang S, Tsao KC, Chen CJ, Hsieh YC, et al. (2013) Bedside immunochromatographic test for Enterovirus 71 infection in children. J Clin Virol 58: 548-552.
- Pozzetto B, Gaudin OG, Aouni M, Ros A (1989) Comparative evaluation of immunoglobulin M neutralizing antibody response in acute-phase sera and virus isolation for the routine diagnosis of Enterovirus infection. J Clin Microbiol 27: 705-708.
- Foo DG, Ang RX, Alonso S, Chow VT, Quak SH, et al. (2008) Identification of immunodominant VP1 linear epitope of Enterovirus 71 (EV71) using synthetic peptides for detecting human anti-EV71 IgG antibodies in Western blots. Clin Microbiol Infect 14: 286-288.
- Routsias JG, Mavrouli MD, Antonaki G, Spanakis N, Tsakris A (2014) Synthetic peptides for efficient discrimination of anti-Enterovirus antibodies at the serotype level. Peptides 58: 52-59.
- Zhang J, Jiang B, Xu M, Dai X, Purdy MA, et al. (2014) Identification of specific antigenic epitope at N-terminal segment of Enterovirus 71 (EV-71) VP1 protein and characterization of its use in recombinant form for early diagnosis of EV-71 infection. Virus Res 189: 248-253.
- Liu CC, Chou AH, Lien SP, Lin HY, Liu SJ, et al. (2011) Identification and characterization of a cross-neutralization epitope of Enterovirus 71. Vaccine 29: 4362-4372.
- He F, Keener TK, Lim XF, Tan Y, Raj KV, et al. (2013) Development of a sensitive and specific epitope-blocking ELISA for universal detection of antibodies to human Enterovirus 71 strains. PLoS One 8: 55517.
- Xu L, Huang KJ, Ho TS, Liu CC, Lee YR, et al. (2013) Monoclonal antibodies for diagnosis of Enterovirus 71. Monoclon Antib Immunodiagn Immunother 32: 386-394.
Citation: Poh CL, Ffrench R, Claridy MD, Anderson D (2016) Immunological Diagnosis of Entervirus 71 in Developing Countries. J Infect Non Infect Dis 2: 014
Copyright: © 2016 Chit Laa Poh, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.

