
In-Vitro Investigation of the Cytotoxic and Genotoxic Effects of Benzimidazole Group Pesticides Benomyl and Carbendazim
*Corresponding Author(s):
Mehtap KaraDepartment Of Pharmaceutial Toxicology, Istanbul University Faculty Of Pharmacy, Istanbul, Turkey
Tel:+09 02124400000,
Email:mehtap.kara@istanbul.edu.tr
Abstract
Fungicides are the most effective method to control fungal microorganisms which cause plant diseases. The benzimidazole group of fungicides acts by inhibiting microtubule formation. Benomyl and its metabolite carbendazim are the most commonly used benzimidazole group systemic agricultural fungus in developing countries. Benomyl is an important teratogenic agent, have toxic effects on male reproductive system and also on the nervous system due to the mechanisms that disrupt the microtubule organization are also frequently encountered. Carbendazim is also known aneugen fungucide. In our study, the cytotoxic effects of benomyl and its metabolite carbendazim were investigated by MTT and NRU tests on human neuroblastoma cell line (SH-SY5Y) and rat kidney epithelial cell line (NRK-52E) and their genotoxic effects were tested by Comet assay. According to the results of cytotoxicity in our study, the LC50 values in SH-SY5Y and NRK52E cell lines were 108.7μM and 25.7μM for benomyl, respectively; and 201.3μM and 1619.5μM for carbendazim, respectively. As a result of our cytotoxicity study, the doses to be used in the genotoxicity assessment were determined for benomyl and carbendazim in both cell lines. According to Comet assay results it has been observed that benomyl and carbendazim have genotoxic effects on SH-SY5Y and NRK52E cell lines.
INTRODUCTION
Fungucides have been commonly used pesticides against fungal diseases in the aim of increase crop production. However adverse effects of fungucides on other species not yet clearly identified. It is important to understand mechanisms that play role under toxic effects for effective hazard identification and risk assessment, increase beneficities of fungucides and also protect non-target species [1]. Benzimidazole fungucides have systemic effects and selectively disrupt tubulin biosynthesis through inhibit α and β tubulin dimerisation that cause disruption in fungal spindle fibril structures. Benomyl and its metabolite carbendazim are widely used benzimidazole fungucides against to crop fungi and believed that these fungucides are nontoxic to other species except male reproductive system [2].
Benomyl is metabolized into functionally active carbendazim and this metabolite is commonly used fungucide from farmers. Benomyl and carbendazim show their toxic effects by inhibiting mitosis through binding β tubulin subunits of microtubules [3-5]. Benomyl binds mammalian neuronal tubulins with low affinity and prevent polymerization of tubulins [6]. Due to weak and slow catabolism of carbendazim, its mostly retained in the tissues [7]. In mammalians, benomyl rapidly absorbed and metabolized through hydroxylation and hydrolysis in the liver and excreted into urine and feces [8]. Carbendazim; absorbed as high as 80-85% after oral exposure and then metabolizes many molecules [7]. Benomyl and carbendazim have severel adverse effects as male reproductive system disruptions, teratogenicity, neurodegeneration, dermal sensitisation, tubular degeneration in kidney, liver toxicity, endocrine disruption and cancer [9-15]. There is only one studyabout benomyl’ toxic effect on SH-SY5Ycells in the literature and no data about carbendazim cytotoxic and genotoxic effect on SH-SY5Ycells [16], and therewithal there is no data on NRK52E cell line. In this study the aim was to investigate cytotoxic and genotoxic effects of benomyl and carbendazim commercial products on SH-SY5Y and NRK52 cell lines.
MATERIALS AND METHODS
Cell culture and cytotoxicity assays
|
NRK-52E MTT Exposure Doses (µM) |
|
|
Benomyl |
10-100 |
|
Carbendazim |
50-1100 |
|
SH-SY5Y MTT Exposure Doses (µM) |
|
|
Benomyl |
6.25-250 |
|
Carbendazim |
25-350 |
|
NRK-52E NRU exposure doses (µM) |
|
|
Benomyl |
10-100 |
|
Carbendazim |
500-1100 |
|
SH-SY5Y NRU exposure doses (µM) |
|
|
Benomyl |
25-250 |
|
Carbendazim |
100-350 |
The rat kidney proximal tubular epithelial cell line (NRK-52E) and Human Neuroblastoma Cell line (SH-SY5Y) were obtained from American Type Culture Collection (ATCC, Manassas, VA). The cells were grown at 37°C in a humidified incubator with 5% CO2 in Dulbecco's Modified Eagles medium consisting of nutrient mixture F12 (DMEM/F12) supplemented with 10% heat-inactivated Fetal Bovine Serum (FBS) and 1% antibiotic (100U/mL penicillin and 100µg/mL streptomycin).
NRK-52E and SH-SY5Y cells were seeded at 104 cells into each well of 96-well plates following disaggregation of cells with trypsin/EDTA. After 24h, the cells were exposed to benomyl and carbendazim doses were shown in table 1. After 24h incubation period, cytotoxicity was assessed using MTT test. Optical densities (OD) of each well were determined at 590nm and compared, against at a reference wavelength of 670nm, using a microplate spectrophotometer system (Epoch, Erlangen, Germany). 1% DMSO were used assolvent control for all assays. All concentrations were tested in triplicates and each test was repeated triple. The absorbance values of samples were compared with those of the solvent controls (1% DMSO) after all values were corrected by subtracting the absorbance value of a blank (negative control). The cytotoxic activity was expressed as an IC50, the concentration of extracts that caused a 50% inhibition of enzyme activity in the cells.
For NRU test a total of 104 cells/well were plated in 96 well tissue-culture plates. After 24h incubationthe cells were exposed to benomyl and carbendazim doses were shown in table 1. The cells were incubated for 24h at 37°C in 5% CO2, then the medium was discarded. The cells were washed twice with PBS and incubated for an additional 3h in the medium supplemented with NR (50µg/ml).The cells were rinsed five three with PBS and 200µl of “fixation solution” (50% ethanol, 1% acetic acid, and 49% distilled water) was added to each well to fix the cells and bring NR into solution. The plates were shaken for 20min, and the absorbance of the solution in each well was measured in a microplate reader at 540nm using a microplate spectrophotometer system (Epoch, Erlangen, Germany). Results were expressed as the mean percentage of cell growth inhibition from three independent experiments. IC50 values represent the concentrations that reduced the mean absorbance of 50% of those in the untreated cells.
Genotoxicity assays
|
NRK-52E Cell Line Exposure Doses |
|||||
|
Benomyl |
10μM |
5μM |
2,5μΜ |
1,25μM |
Control |
|
Carbendazim |
900μΜ |
450μM |
225μM |
112,5μM |
Control |
|
SH-SY5Y Cell Line Exposure Doses |
|||||
|
Benomyl |
60μΜ |
30μM |
15μΜ |
7,5μM |
Control |
|
Carbendazim |
100μΜ |
50μM |
25μM |
12,5μM |
Control |
STATISITCS
MTT and NRU tests results calculated and evaluated with Microsoft Office Excel Programme. Data were analyzed by one-way ANOVA Dunnett t-test and expressed as mean±SE. The level of statistical significance was set at p0.05, and all analyses were performed using the statistical package SPSS version 17.0 for Windows (SPSS Inc., Chicago, IL).
RESULTS
Cytotoxicity results
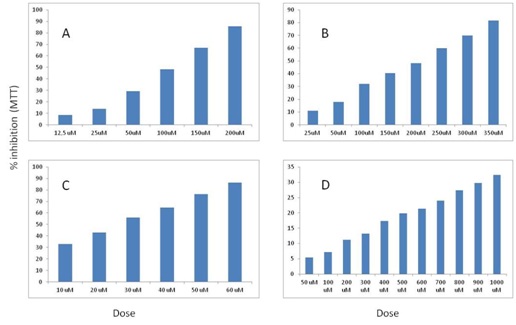 Figure 1: % inhibition values of benomyl and carbendazim on SH-SY5Y and NRK52E cells with MTT. A) Benomyl on SH-SY5Y cells; B) Carbendazim on SH-SY5Y cells; C) Benomyl on NRK52E cells; D) Carbendazim on NRK52E cells.
Figure 1: % inhibition values of benomyl and carbendazim on SH-SY5Y and NRK52E cells with MTT. A) Benomyl on SH-SY5Y cells; B) Carbendazim on SH-SY5Y cells; C) Benomyl on NRK52E cells; D) Carbendazim on NRK52E cells.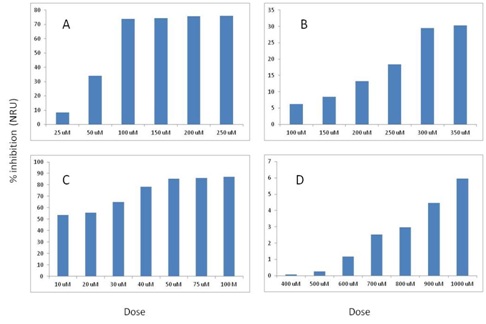 Figure 2: % inhibition values of benomyl and carbendazim on SH-SY5Y and NRK52E cells with NRU. A) Benomyl on SH-SY5Y cells; B) Carbendazim on SH-SY5Y cells; C) Benomyl on NRK52E cells; D) Carbendazim on NRK52E cells.
Figure 2: % inhibition values of benomyl and carbendazim on SH-SY5Y and NRK52E cells with NRU. A) Benomyl on SH-SY5Y cells; B) Carbendazim on SH-SY5Y cells; C) Benomyl on NRK52E cells; D) Carbendazim on NRK52E cells.Genotoxicity results
|
SH-SY5Y |
P Value |
NRK |
P Value |
||
|
Groups |
Mean ±SD |
|
Groups |
Mean ±SD |
|
|
Control |
3,774±0,632 |
|
Control |
4,184±0,377 |
|
|
7,5µM |
3,191±0,607 |
>0,05 |
1,25µM |
5,291±0,617 |
>0,05 |
|
15µM |
4,712±1,372 |
>0,05 |
2,5µM |
5,669±0,364 |
>0,05 |
|
30µM |
5,2349±0,522 |
<0,05* |
5µM |
4,959±0,950 |
>0,05 |
|
60µM |
6,306±0,344 |
<0,05* |
10µM |
3,514±0,238 |
>0,05 |
Table 3: Tail intensity values of Benomyl on SH-SY5Y and NRK cells.
Note: *significantly increased compared to control group.
|
SH-SY5Y |
P Value |
NRK |
P Value |
||
|
Groups |
Mean±SD |
|
Groups |
Mean±SD |
|
|
Control |
3,774±0,632 |
|
Control |
6,467±0,336 |
|
|
12,5µM |
4,235±1,393 |
>0,05 |
112,5µM |
8,926±1,230 |
<0,05* |
|
25µM |
3,869±0,772 |
>0,05 |
225µM |
9,787±0,604 |
<0,05* |
|
50µM |
5,224±0,683 |
>0,05 |
450µM |
12,45±0,932 |
<0,05* |
|
100µM |
5,260±1,453 |
>0,05 |
900µM |
12,19±0,219 |
<0,05* |
Table 4: Tail intensity values of Carbendazim on SH-SY5Y and NRK cells.
Note: *significantly increased compared to control group.
DISCUSSION
The benzimidazole pesticides benomyl and its main metabolite carbendazim, are fungicides that target to microtubules and inhibit microtubule asssembly and perturbing microtubule formation so this resulted with chromosomal assemble disruptions. It has been reported that carbendazim inhibit mitosis by disrupting the polymerization of mammalian tubulin into microtubules and arrest the cell cycle at the G2/M phase in turn induce apoptosis. Benomyl and carbendazim are worldwide used antifungal pesticides. It has been shown in different studies that benomyl and carbendazim cytotoxic effects on pancreas, prostate, colon and breast tissues. And also carbendazim have role on immun system deregulation [18-20].
In our study we found that the IC50 values of benomyl on NRK52E and SH-SY5Y cells were 25,78μM and 108,7μM and carbendazim were 1619,47μM and 201,27μM. DNA damage increased dose dependent with benomyl and carbendazim in NRK cells and in SH-SY5Y cells DNA damage increased in 30 and 60µM groups compared to control.There are different studies in the literature about cytotoxic and genotoxic effects of benomyl and carbendazim on different cells. Chang et al. [20], showed cell proliferation inhibition in human endometrial cells of benomyl and carbendazim with dose dependent . Laryae et al. [18], showed that benomyl have more potent cytotoxic effect than carbendazim on T-cell leukomia, multiple myeloma, small cell lung cancer, renal adenocarcinoma, cervical adenocarcinoma, normal retinal epithelial cells and LNCaP cells. In LNCaP cells IC50 values of benomyl and carbendazim were reported as 15±5.7 and 50±9.0 mmol/l.In another study in cultured rat hepatocytes 35ug/ml benomyl decreased 49% cell viability [21]. Dierickx [22], reported benomyl and carbendazim’s IC50 values in HepG2 cells are 203uM and >1750uM and in Fa32 cell 205uM and >1750uM neutral red uptake inhibition assay. In this study Dierickx classified benomyl more toxic chemical compared to carbendazim, quinalphos, carbaryl, piperonyl butoxide and 1-Aminobenzotriazole.
In another study with benomyl effects on 16HBE14o-(16HBE) human bronchial epithelial cells results indiated that IC50 values of benomyl administration for 24 or 48h are 44.2 and 7.2μM [23]. In human placental trophoblast cell line (HTR-8), compared to control group 2.5 and 5μM benomyl doses reduced cell viability by 5.79% and 6.49% and 5μM carbendazim dose decreased viability by 5.17% [24].
Benomyl and carbendazim classified as IARC group 2B possible human carcinogens. Benomyl is an aneugenic pesticide that disrupt microtubule formation. Benomyl caus micronuclei formation dyring cell division mechanism. It has been reported that 3.2-4.1mM benomyl concentrations associaed with chromosomal abormalities [25]. Lebailly et al. [26], reported that, benomyl administration in human peripheral blood lymphocytes up to 500uM did not increase DNA damage with Comet Assay. 1000mg/kg benomyl induces DNA damage in Japanese quails [27]. It has been demonstrated in several different studies that carbendazim induce DNA damage in different species asDaphnia magna, Eisenia foetida earthworms, Donax faba,mice, rat, in human lymphocytes. In D.magna species it has been demonstrated with comet assay that, carbendazim induce DNA damage cumulative and were seen in all the generations with multigenerational study. In another study, carbendazim induce DNA damage with duration dependent in Eisenia foetida earthworms [26-32].
In conclusion, our in-vitro study results in accordance with different studies about benomyl and carbendazim’s cytotoxic and genotoxic effects. While benomyl and carbendazim usage restricted in many countries, their usage stil continue in many developing countires. Thus deailed studies on these fungusides about its usage currency, accumulation in the environment, detailed mechanistic studies on their toxic effects should be clarified with further studies.
DECLARATION OF CONFLICTING INTERESTS
The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
REFERENCES
- Yang C, Hamel C, Vujanovic V, Gan Y (2011) Fungicide: Modes of Action and possible ?mpact on nontarget microorganisms. ISRN Ecology 1-8.
- Hess RA, Nakai M (2000) Histopathology of the male reproductive system induced by the fungicide benomyl. Histol Histopathol 15: 207-224.
- Ahmed SM, Ismail AA, Houusien AA (2010) Dissipation and persistence of fungicides, carbendazim and metalaxyl in egyptian soil under biotic and abiotic conditions. Journal of Applied Sciences Research 6: 1240-1246.
- McCarroll NE, Protzel A, Ioannou Y, Frank Stack HF, Jackson MA,et al. (2002) A survey of EPA/OPP and open literature on selected pesticide chemicals, III. Mutagenicity and carcinogenicity of benomyl and carbendazim. Mutat Res 512: 1-35.
- Rathinasamy K, Panda D (2006) Suppression of microtubule dynamics by benomyl decreases tension across kinetochore pairs and induces apoptosis in cancer cells. FEBS J 273: 4114-4128.
- Singh P, Rathinasamy K, Mohan R, Panda D (2008) Microtubule assembly dynamics: An attractive target for anticancer drugs. IUBMB Life 60: 368-375.
- World Health Organization (1993) Reproduction, embryotoxicity and teratogenicity. In: World Health Organization (ed.) Environmental Health Criteria 149 Carbendazim.World Health Organization, Geneva, Switzerland.
- Gardiner JA, Kirkland JJ, Klopping HL, Sherman H (1974) Fate of benomyl in animals. J Agric Food Chem 22: 419-427.
- Minta M,Biernacki B (1982) Embryotoxicity of carbendazim in hamsters, rats, and rabbits. Bulletin of the Veterinary Institute in Pulawy (Poland) 25: 42-52.
- Mantovani A, Maranghi F, Ricciardi C, Macrì C, Stazi AV, et al. (1998) Developmental toxicity of carbendazim: Comparison of no-observed-adverse-effect level and benchmark dose approach. Food Chem Toxicol 36: 37-45.
- Minta M, Wilk I, Mudzki J (2004) Embryotoxicity of carbendazim in rat and hamster micromass cultures. Bulletin of the Veterinary Institute in Pulawy (Poland) 48: 481-484.
- Lu SY, Liao JW, K?o ML, Ueng TH, Hwang JS (2006) Antagonistic and synergistic effects of carbendazim and flutamide exposures in utero on reproductive and developmental toxicity in rats. Journal of Food and Drug Analysis 14: 120-132.
- Casida JE, Ford B, Jinsmaa Y, Sullivan P, Cooney A,et al. (2014) Benomyl, aldehyde dehydrogenase, DOPAL, and the catecholaldehyde hypothesis for the pathogenesis of Parkinson’s disease. Chem Res Toxicol 27: 1359-1361.
- Muthuviveganandavel V, Muthuraman P, Muthu S,Srikumar K (2008) Toxic effects of carbendazim at low dose levels in male rats. J Toxicol Sci 33: 25-30.
- Kawaratani Y, Matsuoka T, Hirata Y, Fukata N, Nagaoka Y,et al. (2015) Influence of the carbamate fungicide benomyl on the gene expression and activity of aromatase in the human breast carcinoma cell line MCF-7. Environ Toxicol Pharmacol 39: 292-299.
- McLean WG, Holme AD, Janneh O, Southgate A, Howard CV, et al. (1998) The effect ofbenomyl on neurite outgrowth in mouse NB2A and human SH-SY5Y neuroblastoma cellsin vitro. Neurotoxicology 19: 629-632.
- Singh NP, McCoy MT, Tice RR, Schneider EL (1988)A simple technique for quantitation of low levels of DNA damage in individual cells. Exp Cell Res 175: 184-191.
- Laryea D, Gullbo J, Isaksson A, Larsson R, Nygren P (2010) Characterization of the cytotoxic properties of the benzimidazole fungicides, benomyl and carbendazim, in human tumour cell lines and primary cultures of patient tumour cells. AnticancerDrugs 21: 33-42.
- Wei KL, Chen FY, Lin CY, Gao GL, Kao WY, et al. (2016) Activation of aryl hydrocarbon receptor reduces carbendazim-induced cell death. Toxicol Appl Pharmacol 1: 86-97.
- Chang CC, Hsieh YY, Hsu KH, Tsai HD, Lin WH, et al. (2010) Deleterious effects of arsenic, benomyl and carbendazim on human endometrial cell proliferation in vitro. Taiwan J Obstet Gynecol 49: 449-454.
- Ramírez-Mares MV, Fatell S, Villa-Treviño S, González de Mejía E (1999) Protection of extract from leaves of Ardisia compressa against benomyl-induced cytotoxicityand genotoxicity in cultured rat hepatocytes. Toxicol In Vitro 13: 889-896.
- Dierickx PJ (1999) CYP1/2 Activation and glutathione-dependent cytotoxicity of fourpesticides in hep G2 and Fa32 cells. Toxicol In Vitro 13: 779-783.
- Jang Y, Lee AY, Kim JE, Jeong SH, Kim JS, et al. (2016) Benomyl-induced effects of ORMDL3 overexpression via oxidative stress in human bronchial epithelial cells. Food Chem Toxicol 98: 100-106.
- Zhou J, Xiong K, Yang Y, Ye X, Liu J, et al. (2015) Deleterious effects of benomyl and carbendazim on human placental trophoblast cells. Reprod Toxicol 51: 64-71.
- Langie SA, Koppen G, Desaulniers D, Al-Mulla F, Al-Temaimi R, et al. (2015) Causes of genome instability: The effect of low dose chemical exposures in modern society. Carcinogenesis 36: 61-88.
- Lebailly P, Vigreux C, Godard T, Sichel F, Bar E, et al. (1997) Assessment of DNA damage induced in vitro by etoposide and two fungicides (carbendazim and chlorothalonil) in human lymphocytes with the comet assay. Mutat Res 375: 205-217.
- Khan MZ, Hassan S, Mahmood F, Khan QM, Muhammad G, et al. (2008) Pathological effects of benomyl in male japanese quails (Coturnix japonica). Acta Vet Brno 77: 209-216.
- Silva AR, Cardoso DN, Cruz A, Pestana JL, Mendo S, et al. (2017) Multigenerational effects of carbendazim in Daphnia magna. Environ Toxicol Chem 36: 383-394.
- Huan Z, Luo J, Xu Z, Xie D (2016) Acute toxicity and genotoxicity of carbendazim, main ?mpurities and metabolite to earthworms (Eisenia foetida). Bull Environ Contam Toxicol 96: 62-69.
- JanakiDevi V, Nagarani N, YokeshBabu M, Kumaraguru AK, Ramakritinan CM (2013) A study of proteotoxicity and genotoxicity induced by the pesticide and fungicide on marine invertebrate (Donax faba). Chemosphere 90: 1158-1166.
- Ðiki? D, Mojsovi?-Cui? A, Cupor I, Benkovi? V, Horvat-Knezevi? A, et al. (2012) Carbendazim combined with imazalil or cypermethrin potentiate DNA damage in hepatocytes of mice. Hum Exp Toxicol 31: 492-505.
- Bowen DE, Whitwell JH, Lillford L, Henderson D, Kidd D, et al. (2011) Evaluation of a multi-endpoint assay in rats, combining the bone-marrow micronucleus test, the comet assay and the flow-cytometric peripheral blood micronucleus test. Mutat Res 722: 7-19.
Citation: Kara M, Jannuzzi AT, Yön ? (2019) In-Vitro Investigation of the Cytotoxic and Genotoxic Effects of Benzimidazole Group Pesticides Benomyl and Carbendazim. J Toxicol Cur Res 3: 007.
Copyright: © 2019 Mehtap Kara, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.

