
Influence of STAT3 and STAT6 on Splenic Susceptibility to Infection in Dogs with Visceral Leishmaniasis
*Corresponding Author(s):
Pamela Rodrigues Reina MoreiraSchool Of Agricultural And Veterinary Sciences (FCAV), SãoPaulo State University (UNESP), Department Of Pathology, Theriogenology And One Health, Via De Acesso Prof. Paulo Donato Castellane S/n, 14884-900, Jaboticabal, São Paulo State, Brazil
Tel:+55-16 3209-7368,
Email:pamela_rreina@yahoo.com.br
Abstract
Splenic susceptibility to Leishmania infantum infection in dogs may be related to the imbalance in the ratio between Th1 and Th2 immune responses, which favors the parasite due to the predominance of an immunosuppressive response. This study aimed to compare splenic lesions with immunostaining of the parasite load and STAT3 and STAT6 transcription factors in dogs with visceral leishmaniasis (VL). The spleens of 31 dogs, subdivided into control (C, n=4), asymptomatic (A, n=10), and symptomatic (S, n=17) group animals, were subjected to histopathological and immunohistochemical analysis. Infected dogs (A, S) presented granulomatous inflammation with macrophages parasitized in the red pulp and disorganized white pulp architecture with lymphoid atrophy. STAT3 and STAT6 immunostaining was observed in lymphocytes and macrophages. Parasite load differed (P < 0.05) between groups S and A. White pulp size and detection of STATs did not differ between infected and control groups. In multivariate analysis, a positive association was observed between parasite load and white pulp size and between STATs 3 and 6. Alterations are related to the infected animals (A and S). Splenic susceptibility in VL is possibly associated with white pulp disorganization and the immunosuppressive environment modulated by STAT3 and STAT6, resulting in the persistence of infection.
Keywords
Immunohistochemistry; Immune response; Leishmania infantum; Spleen; Transcription factors.
Introduction
Leishmaniosis is a zoonosis caused by the protozoan of the genus Leishmania spp., commonly described in tropical and subtropical regions of the world [1]. In 2020, 12.838 new cases were reported to the WHO (World Health Organization), most of these in Africa. The three eco-epidemiological hotspots were East Africa with 57% of cases (Ethiopia, Kenya, Somalia, Sudan), India with 18% (Bangladesh, Nepal) and Brazil with 16% of all reported cases in the world [2].
The dog is the primary host and domestic reservoir of Leishmania infantum, the etiologic agent of VL cases reported in Latin America [3]. This zoonosis has an essential impact on public health and has become a model for studying host-parasite interactions and evasion of the host's immune response [4]. Parasite-induced immunosuppression causes an imbalance in cytokine levels of cellular (Th1) and humoral (Th2) immune responses, resulting in suppression of the anti-Leishmania response [5]. The host's ability to control the infection is due to its ability to generate a cellular immune response (Th1), which activates macrophages (via IFN-g) to eliminate intracellular parasites via nitric oxide. On the other hand, the stimulus initiated by the cytokine IL-4 results in the Th2 polarization of the response, with the production of cytokines (IL-5, IL-10, IL-13) responsible for humoral immunity. Furthermore, IL-4 inhibits the Th1 response and activates macrophages by an alternative pathway (arginase I), which results in the persistence of infection [6].
In dogs with VL, the clinical presentation of the disease is variable, depending on the predominance of cytokines produced in systemic inflammation. Th1 immune response predominates in dogs resistant to parasite multiplication in tissues .T lymphocyte (CD4+) phenotypes, activated for a Th1 and Th2 profile, are induced by transcription factors T-bet and GATA3, respectively [7,8]. Expression of GATA3 results from the activation of STAT6, induced after the binding of IL-4 to its receptor (IL-4R) in the cell membrane [9].IL-10 reduces the pro-inflammatory response produced by activated leukocytes [10]. However, many pathogens are favored and multiply within the host cell due to the regulatory function of this cytokine. In canine VL, there are elevated levels of IL-10 in the spleen of infected dogs [11,12]. Donovani stimulates the production of IL-10 and activates STAT3, which directs the expression of IL4Rα to activate macrophages by alternative pathways; this results in immune escape from the nitric oxide-dependent parasiticide action on macrophages [13]. In murine models, the parasite induces the release of arginase I, stimulated by IL-10 and mediated by the expression of STAT3, resulting in the multiplication and survival of the parasite in this phagocyte [14-16]. In hamsters experimentally infected with L. donovani, the transcription factor STAT6 activates macrophages by an alternative pathway [17]. In VL, the spleen is an essential organ for developing the systemic immune response to the parasite [18,19]. However, this organ maintains the infection throughout the disease [20] for presenting disruptions in its architecture due to diffuse granulomatous inflammation and lymphoid atrophy The spleen of symptomatic dogs has more significant tropism of the parasite[21,22], a higher proportion of immunosuppressive macrophages (M2) [22] and T lymphocyte apoptosis [22]. Furthermore, in the splenic tissue of infected dogs, elevated levels of IL-10 were associated with regulatory T lymphocytes infiltrating the splenic tissue [23]. These reports show that parasite prevalence and survival in the canine spleen occur according to the modulation of the splenic microenvironment. Therefore, this study aimed to evaluate the influence of STAT3 and STAT6 on the splenic immune response in VL. Immunodetection of these transcription factors was compared with lesion intensity and splenic parasite load.
Materials and Methods
- Experimental groups
In this study, spleen samples from 31 dogs from the paraffin block archive of the Department of Pathology, Theriogenology and One Health (DPTOH), School of Agricultural and Veterinary Studies (FCAV), São Paulo State University (UNESP), municipality of Jaboticabal, São Paulo State, Brazil were used. Dogs naturally infected with Leishmania spp. (n=27) came from the Zoonosis Control Center of the municipality of Araçatuba (São Paulo State, Brazil), an endemic area for VL [24]. These animals were divided into a symptomatic (S, n=17) and asymptomatic (A, n=10) group [12]. The control group (C, n=4) consisted of canine spleen samples from a non-endemic area for VL [25].
The positive diagnosis selected the animals of the infected group through an immunoenzymatic test (ELISA) and the Dual Path Platform rapid immunochromatographic test (DPP®). The control group was selected from samples from the DPTOH paraffin block file from animals whose clinical history and histopathological analysis excluded systemic or neoplastic diseases and splenic tissue disorders.
- Histopathological analysis
5µm spleen sections were stained with Hematoxylin and Eosin for analysis under light microscopy. In the histological analysis, the lesions in the red pulp and capsule and the proliferative changes in the white pulp were evaluated employing intensity scores of the lesions (absence, mild, moderate, severe). White pulp size (WP, germinal center) was determined by selecting five microscopic fields (10x objective lens), and image analysis software (Olympus CellSens Standard, version 1.18, 2009-2017) was used to measure white pulp extension/field on the Olympus BX50 microscope. The average WP measurements were compared by groups (C, S, and A).
- Immunohistochemical analysis
The immunohistochemical technique was used to identify cells expressing the transcription factors STAT3 (rabbit polyclonal, Abcam; code ab30647) and STAT6 (rabbit polyclonal, Abcam; code ab44718), as well as to determine the tissue parasite load (PL, hyperimmune serum), using serum from a VL-positive dog, according to the protocol modified.
The immunohistochemical protocols for the antibodies consisted of deparaffinized slides in a heated chamber (60°C) for one hour, followed by immersing them in xylol (20 min). After that, the sections were hydrated in solutions of decreasing concentrations of alcohol until they were washed in distilled water. Antigen retrieval was performed by applying heat, a blockade of endogenous peroxidase, a blockade of non-specific proteins, and incubation of primary and secondary antibodies were performed (Table 1). Between steps, the sections were washed with Tris-HCl buffer solution (pH 7.4) and distilled water for 5 minutes each. DAB chromogen (3,3-diaminobenzidine - Dako, code K3468-1) was used to visualize the reaction; counterstaining was done using Harris' hematoxylin, and the slides were mounted with Entellan (Merck). All sections were incubated in a dark and humid chamber (EasyPath®).
|
Primary antibody |
Peroxidase blockade 3 |
nonspecific proteins blockade4 |
Dilution |
Incubation antibodies |
Conjugate |
|
STAT31
|
01 hour |
01 hour |
1:100 |
18 hours (4ºC) |
Envision5
|
|
STAT61
|
01 hour |
01 hour |
1:100 |
18 hours (4ºC) |
Envision5 |
|
Leishmania2 |
30 minutes |
30 minutes |
1:200 |
2 hours (room temperature) |
Advance 6 |
Table 1: Antibodies used in the immunohistochemical analysis of canine spleens.
- Buffer for antigen recovery: Tris EDTA, pH 9,0, for 50 minutes (Pascal pressure chamber, DakoCytomation);
- Buffer for antigen recovery: sodium citrate buffer solution, 10 mM, pH 6,0, for 50 minutes (Pascal pressure chamber, DakoCytomation);
- Blockade of endogenous peroxidase: 8% Methanol + hydrogen peroxide (30 v.v., Synth), room temperature, dark humid chamber;
- Protein Block (DAKO, DakoCytomation, code X0909), room temperature;
- Conjugate: Polymer complex bonded to peroxidase (DAKO, Kit Envision + Dual Link System – HRP, code K406189-2);
- Conjugate: Polymer complex bonded to peroxidase (Kit Advance HRP, Dako, code K4068).
Positive controls were selected according to the tissues suggested by the manufacturer of each antibody. The negative control was made from an antibody diluent with background-reducing components (Dako, code S3022) replacing the primary antibody.The density of cells immunolabeled for each antibody was determined by counting cells/animal in five microscope fields at high magnification (40x objective lens; area = 0.19625 μm2), as described by Moreira et al. (2010), under an optical microscope (Nikon, model E200). Images were captured for analysis using cellSens Standard software (Olympus Corporation), version 1.18 (2009-2017).
- Statistical analysis
In the statistical analysis, the intensity values of splenic lesions were considered in percent intensity (absent, mild, moderate, severe). STAT3, STAT6 immunolabeling means, and parasite load was compared between groups C, A, and S using the non-parametric Kruskal-Wallis test, followed by Dunn's multiple comparison tests. Parasite load comparison between the infected groups (S and A) was made using the non-parametric Mann-Whitney test. All analyses were performed using GraphPad Prism software (version 5.0), considering significant differences when p<0.05. Multivariate analyses (principal component and factor analysis) were performed to describe the joint variation in the traits studied using R Core Team software (2015).
Results
- Histopathological analysis
No lesions were observed in the splenic tissue of animals in the control group (C). In infected dogs, group S (n=17) showed changes in the red pulp, more evident than group A (n=10). Perisplenitis (47.05%; n=8) and granulomatous inflammation (52.94%; n=9) were observed only in group S. Perisplenitis was associated with focal to multifocal thickening of the splenic capsule (Figure 1A), with the presence of parasitized macrophages and evidence of mesothelial cells (reactivity) in the capsule. The granulomatous inflammation corresponded to multifocal to diffuse epithelioid macrophage aggregates predominantly seen in the red pulp. Diffuse distribution occurred only in symptomatic dogs, eventually distorting the splenic architecture. Granulomatous inflammation was noted in the red pulp, sometimes invading the mantle zone and the marginal areas of the white pulp (Figure 1B). In infected dogs, the frequency of macrophages containing intracytoplasmic amastigotes was variable, with a predominant distribution in the red pulp (Figure 1C), mainly in the subcapsular region. The plasma cell infiltrates predominantly moderate (41.18%, n=7) to severe (29.41%, n=5) in group S, usually associated with the presence of plasma cells with Russell bodies in infected dogs (A and S). Myeloid metaplasia, characterized by the presence of hematopoietic precursor cells from the bone marrow, was more evident in group S, predominantly of mild intensity, observed in nine animals (52.94%). In group A, plasma cell infiltration, hemosiderosis, and myeloid metaplasia were predominantly discrete. The infected group showed different intensities of structural disorganization of the white pulp, characterized by reduction or absence of the mantle and marginal areas; this change was most evident in group S, with predominantly moderate intensity (41.17%). The distinction between white and red pulp was poor. However, the germinal centers were increased and evident due to the invasion of macrophages (Figure 1D) and plasma cells, accompanied by a reduction in the lymphoid population in the marginal and mantle zones. Lymphoid rarefaction extending to the periarteriolar sheath due to apoptosis was observed in groups S (mild to moderate; 64.70% of dogs) and A (mild; 30% of dogs). Slight to moderate white pulp hyperplasia was observed in group A.
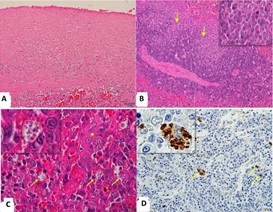 Figure 1: Photomicrographs of the spleen of a dog with visceral Leishmaniasis.
Figure 1: Photomicrographs of the spleen of a dog with visceral Leishmaniasis.
- Note the thickening of the splenic capsule (group S, Obj. lens 20x; Hematoxylin and Eosin). B)
- Note macrophages invading the white pulp (group S, Arrows / Obj. lens 20x; detail, Obj. lens = 40x; Hematoxylin and Eosin).
- Parasitized macrophages (arrows / Detail) with Leishmania in the red pulp (*; Obj. lens 100x, Hematoxylin and Eosin).
- Macrophages immunized to the amastigote forms of the parasite (arrows/details; Obj. lens 20x; detail Obj. lens = 40x; Peroxidase-linked polymeric complex).
- Immunohistochemical analysis
Parasite load immunostaining was cytoplasmic and specific for Leishmania spp. in macrophages in the red pulp (Figure 1D), subcapsular region, capsule and mantle invasion, and marginal areas of the white pulp. The parasite load was higher in group S compared to A (table 2), with highly variable immune intensity. All spleens from dogs in the control group were negative for parasite load.The STAT6 antibody marked the cytoplasm and nucleus of lymphocytes and macrophages from the red and white pulp (Figure 2A). The immunostaining intensity varied within groups but was observed in all animals. In group C, red pulp lymphocytes and macrophages were stained in the cytoplasm and nucleus (Figure 2B). In the infected groups (S and A), staining predominated in the macrophage and lymphocyte nuclei (Figures 2C & 2D).
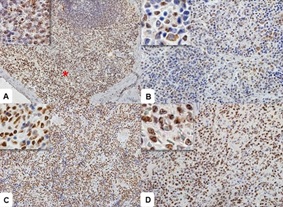 Figure 2: Photomicrographs of STAT6 immunostaining in the spleen of a dog with visceral Leishmaniasis.
Figure 2: Photomicrographs of STAT6 immunostaining in the spleen of a dog with visceral Leishmaniasis.
- Observe immunostaining in the nuclei of red blood cells (*). In detail, observe nuclear immunostaining in the white pulp (Obj. lens 40x). B
- Observe cytoplasmic positivity in macrophages and nuclear positivity in lymphocytes (Detail / Obj. lens 20x).
- Symptomatic dog with intense nuclear staining (Detail, Obj. lens 40x).
- Note the more intense immunoglobulin’s in group A lymphocytes (Detail, Obj. lens 20x). Polymeric complex bound to peroxidase.
Lymphocytes and macrophages were positive for STAT3, marking cytoplasm and nucleus (Figure 3A), mainly in the infected groups. However, this staining was weak in group C (Figure 3B) and more pronounced in group S (Figure 3C). In group A, immunostaining was weak and observed in both the nucleus and cytoplasm of the cells (Figure 3D).
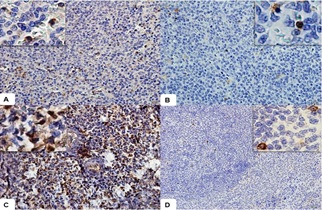 Figure 3: Photomicrographs of STAT3 immunostaining in the spleen of a dog with visceral Leishmaniasis.
Figure 3: Photomicrographs of STAT3 immunostaining in the spleen of a dog with visceral Leishmaniasis.
- Observe immunostaining in red pulp lymphocytes. In detail, observe nuclear and cytoplasmic immunostaining (Obj. lens 20x).
- Observe positive cells in the control group (Detail / Obj. lens 20x).
- In group S, observe the most intense immunostaining in the nucleus of red pulp lymphocytes (Detail, Obj. lens 40x).
- Note the discrete immunostaining of lymphocytes in group A (Detail, Obj. lens 20x). Polymeric complex bound to peroxidase.
STAT3 and STAT6 immunostaining and white pulp size (WP) were higher in the infected groups (A and S). STAT6 showed higher medians in group A (Table 2). There was no significant difference between groups C, A, and S (Kruskal-Wallis and Dunn tests) for the parameters STAT3 (P=0.061), STAT6 (P=0.821), and splenic white pulp size (P =0.052). Only the parasite load (PL, P=0.020) differed between the groups of infected dogs (Mann-Whitney test), being higher in the S group (Figure 4).
|
Groups |
STAT61 |
STAT31 |
PL2 |
WP1 |
|
C |
81,1 |
0,6 |
- |
459,8 |
|
A |
127,2 |
7,0 |
0,2 |
494,4 |
|
S |
79,2 |
4,8 |
2,8 |
663,1 |
|
P value |
0,821 |
0,061 |
0,021 |
0,052 |
Table 2: Medians of immunostaining for STATs antibodies (3 and 6) and parasite load (PL), as well as for the measurement of white pulp (WP) in the spleens of infected and control dogs.
PL = parasite load; WP = White pulp; C = control group; S = symptomatic group; A = asymptomatic group; 1 – Kruskall-Walis non parametric test; 2 – Mann-Whitney non parametric test.
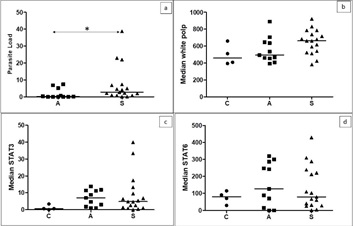 Figure 4: Medians of immunostaining for parasite load.
Figure 4: Medians of immunostaining for parasite load.
(a), white pulp extension (b), STAT3 (c), and STAT6 (d) in splenic tissue from dogs in groups C, A, and S. There was only one statistical difference between groups for parasite load. Kruskall-Wallis non-parametric test (P>0.05; b/c/d). Mann-Whitney non-parametric test (a; P= 0.020).
In the multivariate analysis, the grouping of variables, through Principal Component (PC) analysis, showed the distribution of groups between PCs 1 and 2. Principal component 1 (PC1) explained 38.4% of the variability in the data, and principal component 2 (CP2) explained 28.8% of the variation in the individuals. The control group (C) was grouped in a different region than the animals in groups A and S. This happened due to the joint variation in the characteristics studied that allowed the grouping of sick animals different from the dogs in the control group (Figure 5).
The clustering of variables showed a positive correlation between the parameters parasite load (PL) and white pulp size (WP, r=0.28) and between the transcription factors STAT3 and 6 (r=0.38). These characteristics increased together but in different directions (Figure 6). Increased parasite load is related to increased white pulp size, just as increased STAT6 is associated with STAT3. The joint variation of these parameters indicates that the magnitude of these traits increases in a disease condition.
 Figure 5 - The variance of principal component
Figure 5 - The variance of principal component
- (PC1) relative to principal component
- (PC2) for the traits studied. Labels indicate classification by the group; Group C, Group A, and Group S.
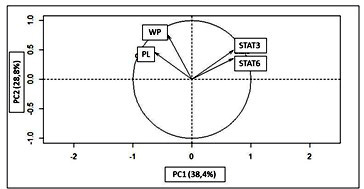 Figure 6: The variance of principal component
Figure 6: The variance of principal component
- (PC1) relative to principal component
- (PC2) for the studied traits. The vectors indicate the relationship between the studied characteristics. WP = white pulp; PL = parasite load; STAT3; STAT6;
Discussion
The lesions found in the spleens of Leishmania infantum-infected dogs in this study were similar to literature reports on parasite load [26] perisplenitis white pulp lymphoid apoptosis and plasma cell infiltrate [27]. All these lesions were observed in the infected groups and with greater intensity in the symptomatic dogs.
The granulomatous inflammation, observed in the red and white pulps, distorted the splenic architecture in group S. This coincides with the literature [28]. The disorganized spleen architecture associated with the parasite-induced immunosuppressive response contributes to the inhibition of the cellular immune response due to inhibition of macrophage activation and failure of antigen presentation. According to Moreira et al. (2016b), splenic macrophages with an immunosuppressive phenotype (M2) favor the survival of the Leishmania spp. in the host. [29] Showed that these macrophages are activated by an alternative (arginase 1) STAT6-dependent pathway and cannot exert microbicidal activity on the parasite.Increased size of splenic germinal centers is usually indicative of white pulp hyperplasia. However, in the present study, median white pulp sizes were associated with lymphoid atrophy and invasion of macrophages and plasma cells in the mantle and marginal areas. Santana et al. (2008) described aggregates of epithelioid macrophages (poorly organized granulomas) in the spleen of dogs with VL. Santana et al. (2008) described aggregates of epithelioid macrophages (poorly organized granulomas) in the spleen of dogs with VL; they also reported structural disorganization of the white pulp. The size of the germinal center and marginal zone compromises the ability to activate the white pulp cellular immune response [30] emphasized that the loss of the marginal zone compromises lymphocyte trafficking in the white pulp and prevents an effective immune response in the chronic phase of the infection. In the present study, immunostaining for STAT6 and STAT3 was positive in the control (C) and infected (S and A) groups. The highest medians for STAT3 occurred in the infected groups (S and A) and for STAT6 in groups A and C, but there were no statistical differences. Osorio et al. (2012) highlighted that the alternative pathway of macrophage activation in the spleens of hamsters infected with L. donovani was induced by STAT6. STAT6 is activated primarily in response to IL-4 and IL-13, while STAT3 responds to IL-10 and IL-6 [31,32] the cytokine IL-10 predominates in the late phase and IL-4 in the early phase of infection. STAT6 stimulates B lymphocyte proliferation through IL-4 by inducing the IL-4R-α receptor. The cytokines IL-4 and IL-10 trigger isolated or synergistic roles for intracytoplasmic activation of STATs (CUMMINGS et al., 2010). In this study, the higher immunostaining of STAT3 and STAT6 in the infected group associated with the parasite in splenic tissue suggests the participation of IL-10 in this response (LANG et al., 2002). High levels of IL-10 have been detected in the spleen of dogs naturally infected with Leishmania infantum. Experimental studies with other protozoa have shown IL-10 overexpression in antigen-presenting cells, inhibiting the release of intracellular pathogens and resulting in diseases with high parasite burden [33,34]. In addition, STAT3 cooperates with STAT6 to promote the differentiation of T lymphocytes into a Th2 profile which predominates in dogs susceptible to leishmaniasis.
Therefore, in the present study, we highlight that an immunosuppressive splenic environment possibly activates STAT3 and STAT6 transcription factors. This fact could negatively regulate the cytotoxic Th1 immune response, contributing to the progression of systemic disease and the maintenance of splenic infection [35].
It can be concluded from this study that the STAT3 and STAT6 transcription factors detected in the splenic tissue of infected animals are associated with the presence of the parasite. STAT possibly influenced the macrophage profile of granulomatous inflammation and the maintenance of an immunosuppressive environment in the spleen. Allied with these factors, disorganization of the white pulp possibly contributed to the failure of the cellular immune response, which shows splenic susceptibility in VL.
Compliance with ethical standards
This study was evaluated and approved by the Ethics Committee for Animal Use of the State University of São Paulo (CEUA-UNESP), Jaboticabal Campus, under protocol no. 009505/17.
Conflict of interest
The authors declare no conflicts of interest.
Acknowledgments
A. Matsui, P.H. Bertolo, and A.P.P. Jacintho were CAPES grantees (Coordination Office for the Improvement of Higher-Education Personnel). P.R.R. Moreira was a FAPESP grantee (The São Paulo Research Foundation, process no.2013/00763-4). The Zoonosis Control Center (Araçatuba, São Paulo State, Brazil) for making the animals available for this study.
References
- Bhardwaj S, Srivastava N, Sudan R, Saha B (2010) Leishmania interferes with host cell signaling to devise a survival strategy. J of Biomed and Biotech 13: 1-13.
- Ruiz-Postigo JA, Jain S, Mikhailov A, Maia-Elkhoury AN, Valadas S, et al. (2021) Global leishmaniasis surveillance: 2019-2020, a baseline for the 2030 roadmap. Weekly Epidemiol Rec 35: 401-419.
- Brasil (2014) Ministério da Saúde. Manual de vigilância e controle da Leishmaniose Visceral. Brasília: Ministério da Saúde p. 122.
- Shio MT, Hassani K, Isnard A, Ralph B, Contreras I, et al. (2012) Host cell signalling and Leishmaniamechanisms of evasion. J of Tropical Med 1-14.
- Barbiéri CL (2006) Immunology of canine leishmaniasis. Parasite Immunology 28: 329-337.
- Cummings HE, Tuladhar R, Satoskar AR (2010) Cyrokines and their STATs in cutaneous and visceral leishmaniasis. J Biomed Biotechnol 10: 6p.
- Szabo SJ, Kim ST, Costa GL, Zhang X, Fathman CG, et al. (2000) A novel transcription factor T-bet directs Th1 lineage commitment. Cell 6: 655-659.
- Kanhere A, Hertweck A, Bathia U, Gökmen MR, Perucha E, et al. (2012) T-bet and GATA3 orchestrate Th1 and Th2 differentiation through lineage-specific targeting of distal regulatory elements. Nature Communications 3: 1268.
- Stritesky GL, Muthukrishnan R, Sehra S, Goswami R, Pham D, et al. (2011) The transcription factor STAT3 is required for T helper 2 cell development. Immunity34: 39-49.
- Sky Ng TH, Britton GJ, Hill EV, Verhagen J, Burton BR, et al. (2013) Regulation of adaptive immunity; the role of interleukin-10. Frontiers in Immunology 4: 1-13.
- Correa APF, Dossi ACS, Vasconcelos RO, Munari DP, Lima VMF (2007) Evaluation of transformation growth factor b1, interleukin-10, and interferon-g in male symptomatic and asymptomatic dogs naturally infected by Leishmania (Leishmania) chagasi. Veterinary Parasitology 143: 267-274.
- Figueiredo MM, Deoti B, Amorim IF, Pinto AJW, Moraes A, et al. (2014) Expression of Regulatory T Cells in Jejunum, Colon, and Cervical and Mesenteric Lymph Nodes of Dogs Naturally Infected with Leishmania infantum. Infection and Immunity 82: 3704-3712.
- Carey AJ, Tan CK, Ulett GC (2012). Infection-induced IL-10 and JAK-STAT. JAKSTAT 1: 159-167
- Iniesta V, Gómez-Nieto LC, Corraliza I (2001) The inhibition of arginase by N(omega)-hydroxy-l-arginine controls the growth of Leishmania inside macrophages. Journal of Experimental Medicine193: 777-784.
- Biswas A, Bhattacharya A, Das PK (2011) Role of cAMP signaling in the survival and infectivity of the protozoan parasite, Leishmania Mole Bio Int 1-9.
- Osorio EY, Travi BL, Cruz AM, Saldarriaga OA, Medina AA, et al. (2014) Growth factor and Th2 cytokine signaling pathways converge at STAT 6 to promote arginase expression in progressive experimental visceral leishmaniasis. PLOS Pathogens 10: e1004165.
- Osorio EY, Zhao W, Espitia C, Saldarriaga O, Hawel L, et al. (2012) Progressive visceral leishmaniasis is driven by dominant parasite-induced STAT6 activation and STAT6-dependent host arginase-1 expression. PLOS Pathogens8: e1002417.
- Santana CC, Vassallo J, Freitas LAR, Oliveira GGS, Pontes-De-Carvalho LC, et al. (2008) Inflammation and structural changes of splenic lymphoid tissue in visceral leishmaniasis: A study on naturally infected dogs. Parasite Immunology 30: 515-524.
- Bronte V, Pittet MJ (2013) The spleen in local and systemic regulation of immunity. Immunity 39: 806-818.
- Strauss-Ayli D, Baneth G, Jaffe CL (2007) Splenic immune responses during canine visceral leishmaniasis. Veterinary Research 38: 547-564.
- Moreira PRR, Fernando FS, Montassier HJ, André MR, Vasconcelos RO (2016) Polarized M2 macrophage in dogs with visceral leishmaniasis. Veterinary Parasitology 226: 69-73.
- Moreira PRR, Franciscato DA, Rossit SM, Munari DP, Vasconcelos RO (2016) Influence of apoptosis on liver and spleen resistance in dogs with visceral leishmaniosis. Brazilian Journal of Veterinary Parasitology 259: 341-347.
- Silva KLO, Andrade MMC, Melo LM, Perosso J, Vasconcelos RO, et al. (2014) CD4+FOXP3+ cells produce IL-10 in the spleens of dogs with visceral leishmaniasis. Veterinary Parasitology 202: 313-318.
- Moreira PRR, Vieira LM, Andrade MMC, Bandarra MB, Machado GF, et al. (2010) Immune response pattern of the popliteal lymph nodes of dogs with visceral leishmaniasis. Parasitol Res 107: 605-613.
- Oliveira TMFS, Furuta PI, Carvalho D, Machado RZ (2008) A study of crossreactivity in serum samples from dog’s positive for Leishmania, Babesia canis and Ehrlichia canis in enzyme-linked immunosorbent assay and indirect fluorescent antibody test. Revista Brasileira de Parasitologia Veterinária17: 7-11.
- Cavalcanti AS, Ribeiro-Alves M, Pereira LOR, Mestre GL, Ferreira ABR, et al. (2015) Parasite load Induces progressive spleen architecture breakage and impairs cytokine mRNA expression in Leishmaniainfantum-naturally infected dogs. PLoS One 104: e0123009.
- Tafuri WL, Barbosa AJ, Michalick MS, Genaro O, França-Silva JC, et al. (1996) Histopathology and immunocytochemical study of type 3 and type 4 complement receptors in the liver and spleen of dogs naturally and experimentally infected with Leishmania (Leishmania) chagasi. Rev Inst Med Trop38: 81-89.
- Bagues NCT, Pinheiro CGM, Bastos LA, Fraga DBM, Verasa PST, et al. (2018) Parasitic load and histological aspects in different regions of the spleen of dogs with visceral leishmaniasis. Comparative immunology, microbiology and infectious diseases 56: 14-19.
- Kong F, Saldarriaga OA, Spratt H, Osorio EY, Travi BL, et al. (2017) Transcriptional profiling in experimental visceral leishmaniasis reveals a broad splenic inflammatory environment that conditions macrophages toward a disease-promoting phenotype. PLOS Pathogens31: 1-34.
- Engwerda CR, Ato M, Cotterell SEJ, Mynott TL, Tschannerl A, et al. (2002) A Role for Tumor Necrosis Factor-α in Remodeling the Splenic Marginal Zone during Leishmania donovani The American Journal of Pathology 161: 429-437.
- Lang R, Patel D, Morris JJ, Rutschman RL, Murray PJ (2002) Shaping gene expression in activated and resting primary macrophages by IL-10. J Immunol 169: 2253-2263.
- Goenka S, Kaplan MH (2011) Transcriptional regulation by STAT 6. Immunol Res 50: 87-96.
- Murray PJ (2006) Understanding and exploiting the endogenous interleukin-10/STAT3-mediated anti-inflammatory response. Current Opinion in Pharmacology 6: 379-386.
- Murray PJ (2006) STAT3-mediated anti-inflammatory signaling. Biochemical Society Transactions 34: 1028-1031.
- Tafuri WL, Santos RL, Arantes RME, Gonçalves R, Melo MN, et al. (2004) An alternative immunohistochemical method for detecting Leishmania amastigotes in paraffin-embedded canine tissues. J of Immunological Methods 292: 17-23.
Citation: Matsui A, Bertolo PHL,Moreira PRR ,Jacintho ARR, Ramos FR, et al. (2023) Influence of STAT3 and STAT6 on splenic susceptibility to infection in dogs with visceral leishmaniasis. J Anim Res Vet Sci 7: 0.42
Copyright: © 2023 Andresa Matsui, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.

