
Journal of Allergy Disorders & Therapy Category: Clinical
Type: Review Article
Innovation in Nasal Measurement: Are We Doing Enough for Patients
*Corresponding Author(s):
Karen Parker DavidsonDoctor Of Heath Administration, Diplomate, Central Michigan University, Michigan, United States
Email:david2k@cmich.edu
Received Date: Jan 07, 2019
Accepted Date: Mar 04, 2019
Published Date: Mar 19, 2019
Abstract
Not all nasal congestion is nasal obstruction and not all nasal obstruction is nasal congestion. Nasal congestion is a common occurrence and one of the most common symptoms in practice found among many diagnoses with various epidemiological patterns that is bothersome for patients. It is often described as fullness, or stuffiness with reduced airflow related often to a disease state. Such conditions contributing to congestion could be chronic rhinitis, allergic rhinitis, rhinosinusitis, polyposis, and non-allergic rhinitis.
The global variation and prevalence of chronic symptoms and the economic impact on the population has not been studied on a global perspective, but has been considered country by country. Among these considerations, an increase in several countries has been recorded, with a more pronounced prevalence in low to mid-income countries. If we look at an example of the epidemiology of a specific disease state where nasal congestion and/or obstruction is present, allergic rhinitis affects one quarter of the world population adverselyimpairing the quality of life, productive work hours, and sleep. In the United States, an estimated 20 million Americans are affected by nasal obstruction, and tens of millions of Americans affected by rhinosinusitis burdening the healthcare system with a cost of $6 billion in healthcare expenditures and one of the costliest physical conditions for healthcare employers. Given the high prevalence of rhinologic disease, to include nasal obstruction and congestion, and the burden of social and economic costs, there continues to be an unmet need in for effective treatment options nationally and globally. Are we doing enough by omitting objective, innovative advancements in nasal measurements to treat patients?
The global variation and prevalence of chronic symptoms and the economic impact on the population has not been studied on a global perspective, but has been considered country by country. Among these considerations, an increase in several countries has been recorded, with a more pronounced prevalence in low to mid-income countries. If we look at an example of the epidemiology of a specific disease state where nasal congestion and/or obstruction is present, allergic rhinitis affects one quarter of the world population adverselyimpairing the quality of life, productive work hours, and sleep. In the United States, an estimated 20 million Americans are affected by nasal obstruction, and tens of millions of Americans affected by rhinosinusitis burdening the healthcare system with a cost of $6 billion in healthcare expenditures and one of the costliest physical conditions for healthcare employers. Given the high prevalence of rhinologic disease, to include nasal obstruction and congestion, and the burden of social and economic costs, there continues to be an unmet need in for effective treatment options nationally and globally. Are we doing enough by omitting objective, innovative advancements in nasal measurements to treat patients?
Keywords
Global health; Innovation; Nasal measurement; Patient care; Treatment
INNOVATION IN NASAL MEASUREMENT: ARE WE DOING ENOUGH FOR PATIENTS?
Nasal congestion is a common symptom seen in clinical practice. A common cause of nasal congestion is allergic rhinitis and Rhinosinusitis. Within the past 20-30 years, the prevalence of allergic rhinitis has increased worldwide from a low of 10 to a high of 40% with disparities due to diagnostic criteria, availability, patient population methodology differences, and the patient population available for studies [1]. Globally, it is approximated that 10-20% of the global population suffers from allergic rhinitis in part due to the increased awareness and testing available for physicians and the public [2]. Within the US population, the prevalence of allergic rhinitis is from 9% to 16%, one of the common atopic condition, and one of the most prevalent condition among the younger population less than 18 years of age with more than 80% of patients suffering from allergic rhinitis by the age of 20 [2].
The objective of this review was to understand how innovation in nasal measurement can address nasal congestion and obstruction, and if we are doing enough to help patients as clinicians treating nasal congestion and obstruction. Secondary data from literature was obtained through a review and examination of the article’s content. The selection of articles for review was based on the study type, article content, the age of the article, and the experience of the end user publishing the information. In an attempt to answer the research question, the following steps in data collection were completed. Over a course of four months, each article was reviewed and examined for specific information to support the objective. To optimize the research, the author chose to focus on the content portion of the literature review relevant to and the validation for the importance of nasal measurements in the allergy patient with strong confidence in the innovation as evident by the enormity of supportive research and literature published over two decades.
Through additional studies, allergic rhinitis with corresponding congestion has become problematic worldwide, and 94% complain of nasal congestion as a primary symptom (Table 1) [3]. Bauchau & Durham, [4] confirmed the findings supporting the problematic condition of allergic rhinitis in a cross-sectional study of 9646 European adults where 40% of the subjects reported having a previous diagnosis, and symptoms, consistent with allergic rhinitis. The European prevalence of rhinitis is similarly seen as a worldwide condition with lifetime prevalence estimates of between 10% and 20% of the population in the USA, UK, Germany, Switzerland and Finland [5]. Using a conservative estimate, it is proposed that allergic rhinitis occurs in approximately 500million people: Over 100million people in Europe and North America., more than 150million in Asia Pacific, greater than 100million in India, Pakistan and surrounding countries, over 75million in Central and South America, more than 30million in Africa, andover 50million in other countries (Table 2). Among these populations, nearly 200million people also have asthma as co morbidity [6].
Specific guidelines in clinical practice have become a form of algorithm for diagnosing, testing, treatment, and outcome measurement tools for rhinitis and nasal congestion. These guidelines help practitioners in their decision-making process and contain updates in science and epidemiology. Guidelines for rhinitis are available; however, in 2008, a Phase 3 update to the original report called Allergic Rhinitis and its Impact on Asthma (ARIA) was published in response to innovation and practice. In 1999, the World Health Organization (WHO) and clinical experts created an initiative in a report form to educate and implement management of rhinitis in an evidence based medicine approach used to treat the nearly 500 million patients affected globally [7].
A phase 4 update of ARIA was completed in 2018, with the Phase 3 ARIA update commencing in 2004. Several chapters of ARIA were extensively reviewed in an evidence based model, and papers were published (or submitted) in peer reviewed journals: tertiary prevention of allergy, complementary and alternative medicine, and pharmacotherapy and anti IgE treatment, allergen specific immunotherapy, links between rhinitis and asthma and mechanisms of rhinitis. There was then a need for a global document based on the published papers to highlight the interactions between the upper and the lower airways and to: Develop an evidence based global document on a key problem of respiratory medicine including diagnosis, epidemiology, common risk factors, management and prevention; propose educational materials for healthcare professionals and patients; meet the objectives of the WHO Global Alliance against Chronic Respiratory Diseases (GARD) in order to help coordinate the efforts of the different GARD organizations towards a better prevention and management of Chronic Respiratory Diseases (CRD), to increase CRD awareness and also to fill some of the gaps in knowledge; focus on the prevention of chronic respiratory and allergic diseases; highlight gaps in knowledge, particularly in developing countries and deprived areas; prepare an executive summary and pocket guide for doctors, patients and healthcare professionals [8].
“Allergic Rhinitis and its Impact on Asthma (ARIA) have evolved from a guideline using the best approach to Integrated Care Pathways (ICP’s) using mobile technology in AR and asthma multimorbidity. The proposed next phase of ARIA is Change Management (CM) with the aim of providing an active and healthy life to rhinitis sufferers and to those with asthma multimorbidity across the life cycle whatever their gender or socio-economic status in order to reduce health and social inequities incurred by the disease. ARIA has followed the 8-step model of Kotter to assess and implement the impact of rhinitis on asthma multimorbidity and to propose multimorbid guidelines. A second change management strategy is proposed by ARIA Phase 4 to increase self-medication and shared decision making in rhinitis and asthma multimorbidity. An innovation of ARIA has been the development and validation of IT evidence-based tools (MASK: Mobile Airways Sentinel Network) that can inform patient decisions on the basis of a self-care plan proposed by the health care professional [3,9].
Beyond the innovation of using advanced IT resources such as clinical instruments, ARIA has evolved from a guideline using the best practices conventionalized with evidence based medicine to Integrated Care Pathways (ICP’s) using mobile application technology in AR and asthma multimorbidity. “The proposed next phase of ARIA is Change Management (CM) with the aim of providing an active and healthy life to rhinitis sufferers and to those with asthma multimorbidity across the life cycle whatever their gender or socio-economic status in order to reduce health and social inequities incurred by the disease”.
The 8-step model of Kotter has been followed by ARIA to propose multimorbidity management guidelines, and to assess and implement the impact of rhinitis on asthma multimorbidity. A second change management strategy proposed by ARIA Phase 4 is to increase shared decision in asthma and rhinitis among healthcareproviders and increase patient self-medication. An innovation of ARIA has been the development and validation of IT evidence-based tools (MASK: Mobile Airways Sentinel Network) that can inform patient decisions on the basis of a self-care plan proposed by the health care professional [3,9].
With nearly 500 million people of the global population suffering or affected by the symptoms of allergic rhinitis, [10] wrote and published a society position statement, EAACI position paper on the standardization of nasal allergen challenges discussing the standardization of nasal allergen challenges. Within this position paper, [10] discussed and elaborated to leading allergists of theworld that nasal measurements had a high actuality in the treatment of allergy disorders.
The literature review formatting the position statement consisted of 786 documents with 173 selected as suitable studies and papers for review and consideration for the position statement. Throughout the examination of the documents, [10] standardized the society position for correctly diagnosing and recommending adequate therapy for allergic rhinitis. Within the section of the application of allergens, the objective assessment of nasal patency describes four techniques to assess and quantify nasal airflow, airflow volume, and airflow ventilation. Of the four techniques and methods mentioned, acoustic rhinometry is one of them, in addition to 4-phase rhinomanometry, PNIF, and anterior acoustic rhinometry. The human eye was never able to estimate the measurements of nasal patency; therefore, suggesting the necessity for objective measurements. Augé et al., (2018) stated, “Acoustic rhinometry is quick and easy to perform, without the need of patient collaboration. It was standardizedin 2005 by the standardization committee on objective assessment of the nasal airway of the European Rhinology Society” [10].
With the innovation of mobile technology to increase patient management, the technology of acoustic rhinometry, in addition to rhinomanometry to measure the airflow function of the upper airway, is used to locate and quantify the level of obstruction within the severity of rhinitis and its effect on asthma [11]. Objective nasal measurements have become an integral part of clinical practice in order to quantify and qualify the level of obstruction beyond the scope of the individual patient directed self-assessments. Beyond the clinical use of the technology, and in conjunction with ARIA, rhinometryand rhinomanometrycan supplement the patient care plan with the cross-sectional area measurements and airway function that has been provided on frequent simultaneous coexistence of inflammatory conditions in the upper airway. These measurements can be integrated into an EMR and reproduced in pdf format for sharing among healthcare providers. The conversion of echo measurements to nasal volume requires mathematical calculations and theoretical assumptions, which is done in real time data analysis by the computer connected to the recording device with four short sound wave firings. In a congested nose, or in the nose with nasal polyps or tumor, the narrowest points are located deeper in nasal cavity, and acoustic rhinometry can detect the position of this point; however, cannot differentiate what is the blocking factor [11]. Acoustic rhinometry is an easy, non-invasive, and non-painful procedure to perform with clear visuals and graphics that examines the entire airway, demonstrates the location and severity of the obstruction, and evaluates the success of treatment and outcomes as shown in the figures. The graphics within the software can be individualized to accommodate the end user and facility as shown in the (Figures 1-8).
Acoustic Rhinometry (AR) by GM Instruments, Ltd [12] remains the only global clinical and research model that has several attractive features relevant to application in the pediatric and veterinary populations, too. Additional measurement products from GM Instruments, LTs. Include the PNIF and the NR6 Rhino manometer [12].
Another method of measurement proposed in the literature has been Computational Fluid Dynamics (CFD). This type of test has been conceptualized and compared to rhinometry and rhinomanometry [12] for ENT use in the literature as far back as 2010, but has never come to fruition as a study due to the cost and the necessary critical improvements needed to become of relevant clinical value; candidly, this type of “industrial” study is way too complex, impractical, and time-consuming to be of value and use in clinical practice [13]. Vogt et al., [13] further commented, “The results of rhinometry do correlate with the symptom of nasal obstruction. Some have denied this. As recently as 2005, it was stated “Surgeons are reluctant to use objective measurements as there is general agreement that objective measurements do not correlate with the patient symptoms of nasal obstruction [13].
The objective of this review was to understand how innovation in nasal measurement can address nasal congestion and obstruction, and if we are doing enough to help patients as clinicians treating nasal congestion and obstruction. Secondary data from literature was obtained through a review and examination of the article’s content. The selection of articles for review was based on the study type, article content, the age of the article, and the experience of the end user publishing the information. In an attempt to answer the research question, the following steps in data collection were completed. Over a course of four months, each article was reviewed and examined for specific information to support the objective. To optimize the research, the author chose to focus on the content portion of the literature review relevant to and the validation for the importance of nasal measurements in the allergy patient with strong confidence in the innovation as evident by the enormity of supportive research and literature published over two decades.
Through additional studies, allergic rhinitis with corresponding congestion has become problematic worldwide, and 94% complain of nasal congestion as a primary symptom (Table 1) [3]. Bauchau & Durham, [4] confirmed the findings supporting the problematic condition of allergic rhinitis in a cross-sectional study of 9646 European adults where 40% of the subjects reported having a previous diagnosis, and symptoms, consistent with allergic rhinitis. The European prevalence of rhinitis is similarly seen as a worldwide condition with lifetime prevalence estimates of between 10% and 20% of the population in the USA, UK, Germany, Switzerland and Finland [5]. Using a conservative estimate, it is proposed that allergic rhinitis occurs in approximately 500million people: Over 100million people in Europe and North America., more than 150million in Asia Pacific, greater than 100million in India, Pakistan and surrounding countries, over 75million in Central and South America, more than 30million in Africa, andover 50million in other countries (Table 2). Among these populations, nearly 200million people also have asthma as co morbidity [6].
Specific guidelines in clinical practice have become a form of algorithm for diagnosing, testing, treatment, and outcome measurement tools for rhinitis and nasal congestion. These guidelines help practitioners in their decision-making process and contain updates in science and epidemiology. Guidelines for rhinitis are available; however, in 2008, a Phase 3 update to the original report called Allergic Rhinitis and its Impact on Asthma (ARIA) was published in response to innovation and practice. In 1999, the World Health Organization (WHO) and clinical experts created an initiative in a report form to educate and implement management of rhinitis in an evidence based medicine approach used to treat the nearly 500 million patients affected globally [7].
A phase 4 update of ARIA was completed in 2018, with the Phase 3 ARIA update commencing in 2004. Several chapters of ARIA were extensively reviewed in an evidence based model, and papers were published (or submitted) in peer reviewed journals: tertiary prevention of allergy, complementary and alternative medicine, and pharmacotherapy and anti IgE treatment, allergen specific immunotherapy, links between rhinitis and asthma and mechanisms of rhinitis. There was then a need for a global document based on the published papers to highlight the interactions between the upper and the lower airways and to: Develop an evidence based global document on a key problem of respiratory medicine including diagnosis, epidemiology, common risk factors, management and prevention; propose educational materials for healthcare professionals and patients; meet the objectives of the WHO Global Alliance against Chronic Respiratory Diseases (GARD) in order to help coordinate the efforts of the different GARD organizations towards a better prevention and management of Chronic Respiratory Diseases (CRD), to increase CRD awareness and also to fill some of the gaps in knowledge; focus on the prevention of chronic respiratory and allergic diseases; highlight gaps in knowledge, particularly in developing countries and deprived areas; prepare an executive summary and pocket guide for doctors, patients and healthcare professionals [8].
“Allergic Rhinitis and its Impact on Asthma (ARIA) have evolved from a guideline using the best approach to Integrated Care Pathways (ICP’s) using mobile technology in AR and asthma multimorbidity. The proposed next phase of ARIA is Change Management (CM) with the aim of providing an active and healthy life to rhinitis sufferers and to those with asthma multimorbidity across the life cycle whatever their gender or socio-economic status in order to reduce health and social inequities incurred by the disease. ARIA has followed the 8-step model of Kotter to assess and implement the impact of rhinitis on asthma multimorbidity and to propose multimorbid guidelines. A second change management strategy is proposed by ARIA Phase 4 to increase self-medication and shared decision making in rhinitis and asthma multimorbidity. An innovation of ARIA has been the development and validation of IT evidence-based tools (MASK: Mobile Airways Sentinel Network) that can inform patient decisions on the basis of a self-care plan proposed by the health care professional [3,9].
Beyond the innovation of using advanced IT resources such as clinical instruments, ARIA has evolved from a guideline using the best practices conventionalized with evidence based medicine to Integrated Care Pathways (ICP’s) using mobile application technology in AR and asthma multimorbidity. “The proposed next phase of ARIA is Change Management (CM) with the aim of providing an active and healthy life to rhinitis sufferers and to those with asthma multimorbidity across the life cycle whatever their gender or socio-economic status in order to reduce health and social inequities incurred by the disease”.
The 8-step model of Kotter has been followed by ARIA to propose multimorbidity management guidelines, and to assess and implement the impact of rhinitis on asthma multimorbidity. A second change management strategy proposed by ARIA Phase 4 is to increase shared decision in asthma and rhinitis among healthcareproviders and increase patient self-medication. An innovation of ARIA has been the development and validation of IT evidence-based tools (MASK: Mobile Airways Sentinel Network) that can inform patient decisions on the basis of a self-care plan proposed by the health care professional [3,9].
With nearly 500 million people of the global population suffering or affected by the symptoms of allergic rhinitis, [10] wrote and published a society position statement, EAACI position paper on the standardization of nasal allergen challenges discussing the standardization of nasal allergen challenges. Within this position paper, [10] discussed and elaborated to leading allergists of theworld that nasal measurements had a high actuality in the treatment of allergy disorders.
The literature review formatting the position statement consisted of 786 documents with 173 selected as suitable studies and papers for review and consideration for the position statement. Throughout the examination of the documents, [10] standardized the society position for correctly diagnosing and recommending adequate therapy for allergic rhinitis. Within the section of the application of allergens, the objective assessment of nasal patency describes four techniques to assess and quantify nasal airflow, airflow volume, and airflow ventilation. Of the four techniques and methods mentioned, acoustic rhinometry is one of them, in addition to 4-phase rhinomanometry, PNIF, and anterior acoustic rhinometry. The human eye was never able to estimate the measurements of nasal patency; therefore, suggesting the necessity for objective measurements. Augé et al., (2018) stated, “Acoustic rhinometry is quick and easy to perform, without the need of patient collaboration. It was standardizedin 2005 by the standardization committee on objective assessment of the nasal airway of the European Rhinology Society” [10].
With the innovation of mobile technology to increase patient management, the technology of acoustic rhinometry, in addition to rhinomanometry to measure the airflow function of the upper airway, is used to locate and quantify the level of obstruction within the severity of rhinitis and its effect on asthma [11]. Objective nasal measurements have become an integral part of clinical practice in order to quantify and qualify the level of obstruction beyond the scope of the individual patient directed self-assessments. Beyond the clinical use of the technology, and in conjunction with ARIA, rhinometryand rhinomanometrycan supplement the patient care plan with the cross-sectional area measurements and airway function that has been provided on frequent simultaneous coexistence of inflammatory conditions in the upper airway. These measurements can be integrated into an EMR and reproduced in pdf format for sharing among healthcare providers. The conversion of echo measurements to nasal volume requires mathematical calculations and theoretical assumptions, which is done in real time data analysis by the computer connected to the recording device with four short sound wave firings. In a congested nose, or in the nose with nasal polyps or tumor, the narrowest points are located deeper in nasal cavity, and acoustic rhinometry can detect the position of this point; however, cannot differentiate what is the blocking factor [11]. Acoustic rhinometry is an easy, non-invasive, and non-painful procedure to perform with clear visuals and graphics that examines the entire airway, demonstrates the location and severity of the obstruction, and evaluates the success of treatment and outcomes as shown in the figures. The graphics within the software can be individualized to accommodate the end user and facility as shown in the (Figures 1-8).
Acoustic Rhinometry (AR) by GM Instruments, Ltd [12] remains the only global clinical and research model that has several attractive features relevant to application in the pediatric and veterinary populations, too. Additional measurement products from GM Instruments, LTs. Include the PNIF and the NR6 Rhino manometer [12].
Another method of measurement proposed in the literature has been Computational Fluid Dynamics (CFD). This type of test has been conceptualized and compared to rhinometry and rhinomanometry [12] for ENT use in the literature as far back as 2010, but has never come to fruition as a study due to the cost and the necessary critical improvements needed to become of relevant clinical value; candidly, this type of “industrial” study is way too complex, impractical, and time-consuming to be of value and use in clinical practice [13]. Vogt et al., [13] further commented, “The results of rhinometry do correlate with the symptom of nasal obstruction. Some have denied this. As recently as 2005, it was stated “Surgeons are reluctant to use objective measurements as there is general agreement that objective measurements do not correlate with the patient symptoms of nasal obstruction [13].
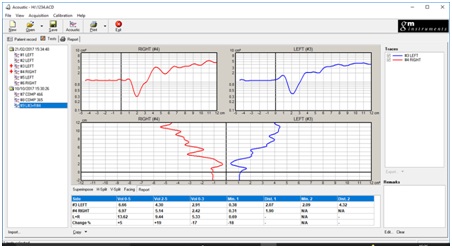 Figure 1: Report View demonstrating the location and severity of the nasal obstruction comparing right to left.
Figure 1: Report View demonstrating the location and severity of the nasal obstruction comparing right to left.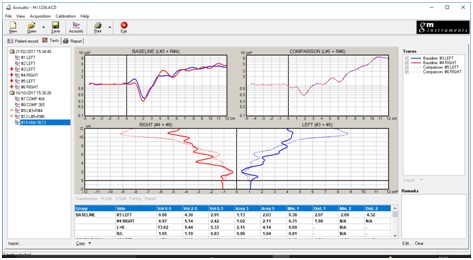
Figure 2: Analysis view-comparing a baseline left/right result with a follow on test.
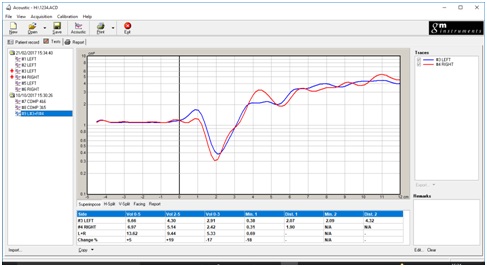 Figure 3: Superimpose a right /left result.
Figure 3: Superimpose a right /left result.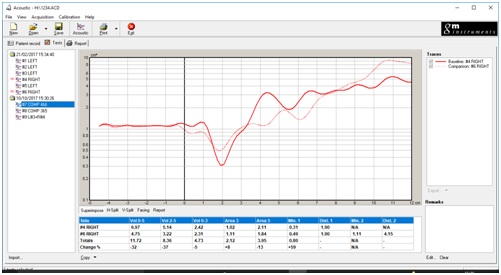
Figure 4: Superimpose 2 x right side results.
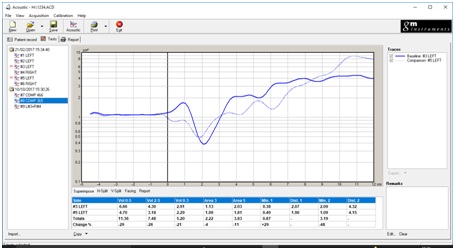
Figure 5: Superimpose 2 x left side results.
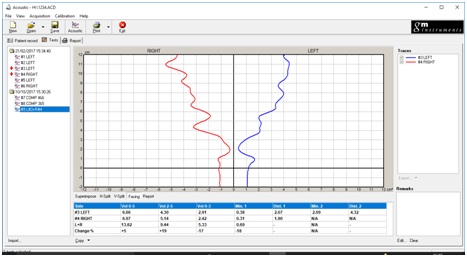
Figure 6: Facing view.
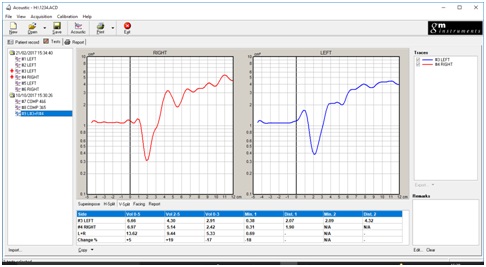
Figure 7: Vertical split.
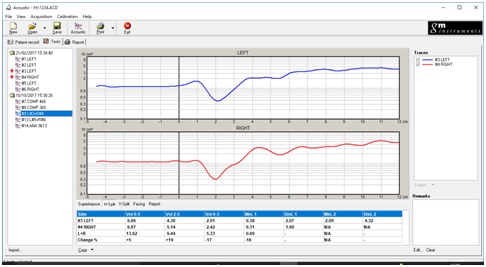
Figure 8: Horizontal split.
CONCLUSION
The nose can be considered as an organ with an interdependent relationship with the lungs. The importance of nasal measurement, in addition to the ARIA guidelines in conjunction with the WHO recommendations, could be concluded as a trilogy:
1. It provides feedback through real time data for the doctor to refine surgical and medical techniques for results and patient outcomes
2. Nasal measurements quantify improvement of technique and patient outcomes, and if changes in technique are necessary
3. It provides evidentiary documentation of surgical outcomes, pre and post op, should the patient have unnecessary claims or unmet expectations requiring additional testing, which can be inconvenient, expensive, and cause third party payors to evaluate the clinician and practice of defensive medicine
1. It provides feedback through real time data for the doctor to refine surgical and medical techniques for results and patient outcomes
2. Nasal measurements quantify improvement of technique and patient outcomes, and if changes in technique are necessary
3. It provides evidentiary documentation of surgical outcomes, pre and post op, should the patient have unnecessary claims or unmet expectations requiring additional testing, which can be inconvenient, expensive, and cause third party payors to evaluate the clinician and practice of defensive medicine
DISCUSSION
Several health organizations and professional societies classify multi-morbidities associated with upper respiratory disease, such as allergic rhinitis, together, and provide recommendations for diagnosis and treatment using rhinometry. Rhinometry has also been established in rhinology and allergy, and continues to be a promising clinical tool in specialties such and pulmonology, sleep, and plastic surgery, particularly in the pediatric age group at the global level, but are we doing enough for patients? The innovation and incorporation of nasal measurements into clinical practice can address the global variation and prevalence of chronic symptoms, and potentiallydecrease the economic impact on the population which has not been studied on a global perspective, but has been considered in the EU, Asia, Australia, the Middle East, UK, Canada, but not the United States.
LIMITATIONS
Limitations of developing this innovation are none as the technology has been on the market for nearly two decades; however, it is becoming more present in the US market from the EU and other countries. Limitations may result in the early adoption by healthcare providers as they have used subjective, patient directed and visual assessment tools, such as endoscopes.
REFERENCES
- Schoenwetter WF (2000) Allergic rhinitis: Epidemiology and natural history. Allergy Asthma Proc 21: 1-6.
- Lundback B (1998) Epidemiology of rhinitis and asthma. Clin Exp Allergy 28: 3-10.
- Meltzer EO, Hamilos DL, Hadley JA, Lanza DC, Marple BF, et al. (2004) Rhinosinusitis: establishing definitions for clinical research and patient care. J Allergy Clin Immunol 114: 155-212.
- Bauchau V, Durham SR (2004) Prevalence and rate of diagnosis of allergic rhinitis in Europe. Eur Respir J 24: 758-764.
- Naclerio RM (1991) Allergic rhinitis. N Engl J Med 325: 860-869.
- Masoli M, Fabian D, Holt S, Beasley R, (2004) The global burden of asthma: executive summary of the GINA Dissemination Committee report. Allergy 59: 469-478.
- Bousquet J, Khaltaev N, Cruz AA, Denburg J, Fokkens WJ, et al. (2008) Allergic Rhinitis and its Impact on Asthma (ARIA) 2008*. Allergy 63: 8-168.
- Bousquet J, Dahl R, Khaltaev N (2007) Global Alliance against Chronic Respiratory Diseases. Allergy 29: 233-239.
- Bousquet J, Hellings PW, Agache I, Amat F, Annesi-Maesano I, et al. (2018) MASK group (2018, September). J Allergy Clin Immunol 28.
- Augé J, Vent J, Agache I, Airaksinen L, Campo Mozo P, et al. (2018) EAACI Position paper on the standardization of nasal allergen challenges. Allergy 73: 1597-1608.
- Davidson K (2017). Acoustic Rhinometry in Role of Sinus Disease and Asthma. J Lung Pulm Respir Res 4: 00128.
- GM Instruments Ltd, Scotland, UK.
- Vogt K, Shah-Hosseini K, Ralph Mösges R, John Pallanch J, Nasse W (2010) New parameters in 4-phase-rhinomanometry relations between objective findings and the sensation of obstruction. In: Vogt K, Jalowayski AA (eds.). A statistical evaluation of 1580 cases. Rhinology 4-phase rhinometry basics and practice, USA.
Citation: Davidson KP (2019) Innovation in Nasal Measurement: Are We Doing Enough for Patients? J Allergy Disord Ther 5: 011.
Copyright: © 2019 Karen Parker Davidson, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.

Journal Highlights
© 2026, Copyrights Herald Scholarly Open Access. All Rights Reserved!
