
Lactobacillus Strains Treatment on Commercial Packaging Paper as Preliminary Study For Extending The Shelf-Life Of Chicken Meat
*Corresponding Author(s):
Stefania SilviSchool Of Biosciences And Veterinary Medicine, Via Gentile III Da Varano, 62032, Camerino (MC), University Of Camerino, Italy
Tel:+39 0737402707,
Email:stefania.silvi@unicam.it
Abstract
Lactobacillus plantarum IMC 509 and SYNBIO® (1:1 combination of Lact. rhamnosus IMC 501® and Lact. paracasei IMC 502®), were investigated as natural anti-spoilage agents sprinkled on commercial packaging, polyethylene (PE) laminated paper sheet, to extend raw chicken breast meat shelf-life at 4°C. Slices of chicken breast meat, wrapped in commercial packaging papers sheet sprayed by Lactobacillus cell suspensions, were analyzed at 0, 2, 5, 7 days of storage. Total aerobic mesophilic bacteria, pH value, sensory changes and biogenic amines (BAs) production were checked. Lactobacillus strains viability on paper packaging was also monitored. The best ability of preserving meat parameters was observed in Lactobacillus sprayed PE laminated papers compared to control paper. The BAs extracted from meat preserved into Lact. plantarum IMC 509 sprayed PE laminated paper sheet were significantly less than other samples. Lact. plantarum IMC 509 showed a high stability, keeping its viability in all paper surfaces. It reduced both spoilage microbial growth and BAs accumulation, providing further evidence for its suitability to be used in packaging application. Lactobacillus strains may be assumed as bio-preservatives applied on papers sheet to extend chicken meat shelf-life without affecting the flavor.
Keywords
Active packaging; Lact. paracasei IMC 502®; Lact. plantarum IMC 509; Lact. rhamnosus IMC 501®; Meat preservation
INTRODUCTION
Poultry meat is a valued food for nutrition due to its high contents of digestible proteins, unsaturated lipids, vitamins (especially B-group), minerals and low carbohydrate contents. Its moderate energy content has drawn a lot of attention from nutritionists, athletes, dieters and healthy eaters [1]. The typical characteristics (high moisture content, moderate pH value, nutrient-rich) [2] of poultry meat make it an easy spoiling matrix by certain microbes, such as psychrotrophic bacteria [3]. Microorganisms, which are introduced into meat from the natural environment or by improper handling of market operators, air or contaminated water, may survive during handling and processing and multiply quickly under high temperature or temperature fluctuation conditions [4,5]. The effect of all these factors may lead to the appearance of undesirable colour, texture, flavour, odours and slime on meat surface. The 58.5 % of all food spoilage bacteria species affecting meat surface is composed by Pseudomonas spp. [6] some bacteria strains, especially, Escherichia spp., Enterobacter spp., Pseudomonas spp., Salmonella spp., Shigella spp., Clostridium perfringens, Streptococcus spp., Lactobacillus spp. and Leuconostoc spp. have been shown to be linked to histamine production [7]. Histamine is one of the biogenic amines (BAs) described as a group of low molecular weight, heat stable, non-volatile, basic nitrogenous compounds with biological activity [8]. BAs are mainly created by microbial decarboxylation of amino acids in foodstuffs. Therefore knowing the levels of BAs in foods is an important aspect to assess the health hazard arising from food consumption and can be used as food quality markers [9].
Lactic acid bacteria (LAB) have been isolated from various food and human sources, many strains are defined as probiotics with well-characterized molecular, genome structure and secretory systems. They are widely applied as natural food bio-preservatives [10] or starter cultures in various kinds of food products since ancient times [11] The main antimicrobial mechanisms involved in the preservation process are through competing nutrients and space with spoilage bacteria, the production of a wide variety of antimicrobial substances such as organic acids, hydrogen peroxide, carbon dioxide, ethanol, bacteriocins. The production of organic acids lowered the surrounding pH value, thus creating unfavourable growth environment for spoilage bacteria [12]. Most studies did focus on the application of probiotic strains as additives on/in food to enhance food safety or extend food shelf-life [13,14]. However, few of them shed light on utilizing probiotics as preservatives which being applied as a part of active packaging material.
This study aimed to evaluate the versatility of some Lactobacillus strains, already characterized as probiotic bacteria [15,16] being applied on commercial packaging paper in order to extend fresh chicken breast meat storage time without affecting meat quality. The choice of commercial packaging paper sheet was a preliminary approach to investigate the feasibility of such a kind of probiotic treatment. The following parameters were used to assess the effect of PE laminated paper sheets with PE surface sprayed with probiotics, on chicken breast meat qualities: (a) the total viable count of aerobic mesophilic bacteria, (b) the levels of BAs, especially some specific indexes as chicken breast fillet freshness markers, (c) the organoleptic characteristics, (d) the viability of probiotic strains over paper during meat storage period and longer.
MATERIALS AND METHODS
Preparation of probiotic strain PE laminated paper sheet
The addition of probiotics on paper sheet was realized by spraying bacterial cells suspension onto internal surface (PE film) of a commercial PE laminated paper sheet (40 cm x 30 cm), kindly provided by a local supermarket (Camerino, Italy). Before the spraying process, all PE surface of packaging paper sheet was sterilized by UV lamp up to 3 hours under Biohazard hood (FASTER, Milan, Italy).
0.5 grams of Lact. plantarum IMC 509 and SYNBIO® lyophilized powders (1011 CFU/g) (SYNBIOTEC Srl, Camerino, Italy) were dissolved in 10 ml of sterile PBS buffer. These probiotic strains [15] were chosen basing on their well studied safeness, antimicrobial properties and adaptation capability [16]. After spraying a drying process was done in oven (Heraeus, Hanau, Germany) at 45°C until the weight reached a constant value.
Meat preparation in probiotic strains PE laminated paper sheets
Slices of fresh breast meat derived from one chicken, purchased from a local supermarket, were immediately transferred to the laboratory, where they were further divided, wrapped and stored in previously prepared PE laminated paper sheet: a) sterile packaging PE laminated paper sheet, as control sample (CTR); b) packaging PE laminated paper sprayed with Lact. plantarum IMC 509, labelled as LP sample; c) packaging PE laminated paper sprayed with SYNBIO®, as SYN sample. Each sample of two slices of breast meat (about 100 g) was stored at 4 °C. At days 0, 2, 5 and 7, microbiological, chemical and sensorial analysis were conducted.
Enumeration of total aerobic mesophilic bacteria
Five grams of meat were taken from each sample and subsequently homogenized in 45 ml of saline solution in a sterile Stomacher bag (Stomacher® 80, Seward, UK). Serial dilutions were made for each replicate sample and were further plated on Plate count agar (PCA - Oxoid, Basinstoke, UK) for enumerating total aerobic mesophilic bacteria. Incubation process was done at 35 ± 1°C for 24-48 h in aerobic condition.
Biogenic amines quantification and determination of freshness indexes
Materials and standards
The BAs studied are reported in Table 1. All of them and 1,7-diaminoheptane used as internal standard (C7H18N2, CAS No. 646-19-5) and dansyl chloride (C12H12ClNO2S, CAS No. 605-65-2) were supplied by Sigma-Aldrich (Milano, Italy).
|
Biogenic amine |
Code |
Formula |
CAS No. |
|
Spermine tetrahydrochloride |
SPE |
C10H26N4 4HCl |
306-67-2 |
|
Spermidine trihydrochloride |
SPD |
C7H17N3 3HCl |
334-50-9 |
|
Cadaverine dihydrochloride |
CAD |
C5H14N2 2HCl |
1476-39-7 |
|
Putrescine dihydrochloride |
PUT |
C4H12N2 2HCl |
333-93-7 |
|
Histamine dihydrochloride |
HIS |
C5H9N3 2HCl |
56-92-8 |
|
Tyramine hydrochloride |
TYR |
C8H11NO.HCl |
60-19-5 |
|
2-phenylethylamine hydrochloride |
PHE |
C8H11N.HCl |
156-28-5 |
|
Tryptamine hydrochloride |
TRY |
C10H12N2 HCl |
343-94-2 |
Table 1: Biogenic amines monitored in the study.
Individual stock solutions of BAs were prepared by dissolving 10 mg of each compound in 10 ml of HCl 0.1 mol l-1 (Merck, Darmstadt, Germany) and stored at 4 °C. Standard working solutions at various concentrations were daily prepared by appropriate dilution of different aliquots of the stock solutions with 0.1 mol l-1 HCl. HPLC-grade methanol and sodium sulphate ?99 % were supplied by Sigma-Aldrich. HPLC-grade acetonitrile and methanol were supplied by Merck. All the solvents and solutions were filtered through a 0.45 µm PTFE filter from Supelco (Bellefonte, PA) before use. Cartridges Discovery SPE DSC-18 Silica Tube (6 ml, 1 g) were from Supelco.
Biogenic amines extraction and analysis
The extraction and analysis of BAs was performed following the methods of [17], with slight modifications. Slices of chicken samples were grinded with a blender and then 5 g of sample were homogenized with 25 ml of 5 % TCA for 2 minutes using an Ultra-Turrax S 18N-10 G (IKA-Werke Gmbh & Co., Staufen, Germany). The obtained homogenate was centrifuged at 2500 rpm for 10 min. Due to their lack of chromophores, BA once extracted needed to be derivatized before analysis by liquid chromatography. Briefly, 1 ml of the supernatant TCA extract was derivatized with 300 µl of a saturated NaHCO3 solution, 200 µl of a NaOH solution (2 mol l-1) and 2 ml of dansyl chloride solution (10 mg ml-1 acetone). Dansylating reaction was conducted under magnetic stirring, in the dark at 45 °C for 45 min. After derivatization, the residual dansyl chloride was neutralized by adding 100 µl of 28 % NH4OH. The mixture was evaporated to 1.5 ml under flow of N2. The aqueous residue was purified by solid phase extraction (SPE) using a SPE DSC-18 cartridge (6 ml, 1 g), which was activated with 4 ml of acetonitrile and conditioned with 4 ml of Milli-Q water using a vacuum system. The aqueous residue was then loaded onto the cartridge at a flow rate -1. The cartridge was then washed with 4 ml of water and thoroughly dried under vacuum.
Analytes were finally eluted from the cartridge using 4 ml of acetonitrile. The eluting solution was filtered on 0.45 µm PTFE filter and analysed in HPLC-DAD.
The analysis of BAs was performed through HPLC-DAD from Agilent 1100 series (Agilent Technologies, Santa Clara, CA, USA). The injection volume was 20 µl. The separation of analytes was performed on an analytical column Gemini C18 (250 x 3.0 mm, 5 µm) preceded by a security guard column C18 (4 x 3 mm, 5 µm), both from Phenomenex (Torrance, CA, USA). The mobile phase used for analytes separation was made of water (A) and methanol/acetonitrile (70:30, v/v) (B), at a flow rate of 0.5 ml min-1. The gradient program was: 0 min 60 % B, 0–10 min 70% B, 10–20 min 90 % B, 20–26 min 100 % B, 26–30 min 100 % B, 30–35 min 60 % B and finally 35–50 min 100 % B. Analytes were detected at 254 nm.
In addition, specific indexes were determined as freshness markers: Biogenic Amine Index (BAI), Chemical Quality Index (CQI), Spermidine/Spermine ratio (SPD/SPM) and the Total of the monitored Biogenic Amines (Total BAs). These indexes were obtained according to the following formula:
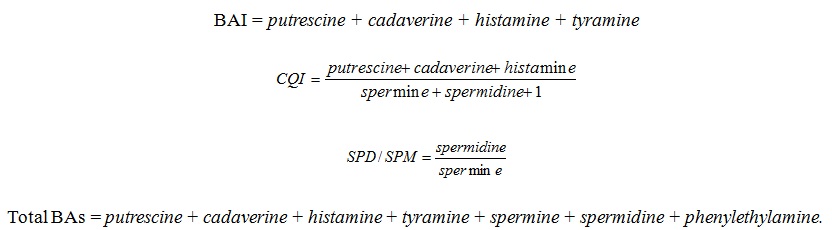
The C.Q.I was proposed by [18] to evaluate the quality of fish and seafood. The B.A.I was created by Veciana- [19] to improve the C.Q.I. The SPD/SPM ratio was proposed by [20] and is considered suitable to assess the chicken meat quality [17]. The Total BAs was used to have more ample vision on the BAs evolution in the different type of samples.
pH measurement
Twenty grams of meat sample were chopped and subsequently transferred into a sterile stomacher bag. The pH of each sample at every time points were measured in triplicate by an electronic pH meter (Mettler Toledo, Columbus, UK) equipped with a probe for solids.
Chicken meat sensory characteristic evaluation
Ten members were selected and trained on meat description following the methods reported by [21] and to familiarize the specific vocabulary and corresponding characteristic of meat. The panellists were asked to judge the aspect (presence of slime), odour, colour and elasticity according to its intensity, they were also asked for the overall acceptability.
The sensory evaluation was conducted in the open laboratory, where each sample was served on a clean white plate, with its corresponding code. The sensory analysis was based on a three-point hedonic scale ranging from 1 (poor) to 3 (excellent) (Table 2).
|
Attributes |
Description |
Values* |
|
Aspect |
Without slime |
3 |
|
Present in some parts slime |
2 |
|
|
All surface with slime |
1 |
|
|
Odour |
Characteristic |
3 |
|
Off-odours |
2 |
|
|
Foreign |
1 |
|
|
Colour |
Pink |
3 |
|
Dark pink |
2 |
|
|
Pale pink/yellow |
1 |
|
|
Elasticity |
Fast return |
3 |
|
Slow return |
2 |
|
|
No return |
1 |
|
|
Overall acceptability |
Excellent |
3 |
|
Acceptable |
2 |
|
|
Unacceptable |
1 |
Table 2: Sensorial attribute values for raw chicken meat (modified from [21]).
* Intensity: 1-3.
Probiotic strains viability on packaging PE laminated paper sheet
To investigate the viability of probiotic bacteria on the packaging materials surface, at each time point, paper sheet of 9 cm2 was cut from both surfaces: one that was in contact with the meat and another that was not. Sterile cotton swabs wetted with saline solution were used to brush the cut packaging surface and subsequently the swab heads were transferred into tube with 5 ml saline solution, vortexing [22] and serial dilutions were made and plated on corresponding de Man, Rogosa, Sharpe, modified with the addition of vancomycin and gentamicin, agar plates (MRS – VWR International, Milan, Italy [23] then incubated at 36 ± 1 °C for 48 h in aerobic condition. The survival rate of each probiotic strains was also calculated by using the following formula:
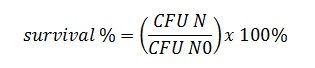
CFU N is the count (CFU ml-1) of probiotic strain on packging sheet at each sampling time; CFU N0 is the count (CFU ml-1) of probiotic strain on PE laminated paper sheet at the preparation day. The value was expressed as percentage.
Statistical analysis
All the microbiological and chemical analyses were carried out in triplicate. The results are expressed as mean ± SD. The Student’s t test was used to assess the statistical significance of the differences between the chicken samples wrapped in the different packaging. Differences were considered significant for P < 0.05.
RESULTS
Total aerobic mesophilic bacteria on preserved chicken meat
Although the initial number of aerobic mesophilic bacteria was already quite high (around 6 log CFU g-1), the counts arose rapidly in the meat wrapped inside the three packaging systems during the first two days, increasing 4 log in SYN-packed meat, 3 log in CTR-packed meat and 2 log increment in LP-packed meat (Figure 1).
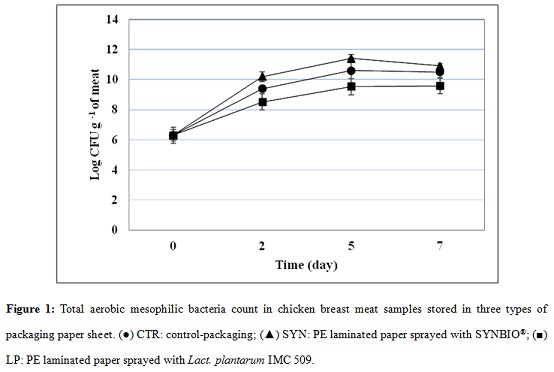
The trend slowed down in the following storage days, with around 9.5 log CFU g-1 in LP-meat and around 10.7 log CFU g-1 in both CTR and SYN-packed meat. Chicken slices in LP-packaging had the lowest mesophiles counts vs CTR sample during time, though the difference was not significant.
BAs concentration and chemical indexes
Six of the 8 monitored BAs were detected in the chicken samples (PUT, CAD, HIS, TYR, SPD and SPM) (Table 3). SPD and SPM presented the highest concentrations at the beginning of the experiment (t0) from all types of packaging prototypes, with SPM as the most abundant BA (53.6 – 58.1 mg kg-1). The levels of PUT, CAD, HIS and TYR were low from t0 to t2, but intensely increased from t5, whereas SPD and SPM levels decreased or remained constant during the chicken storage. PUT, CAD and HIS presented the highest increment, with HIS showing the highest levels (853 ± 30 and 390 ± 12 mg kg-1) in chicken samples packed in SYN and LP-packaging respectively. PUT exceeded 100 mg kg-1 after 7 days of storage (113 – 126 mg kg-1), while CAD was in the range of 212 – 294 mg kg-1 at the end of the study (t7).
|
Biogenic amines |
t0 |
t2 |
t5 |
t7 |
||||
|
SYN |
LP |
SYN |
LP |
SYN |
LP |
SYN |
LP |
|
|
Putrescine
|
0.99 ± 0.1 |
0.68 ± 0.1 |
64.62 ± 5.1 |
5.45 ± 0.4 |
101.55 ± 2.2 |
122.19 ± 1.0 |
113.42 ± 4.6 |
125.96 ± 1.6 |
|
Cadaverine
|
0.74 ± 0.1 |
0.14 ± 0.0 |
151.94 ± 10.1 |
20.46 ± 2.1 |
237.84 ± 3.9 |
186.35 ± 0.5 |
294.48 ± 15.2 |
212.43 ± 3.0 |
|
Histamine
|
1.78 ± 0.1 |
3.79 ± 0.4 |
402.76 ± 38.1 |
13.41 ± 0.4 |
665.98 ± 78.3 |
390.29 ± 12.8 |
853.10 ± 30.2 |
252.58 ± 23.1 |
|
Tyramine
|
0.09 ± 0.0 |
0.02 ± 0.0 |
20.38 ± 1.8 |
6.97 ± 0.1 |
33.37 ± 0.3 |
24.69 ±0.1 |
32.74 ± 0.9 |
17.94 ± 2.2 |
|
Spermidine
|
10.66 ± 0.0 |
14.91 ± 0.7 |
24.96 ± 1.9 |
14.99 ± 0.9 |
26.70 ± 3.1 |
6.82 ± 0.2 |
3.83 ± 0.2 |
4.45 ± 0.6 |
|
Spermine |
53.60 ± 0.4 |
58.18 ± 1.6 |
60.30 ± 5.6 |
58.08 ± 2.0 |
46.90 ± 2.4 |
54.06 ± 4.6 |
41.05 ± 4.7 |
39.74 ± 1.3 |
Table3: Levels of the monitored biogenic amines (mg kg-1) in chicken meat during the storage in tested packaging.
SYN: packaging PE laminated paper sprayed with SYNBIO®
LP: packaging PE laminated paper sprayed with Lact. plantarum IMC 509
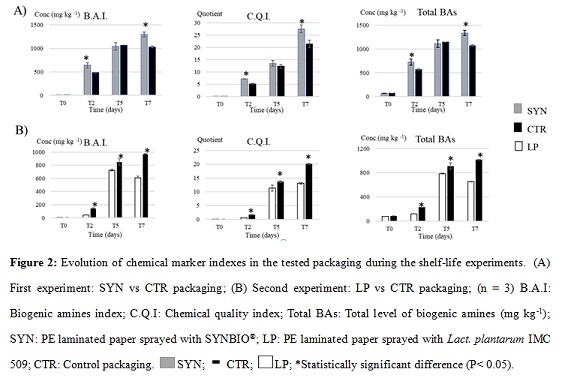
BA concentrations were used to determine chemical indexes assessing the effects of probiotic packaging on the Chicken quality during storage (Figure 2). Considering SYN vs CTR, the levels of BAs throughout the storage, tended to be higher in chicken samples wrapped in SYN than in CTR-packaging.
The CQI levels, starting with similar values (0.064 and 0.065) at t0, increased during chicken meat shelf-life, but tended to be higher in SYN-packaging samples respect to CTR from t2 until t7 (27.57 and 21.35). These differences were statistically significant (P? 0.05) at t2, with C.Q.I levels of 7.18 and 5.16 for samples in SYN and CTR-packages respectively. From t2, BAI levels and total BAs concentrations have the tendency to be higher in samples wrapped in SYN than in CTR-packaging. The gaps were statistically significant (P? 0.05) at the end of the study (t7) with B.A.I levels of 1294 and 1027 mg kg-1 from SYN and CTR respectively (Figure 2A). Unlike the SYN-packaging, from t2 to t7, the levels of PUT, CAD, HIS and TYR increased during the storage, but remained lower during each monitored day in chicken samples stored in LP-packaging respect to CTR.
Figure 2B reports the evolution of CQI, BAI, SPD/SPM, and Total BAs during the monitored days in chicken samples in LP and CTR-packaging. LP-packaged meat presented a reduction of the BAs increment during storage. CQI, BAI and Total BAs have the tendency to be lower in PL-packaged samples than in CTR-samples. These differences were statistically significant (P< 0.05) at t2 (CQI: 0.53 vs 1.75; B.A.I: 46.4 vs 149 mg kg-1) and t7 (CQI: 13.1 vs 20.1; B.A.I: 609 vs 970 mg kg-1).
pH of meat during storage
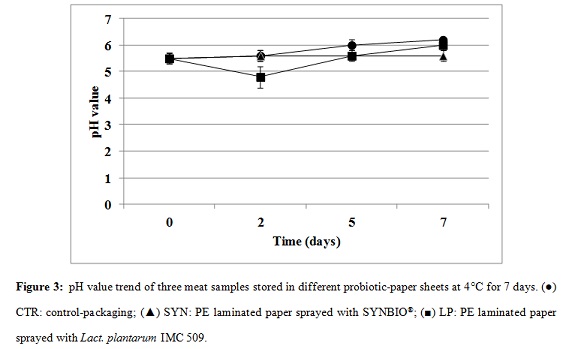
One of the poultry meat spoilage indicators is the sour smell on meat surface which makes it utterly repellent [24]. Whereas the pH values of all samples at t0 were around 5.5 ± 0.2, the pH showed relatively different variations afterwards (Figure 3). The pH value of CTR-meat exhibited an increment after 2 days storage, from 6 ± 0.2 at t5 to 6.2 ± 0.1 at t7. However, the meat pH decreased to 4.8 ± 0.4 after two days stored in LP-package sheet, while it restored to initial pH value after 3 days in fridge, reaching 6.0 ± 0.2 at t7. SYN-package had no effect on meat pH values during 7 days storing.
Changes in sensory characteristics
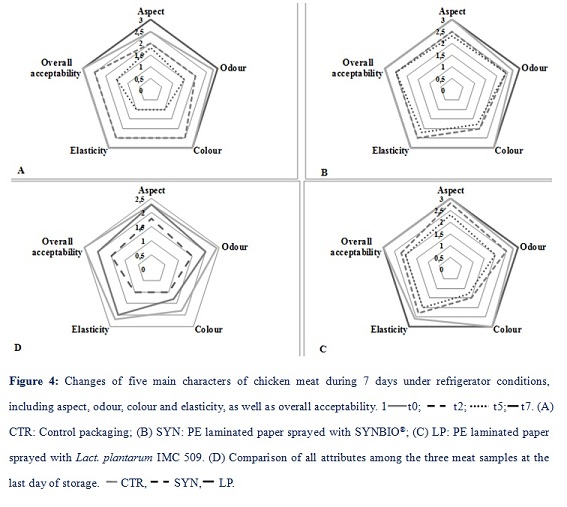
All samples exhibited optimal sensorial qualities, having maximum scores in all attributes at t0 (Figure 4 –A, B, C). After two days at 4°C, a slightly decrement was found in meat odour in all three samples, in addition, meat stored in CTR PE laminated paper sheet also lost a bit at its aspect. At t5, all meat samples showed reduced scores in all characters, notably, a larger decrement was found in meat colour, when it was in LP-packaging. Figure 4-D shows all the attributes in the three meat samples at the last day of storage. Compared to CTR, meat in both probiotic-packaging paper sheets showed a better preservation of main meat characteristics. Furthermore, SYN-packaging seemed to be better more efficient than LP-packaging in preserving aspect, odour, colour and elasticity. Looking at the overall acceptability, after 7 days, meat preserved inside SYN-package was still positively accepted by all panellists, while meat in LP-package had less scores.
Probiotics viability on packaging paper sheet
Table 4 shows the viability of probiotic strains and the survival rate (%), which was calculated after recovering from both paper sheet area touched with meat and area not in touch with meat during storage period. The SYNBIO® combination and the Lact. plantarum IMC 509 strain showed excellent survival rate during the study period. SYNBIO®, showed an updrawn trend in its cell counts after 5 days (18 times over 100 %), and further increased after 7 days of its initial value on areas which were not in touch with meat surface (30 times over 100 %). On the other hand from the area which is in contact with meat, its survival rates was firstly increased 15 times over 100 % after 2 days of storage and this percentage was nearly kept up to day 5. At day 7, a 40 time over 100 % survival rate was observed. Lact. plantarum IMC 509 strain demonstrated a lower but a more stable viability from beginning time point to the end, in particular on the sampling area in contact with meat (Table 4).
|
Bacterial strains |
Time point (days) |
Bacterial cell counts (CFU ml-1)* |
Survival rate (%) |
|||
|
Not in contact with meat |
|
|||||
|
SYNBIO® |
0 |
(1.0 ± 0.0) x 102 |
100 |
|||
|
2 |
(1.0 ± 0.0) x 102 |
100 |
||||
|
5 |
(1.8 ± 0.1) x 103 |
>> 100 |
||||
|
|
7 |
(3.0 ± 0.2) x 105 |
>>> 100 |
|||
|
|
|
|
|
|||
|
Lact. plantarum IMC 509 |
0 |
(2.0 ± 0.0) x 108 |
100 |
|||
|
2 |
(1.7 ± 0.4) x 108 |
85 |
||||
|
5 |
(3.7 ± 0.1) x 107 |
20 |
||||
|
|
7 |
(5.8 ± 0.0) x 106 |
3 |
|||
|
|
|
|
|
|||
|
In contact with meat |
|
|||||
|
|
|
|
|
|||
|
SYNBIO® |
0 |
(1.0 ± 0.0) x 102 |
100 |
|||
|
2 |
(1.5 ± 0.1) x 105 |
>> 100 |
||||
|
5 |
(0.5 ± 0.2) x 105 |
>> 100 |
||||
|
|
7 |
(1.0 ± 0.0) x 106 |
>>> 100 |
|||
|
|
|
|
|
|||
|
Lact. plantarum IMC 509 |
0 |
(3.5 ± 0.7) x 106 |
100 |
|||
|
2 |
(3.4 ± 0.3) x 106 |
97 |
||||
|
5 |
(1.6 ± 0.3) x 106 |
46 |
||||
|
|
7 |
(2.3 ± 1.3) x 106 |
66 |
|||
Table 4: Cell count values (expressed as CFU ml-1) of probiotic bacterial strains, monitored at 0, 2, 5 and 7 days on paper sheets, from both paper sheet area touched with meat and area not in touch with meat. The respective survival rate (%) is also reported.
*mean value ± SD
DISCUSSION
The packaging materials sprayed with the two selected probiotic suspensions showed different effect on the growth of total aerobic mesophilic bacteria in the meat. Differences were observed among three meat samples, with the higher amount of mesophiles in SYN-wrapped meat compared to CTR sample, while lower amounts of mesophiles were found in LP-wrapped meat. This trend put in evidence a sort of inhibitory activity of Lact. plantarum IMC 509 toward common spoilage bacteria, while the probiotic combination SYNBIO® showed promoting effects on the growth of meat microbes compared to CTR sample.
The synthesis and accumulation of BAs during chicken storage are in accordance with related studies [25] and are associated to the presence of microorganisms with decarboxylating activity. Enterobacteriaceae have been associated principally with the production of PUT and CAD [26] while LAB strains of Enterococcus spp. and Lactobacillus spp. are the main producers of TYR [27]. Enterobacteriaceae and LAB strains are reported to be histamine-producing bacteria [28].
High oral intake of BAs in food can be hazardous to human health inducing adverse reactions such as nausea, headaches, rashes and change in blood pressure [29] However, the only BA for which maximum levels have been regulated by the EU and the USA is histamine. FDA limits HIS levels to 500 mg kg1 [30] while the Commission Regulation (EU) setup the maximum levels of HIS to 200 and up to 400 mg kg-1 in fish and fishery products respectively [31-33] suggested the maximum tolerable levels of PUT and CAD in Austrian fish products to be respectively 170 and 510 mg kg-1.
Our results from chemical indexes were contrary to the effects expected proving by this fact that the probiotic strains chosen in the SYN-packaging were not able to prevent the formation of high levels of BAs. Indeed, they have rather increased the BAs synthesis in chicken during storage. Lact. rhamnosus IMC 501® and Lact. paracasei IMC 502®, which were combined in SYN-packaging, possess inhibitory activity towards the multiplication of pathogenic microorganisms and are highly competitive due to their production of several antimicrobial compounds such as bacteriocins [34] Moreover, other studies reported that Lact. rhamnosus was suitable for use as starter cultures in fermenting dry sausage allowing the BAs levels to remain low during the ripening [35] However, although having antimicrobial activities, these strains caused a higher increment of BAs levels in chicken during the experiment. This could be explained by the fact they possess also BA-producing properties. Indeed, Lact. paracasei has been reported as a BAs producing microorganism especially for PUT, CAD and TYR [36,37] also reported the production of BAs by Lact. rhamnosus. These results could imply that the BAs accumulation in chicken is not determined by the antimicrobial activity of the probiotic strains but by their positive amino-acid decarboxylase activity.
According to Mietz and Karmas (1997) [18] formula, a CQI < 1 indicates a good tuna quality, between1-10, tuna quality is borderline, while a CQI > 10 indicates a decomposed tuna [38]. Although these ranges have been defined for fish, they could be applied on chicken to comment the effect of the LP- packaging on chicken quality during storage. Indeed at t2, LP-packed samples were still in good quality with a CQI < 1 (0.53) while CTR samples were already in a borderline state (CQI = 1.75). According to the same CQI ranges, at the end of the study, the LP-packed samples were closed to the borderline state (CQI = 13.1) while the CTR samples were in advanced state of decomposition (CQI = 20.1).
Considering the Total BAs, at the end of the study, the LP-packaging allowed a 35.8 % reduction of BAs levels (mg kg-1) compared to the CTR-packaging. This reduction seems to be clearly caused by the probiotic strain used during LP-packaging preparation. Indeed, besides its antimicrobial activities, Lact. plantarum also demonstrated to possess the ability to reduce the BAs accumulation in chicken during storage. This ability can be explained by the amino-oxidase activity, which is reported in different LAB strains such as Lact. sakei and Lact. pentosus [39]. The amino-oxidase activity of these strains allows them to degrade BAs once they have been synthesized in the food matrix and thus, they can prevent the formation of high BAs levels in chicken. Different authors have reported the amino-oxidase activity of Lact. plantarum [40]. Moreover, it has been used in various studies to reduce the BAs accumulation in different food matrix such as wine and sausage [41,42] similar results were observed in the present study, however it is important to note that it is the first time that Lact. plantarum is used simultaneously as an antimicrobial agent and also as BA-degrading microorganism in active packaging for chicken. The variations of meat samples pH value demonstrated the effects of different Lactobacillus species on fresh chicken meat acidity. A sharp decrease in the pH value of LP-package stored chicken meat was observed after 2 days. This decrement was also observed by [43].
The sensory evaluations of the chicken meat samples were positive, considering the packaging materials with both probiotic strains; especially the combination product seemed to greatly maintain the sensorial characteristics of meat.
Both probiotics have a good capacity to maintain their viability on paper sheets, but Lact. plantarum IMC 509 had a higher stability, maintaining its count during time, even if showed a decrease in the area without contact. SYNBIO® had a high increase in the meat contact area. It may be explained by the fact that SYNBIO® strains, when touch meat surface, use meat nutrients not only to survive, but also to grow, facilitating meat spoilage process. A further investigation showed that both probiotic strains were still viable on the packaging paper sheets after 50 days (data not shown).
CONCLUSION
In summary, between the different probiotic PE laminated paper sheets in this preliminary study, both the SYN and LP-packaging showed some promising characteristics for extending the shelf-life of chicken meat, using a commercial packaging PE laminated paper sheet.
In particular, when taking into account the total aerobic mesophiles bacteria, the lower bacterial counts demonstrated the inhibition capability of Lact. plantarum IMC 509 toward common spoilage bacteria, while the probiotic product SYNBIO® showed promoting effects on meat microbes when compared to control. In addition the determination of the BAs levels put in evidence that Lact. plantarum IMC 509 possess an interesting activity as BA-degrading microorganism.
The high bacteria counts on meat at the beginning of the study may hamper the inhibition effects exerted by probiotic strains. Thus, to have a better understanding of the predicted effects, chicken samples with low microbial counts should be applied for further studies.
At last, Lact. plantarum IMC 509 had a high stability on the paper maintaining its survival rate during time and not affecting the microbial count of the meat. Keeping viable for such a high value during the whole study period is noteworthy, in this way they can work as a preservative for food.
Therefore, the differences generated from SYN and LP-packaging, highlighting the fact that the choice of an appropriate probiotic strain in meat active packaging development, not only depends on its antimicrobial activity, but also on its amino-oxidase activity. These properties, could work synergistically to implement bio-preserve packaging to maintaining chicken meat quality for longer period of time, by reducing both the pathological microbial growth and the BAs accumulation during chicken meat shelf-life.
ACKNOWLEDGEMENTS
This work was supported by the University of Camerino and Synbiotec Srl, Italy (MIUR contract n. 1-2895), Technical University of Cluj Napoca and Ceprohart Braila, Romania (UEFISCDI 72/2017), National Institute of Chemistry Ljubljana, Slovenia (MIZS 4126), and Andaltec, Spain (MINECO PCIN-2017-037), in the frame of GRAFOOD project.
CONFLICT OF INTEREST STATEMENT
The authors declare that there are no conflicts of interest.
REFERENCES
- Marangoni F, Corsello G, Cricelli C, Ferrara N, Ghiselli A, et al. (2015) Role of poultry meat in a balanced diet aimed at maintaining health and wellbeing: an Italian consensus document. Food Nutri. Res 59: 27606.
- Motarjemi Y, Moy G, Todd E (2013) Encyclopedia of food safety. In: Academic Press.
- Carrizosa E, Benito MJ, Ruiz-Moyano S, Hernández A, Villalobos MDC, et al (2017) Bacterial communities of fresh goat meat packaged in modified atmosphere. Food Microbiol 65: 57-63.
- Remenant B, Jaffrès E, Dousset X, Pilet M, Zagorec M (2015) Bacterial spoilers of food: Behavior, fitness and functional properties. Food Microbiol. 45: 45-53.
- Rouger A, Tresse O, Zagorec M (2017) Bacterial Contaminants of Poultry Meat: Sources, Species, and Dynamics. Microorganisms 5: 50.
- Lee HS, Kwon M, Heo S, Kim MG, Kim GB (2017) Characterization of the biodiversity of the spoilage microbiota in chicken meat using next generation sequencing and culture dependent approach. Korean J. Food Sci.An 37: 535-541.
- Santos M, Draisci H, Giannetti R, Boria L, Lucentini L, et al. (1998) Amino acid decarboxylase capability of microorganisms isolated in Spanish fermented meat products. J. Food Microbiol 39: 227-230.
- Stadnik J, Dolatowski Z (2010) Biogenic amines in meat and fermented meat products. ACTA Pol. Technol. Aliment 9: 251-263.
- Ekici K, Omer AK (2018) the determination of some biogenic amines in Turkish fermented sausages consumed in Van. Toxicol Rep 5: 639-643.
- Coman MM, Cecchini C, Verdenelli MC, Silvi S, Orpianesi C, et al. (2012) Functional foods as carriers for SYNBIO®, a probiotic bacteria combination. J. of Food Micr 157: 346-352.
- Laranjo M, Elias M, Fraqueza MJ (2017) the use of starter cultures in traditional meat products. Food Qual. 2017: 1-18.
- Reis JA, Paula AT, Casarotti SN, Penna ALB (2012) Lactic Acid Bacteria Antimicrobial Compounds: Characteristics and Applications. Eng Rev. 4: 124-140.
- Kamarudheen N, George C, Priya CL, Bhaskara Rao KV (2014) Biopreservation of meat by probiotic bacteria isolated from dairy products. Der Pharmacia Lettre 6: 266-271.
- Park YH, Hamidon F, Rajangan C, Soh KP, Gan CY, et al. (2016) Application of Probiotics for the Production of Safe and High-quality Poultry Meat. Korean J. Food Sci. Anim. Res 36: 567-576.
- Verdenelli MC, Silvi S, Cecchini C, Orpianesi C, Cresci A (2011) Influence of a combination of two potential probiotic strains, Lactobacillus rhamnosus IMC 501® and Lactobacillus paracasei IMC 502® on bowel habits of healthy adults. Lett. Appl. Microbiol 52: 596-602.
- Coman MM, Verdenelli MC, Cecchini C, Silvi S, Orpianesi C, et al. (2014) in vitro evaluation of antimicrobial activity of Lactobacillus rhamnosus IMC 501®, Lactobacillus paracasei IMC 502® and SYNBIO® against pathogens. Appl. Microbiol 117: 518-527.
- Sirocchi V, Caprioli G, Cecchini C, Coman MM, Cresci A, et al. (2013) Biogenic amines as freshness indexof meat wrapped in a new active packaging system formulated with essential oils of Rosmarinus officinalis. J.Food Sci. Nutr 64: 921-928.
- Mietz JL, Karmas E (1977) Chemical quality index of canned tuna as determined by high?pressure liquid chromatography. of Food Sci 42: 155-158.
- Veciana-Nogués MT, Mariné-Font A, Vidal-Carou MC, (1997) Biogenic amines as hygienic quality indicators of tuna. Relationships with microbial counts, ATP-related compounds, volatile amines, and organoleptic changes. Agr. Food Chem 45: 2036-2041.
- Silva CM, Glória MBA, (2002) bioactive amines in chicken breast and thigh after slaughter and during storage at 4±1°C and in chicken-based meat Food Chem 78: 241-248.
- Baston O, Barna O (2010) Raw chicken leg and breast sensory evaluation. Annals. Food Sci. Tech 11: 25-30.
- Satyada R, Sandle T (2016) Releasing capacity of pre-sterile cotton swabs for discharging sampled microorganisms. European Journal of Parenteral and Pharmaceutical Sciences 21: 121-128.
- Silvi S, Verdenelli MC, Cecchini C, Coman MM, Bernabei S, RosatiJ De, Leone R, Orpianesi C, Cresci A (2014) Probiotic-enriched foods and dietary supplement containing SYNBIO® positively affects bowel habits in healthy adults: an assessment using standard statistical analysis and Support Vector Machines. Int J Food Sci Nutr 65: 994-1002.
- McGlynn W (1992) The Importance of Food pH in Commercial Canning Operations. 118: 1-8.
- Triki M, Herrero A, Jiménez-Colmenero F, Ruiz-Capillas C (2018) Quality Assessment of Fresh Meat from Several Species Based on Free Amino Acid and Biogenic Amine Contents during Chilled Storage. Foods 7: 132.
- Curiel JA, Ruiz-Capillas C, de Las Rivas B, Carrascosa AV, Jiménez-Colmenero F, et al. (2011) Production of biogenic amines by lactic acid bacteria and enterobacteria isolated from fresh pork sausages packaged in different atmospheres and kept under refrigeration. Meat Sci 88: 368-373.
- Ladero V, Calles-Enríquez M, Fernández M, Alvarez M (2010) Toxicological effects of dietary biogenic amines. Nutr. & Food Sci 6: 145-156.
- Comas-Basté O, Latorre-Moratalla ML, Sánchez-Pérez S, Veciana-Nogués MT, del Carmen Vidal-Carou M (2019) Histamine and Other Biogenic Amines in Food. From Scombroid Poisoning to Histamine Intolerance. In: Biogenic Amines. Intech Open.
- Paulsen P, Bauer S, Bauer F (2019) Biogenic amines and polyamines in foods of animal origin. In: Smulders FJM, Rietjens IMCM, & Rose MD (Eds.) Chemical hazards in foods of animal origin. (Food safety assurance and veterinary public health; Vol. 7). Wageningen Academic Publishers, Wageningen, page nos. 323-328.
- Sagratini G, Fernandez-Franzón M, Berardinis F, Font G, Vittori S, et al. (2012) Simultaneous determination of eight underivatised biogenic amines in fish by solid phase extraction and liquid chromatography–tandem mass spectrometry. Food Chem 132: 537-543
- H.E.E. Commission, Commission Regulation (EU) (2013) No 1019/2013 of 23/10/2013 amending Annex I to Regulation (EC) No 2073/2005 as regards histamine in fishery products. Off J Eur Commun, L282, page nos. 46-47.
- Sørensen KM, Aru V, Khakimov B, Aunskjaer U, Engelsen SB, (2018) Biogenic amines: a key freshness parameter of animal protein products in the coming circular economy. Op. in Food Sci. 22: 167-173.
- Rauscher-Gabernig E, Gabernig R, Brueller W, Grossgut R, Bauer F, et al. (2012) Dietary exposure assessment of putrescine and cadaverine and derivation of tolerable levels in selected foods consumed in Austria. Food Res. Tech 235: 209-220.
- Verdenelli MC, Ghelfi F, Silvi S, Orpianesi C, Cecchini C, et al. (2009) Probiotic properties of Lactobacillus rhamnosus and Lactobacillus paracasei isolated from human faeces. Eur J Nutr 48: 355-363.
- Erkkilä S, Suihko ML, Eerola S, Petäjä E, Mattila-Sandholm T (2001) Dry sausage fermented by Lactobacillus rhamnosusInt. J. Food Microbiol 64: 205-210.
- Benkerroum N (2016) Biogenic amines in dairy products: origin, incidence, and control means. Comprehensive Reviews in Food Sc. Food Saf.15: 801-826.
- Lorencová E, Bu?ková L, Matoulková D, Dráb V, Pleva P, et al. (2012) Production of biogenic amines by lactic acid bacteria and bifidobacteria isolated from dairy products and beer. Int J Food Sci Tech 47: 2086-2091.
- Barbosa RG, Gonzaga LV, Lodetti E, Olivo G, Costa ACO, et al. (2018) Biogenic amines assessment during different stages of the canning process of skipjack tuna (Katsuwonus pelamis). J. Food Sc Tech 53: 1236-1245.
- Lorenzo JM, Munekata PES, Domínguez R (2017) Role of autochthonous starter cultures in the reduction of biogenic amines in traditional meat products. Op in Food Sci 14: 61-65.
- Alvarez MA, Moreno-Arribas MV (2014) the problem of biogenic amines in fermented foods and the use of potential biogenic amine-degrading microorganisms as a solution. Food Sc. & Tech 39: 146-155.
- Garcia-Ruiz A, González-Rompinelli EM, Bartolome B, Moreno-Arribas MV (2011) Potential of wine-associated lactic acid bacteria to degrade biogenic amines. Int. J. Food Microbiol 148: 115-120.
- Zhang Q, Lin S, Nie X, (2013) Reduction of biogenic amine accumulation in silver carp sausage by an amine-negative LactobacillusFood Contr 32: 496-500.
- Sun Q, Chen Q, Li F, Zheng D, Kong B (2016) Biogenic amine inhibition and quality protection of Harbin dry sausages by inoculation with Staphylococcus xylosus and Lactobacillus plantarum Food Contr 68: 358-366.
Citation: Huang X, Kamgang NF, Coman MM, Peter A, Talasman CM, et al. (2020), Lactobacillus strains treatment on commercial packaging paper as preliminary study for extending the shelf-life of chicken meat, J Biotech Res Biochem 3: 007.
Copyright: © 2020 Huang X8, , et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.

