
Mechanistic and Efficacy Study on Effects of Fibroin Enzymatic Hydrolysate (FEH) on Memory and Learning Impairments Induced by Scopolamine in Mice
*Corresponding Author(s):
Sidney J StohsSchool Of Pharmacy And Health Professions, Creighton University Medical Center, Omaha, United States
Tel:+1 1 2142156655,
Email:sid.stohs9@gmail.com
Abstract
Previous studies have indicated that the oral administration of a silk Fibroin Enzymatic Hydrolysate (FEH) is beneficial in supporting memory and learning. In this study, the effects of oral administration of a FEH on scopolamine-induced memory and learning impairment in mice were investigated to assess the mechanism involved. Mice were tested for memory and learning using the Morris water maze, passive avoidance and Y-maze tests. Acetylcholine (Ach), Acetylcholine Esterase (AChE), Brain-Derived Neurotrophic Factor (BNDF), and cytokines Tumor Necrosis Factor alpha (TNF-α), Interleukin-1 beta (IL-1β) and interleukin-6 (IL-6) were measured in the brain hippocampus. Protein expression of BDNF-associated and p75 apoptotic signaling pathways were determined to investigate mechanistic effects of FEH. Scopolamine was given intraperitoneally 1.0 mg.kg daily for three weeks. Daily FEH treatment (10, 50and 150 mg/kg b.w.) for three weeks inhibited scopolamine-induced memory and learning impairment. In addition, FEH treatment increased ACh and BDNF levels, and reduced the levels of IL-1β, TNF-α and IL-6 in brain hippocampal tissue of mice with scopolamine-induced memory and learning impairment. Furthermore, FEH treatment enhanced the expression of phosphorylated cAMP response element-binding protein (p-CREB) and BDNF via the stimulation of the phosphatidylinositol-3-kinase/protein kinase B/mammalian target of rapamycin/postsynaptic density protein 95 (p-PI3K/p-AKT/mTOR/PSD95) pathway, and activation of Extracellular Signal-Regulated Kinases (ERK) and Ca2+/calmodulin-dependent protein kinase II (CaMKII). These results provide extensive information regarding the protective mechanism of action of FEH, and support the potential of FEH supplementation to prevent learning and memory impairment.
Keywords
Amyloid beta plaque; Fibroin enzymatic hydrolysate; Inflammation; Memory and learning; Neurofibrillary tangles; Neurodegenerative diseases; Oxidative damage
Abbreviations
AB: Amyloid beta
FEH: Fibroin Enzymatic Hydrolysate
BDNF: Brain-Derived Neurotrophic Factor
DO: Donepezil
TNF-α: Tumor Necrosis Factor-alpha
IL: Interleukin
Ach: Acetylcholine
AchE: Acetylcholinesterase
(NF-κB): Nuclear Factor-kappa B
(TrkB): Tropomyosin receptor kinase B
(PI3K): Phosphatidylinositol 3-kinase
(PI3K): Protein kinase B x
(P-AKT): Phosphorylated protein kinase B
(mTOR): Mammalian Target Of Rapamycin
Cyclic Adenosine Monophosphate [cAMP] response element-binding protein (CREB)
(JNK): c-Jun N-terminal Kinase
(p-ERK 1/2): Phosphorylated extracellular signal-regulated kinase 1/2, P75
(TRAF6): TNF receptor-associated factor 6
(Bcl2): B-cell lymphoma 2
(Bax): Bcl2-associated X protein
(PSD 95): Postsynaptic Density protein 95
Introduction
The central nervous system plays a crucial role in memory and learning [1]. Impairment of the central nervous system causes memory and learning loss that can induce dementia and even lead to Alzheimer's disease [2]. General dementia symptoms are memory and learning loss, disorientation and inappropriate behavior, changes in personality and language problems. Currently, approximately 50 million people suffer from dementia worldwide, and the number of patients with memory and learning loss is expected to increase in the future [3].
Learning and memory formation involve the activation of neurotransmitters as Acetylcholine (ACh), γ-Aminobutyric Acid (GABA), dopamine and serotonin that have crucial roles as intercellular messengers in the nervous system [4,5]. Damage including oxidative damage to the nervous system, especially to the cholinergic system, is associated with memory loss and learning impairment [6]. The cholinergic neuron secretes ACh, which plays a role in encoding new information. Thus, reduced cholinergic neurons and choline acetyltransferase activity for the synthesis of ACh causes memory loss and learning impairment [4-6].
Studies have demonstrated that induction of neuro-inflammation is associated with memory and learning loss via neurodegeneration [7,8]. The imbalance between the production of reactive oxygen species and antioxidant defense mechanisms contribute significantly to the pathogenesis of neurodegeneration and progression of diseases associated with memory loss and learning ability as Alzheimer’s. Huang et al. reported that the anti-inflammatory drug ibuprofen suppressed inflammation in the brain and improved cognitive performance [8]. Base on these mechanisms, Acetylcholinesterase (AChE) inhibitors and anti-inflammatory drugs are used to improve memory loss symptoms [6-8]. In addition, various nutrients and Nutraceutical have been studied for the improvement of memory loss and modulation of neurodegeneration [9].
Dietary proteins and amino acids are important for the precursors of neurotransmitters including norepinephrine, dopamine and epinephrine [10]. Silk peptide, from the cocoons produced by silkworms, has been proposed as an amino acid supplement with biological activities such as anti-inflammatory, immune-regulatory, anti-tumor, and bone formation functions [11-13].
Several studies have observed that a specifically prepared, proprietary silk protein Enzymatic Hydrolysate (FEH) improves cognitive functions in healthy humans [14,15]. However, the mechanisms involved in mediating these effects have not been identified [16]. A previous study has shown that FEH significantly ameliorated amyloid beta induced learning impairment and memory loss in mice, and reduced the levels of inflammatory cytokines [17]. In this study, the ability of orally administered FEH to enhance memory and learning in scopolamine-induced memory and learning impaired mice, and the associated mechanisms were investigated in brain tissue of mice.
Materials And Methods
Treatment and induction of memory impairments induced by scopolamine
This study was conducted at the Brain Research Laboratory, Department of Oriental Medicinal Sciences, Kyung Hee University, Seoul 02447 and Republic of Korea. The experimental protocol was approved by the Kyung HeeUniversity’s Animal Care and Use Review Committee. The silk fibroin protein hydrolysate (FEH) was procured from Sunbio Corporation (Long Beach, CA, USA). Briefly, the FEH was prepared by protease hydrolysis and isolated by gel-filtration column chromatography (Sephadex G-25, Pharmacia, Sweden) as previously described [17]. The final product (FEH) consists of polypeptides with a median molecular weight of approximately 2750 Daltons. The primary amino acids inthepeptidecomponents include glycine (42.5 %), alanine (28.2 %) and serine (9.4 %). Scopolamine and Donepezil (DO) were obtained from Sigma Chemical Co.
C57BL/6 mice were obtained from Searon-bio (Uiwang-si, Korea) and were housed in a cages with 8 animals per cage (22 ± 2 °C, humidity of 55 ± 5%, and a 12-h light/dark cycle), provided AIN 93G diet and water ad libitum, and allowed to adapt for 7 days. The animals were randomly divided into five groups (n = 8) which included normal placebo control, scopolamine control (1 mg/kg, i.p.), Donepezil [DO] positive control (scopolamine 1 mg/kg i.p. + DO 2 mg/kg p.o.), FEH 10 (scopolamine 1 mg/kgi.p. + FEH 10 mg/kg body weight (b.w.) p.o.), FEH 50 (scopolamine 1 mg/kg i.p. + FEH 50 mg/kg b.w, p.o.), and FEH 150 (scopolamine 1 mg/kg i.p. + FEH 150 mg/kg b.w, p.o.). DO is an acetylcholine esterase inhibitor that enhances memory and learning in rodents [18] while scopolamine is well known to impair memory and learning [19]. The FEH and DO were given at the same time that the scopolamine was administered i.p. The animals were treated once daily, six days per week for three weeks. Mice were tested for learning and memory abilities and were euthanized by cervical dislocation at the end of three weeks.
Morris water maze test
The water maze was a circular pool (140 cm in diameter and 60 cm in height) with a water depth of 25 cm and 23 ± 1°C. Visual cues were placed on the edge of the pool. This test was conducted according to the procedure of Hagaki et al., [20]. The pool was divided into four quadrants with an escape platform placed in the middle of one quadrant, 1.0 cm below the water surface.
Mice were trained for five days with four training sessions per day. During the training, the time required for a mouse to locate the platform was measured within 60 s (escape latency). If a mouse was unable to reach the platform within 60 s, it was guided to the platform and scored with a latency of 60 s. The latency time to reach the hidden platform and the retention time in the target quadrant were analyzed by Smart System 3.0 video tracking software (Panlab, Harvard Apparatus, Holliston, MA USA).
Passive avoidance test
Mice were placed in a shuttle box (250 X 250 cm) with a small dark chamber and a bright chamber (Scitech Korea Inc, Seoul, Republic of Korea) according to the procedure of Malikowsha et al., [21]. A mouse was placed in the bright chamber; the guillotine door was opened and after entry into the dark compartments the door automatically. The subject received a low-intensity electric shock (75 V, 0.5 mA 50 Hz) in the dark compartment which induced fear conditioning and learning. Each experimental animal again was placed in the bright light chamber after 8 hours, and the time to enter the dark chamber was determined.
Y-maze test
This test was conducted according to the procedure of Kraeuter et al., [22] after three weeks of treatment, and consisted of three extending plastic (80 cm) arms arranged at 120º positions. Mice were randomly placed in one arm and permitted to freely move freely for 8 min. among the arms of the device. Alternation was defined as a sequential visit to each of the three different arms. The spontaneous alternation percentage was defined as the percentage of actual alternations relative to the total number of possible arm entries.
Measurement of Ach, AChE, BNDF, and cytokines TNF-α, IL-1β and IL-6 in the brain
Brain hippocampal tissues were obtained from all mice following three weeks of treatment, and ELISA kits were used to determine the levels of ACh, AChE (BioVision Inc., Milpitas, CA USA), BNDF (MyBioSource Inc., San Diego, CA USA);, and the cytokines TNF-α, IL-1β and IL-6 (R&D Systems, Minneapolis, MN USA) based on the manufacturer’s instructions.
Western blot assays
Following treatment, brain hippocampal tissues were placed in radio immune precipitation assay RIPA) buffer (Sigma-Aldrich Korea, Seoul, Republic of Korea) and centrifuged at 124,000 × g and 4°C. For 20 min.Proteins were separated on sodium dodecyl sulfate-polyacrylamide gel (SDS-PAGE, 10%) and transferred onto a polyvinylidene difluoride membrane. Following incubation with a blocking buffer for one hour, the membranes were incubated overnight at 4°C in primary antibody to phosphoinositide 3-kinase (p-PI3K), protein kinase B (p-AKT), mammalian target of rapamycin (mTOR), postsynaptic density protein 95 (PSD95), extracellular signal-regulated kinases 1/2 (ERK1/2), Ca2+/calmodulin-dependent protein kinase II (CaMK II), phosphorylated cAMP response element-binding protein (p-CREB) and β-actin (Cell Signaling Technology, Beverly, MA, USA), followed by a two hour incubation with an secondary antibody (Cell Signaling Technology, Beverly, MA, USA) at room temperature. The immunoreactive protein bands were detected using chemiluminescence (ECL) western blotting detection reagents (Bio-Rad, Hercules, CA, USA) and CS Analyzer 3.0 software (Ez-Capture II, ATTO, Tokyo, Japan)) was used for quantification.
Immunohistochemistry (IHC) Assays
Brain hippocampal tissues from the mice in each treatment group were fixed in 10% buffered formalin and embedded in paraffin. The paraffin blocks were cut at 4 µm thickness, and sections were stained with rabbit anti-BNDF antibody and anti-CREB antibody (Abcam, Cambridge, UK). Microscopic image analysis was performed to assess color changes.
Statistical analysis
All results were expressed as mean ± Standard Deviation (SD) and a one-way ANOVA or t-test using SPSS statistical procedures (SPSS PASW Statistic 23.0, SPSS Inc. Chicago, IL, USA) was used for statistical analysis. Differences between groups were determined by using Duncan’s multiple range test. P-Values less than 0.05 were considered significant. Values with non-identical superscripts were significantly different (P < 0.05).
Results
To assess the effect of FEH on scopolamine-induced impairment of memory acquisition and retention in mice, the Morris water maze (Figure 1), passive avoidance (Figure 2A) and Y-maze tests (Figure 2B). Were employed. Scopolamine induced a delay in escape latency and reduced the time spent in the target quadrant in the Morris water maze test (Figure 1). However, oral administration of the positive control DO as well as FEH for three weeks significantly suppressed scopolamine-induced memory and learning impairment (P < 0.05). A significant enhancement of memory and learning was produced by the lowest dose (10 mg/kg) of FEH with small but non-significant dose-dependent effects of FEH at higher doses (Figure 1).
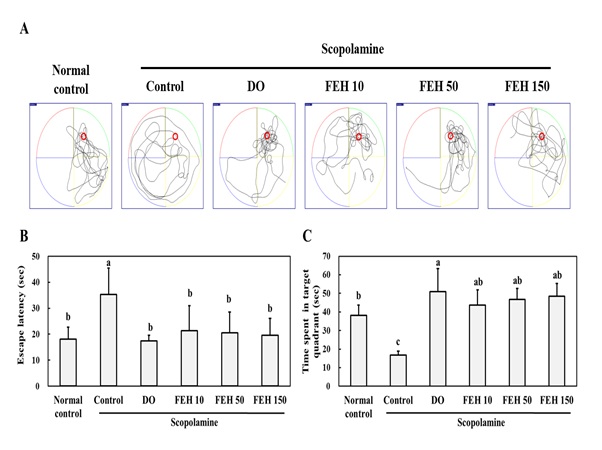 Figure 1: Effect of Fibroin Enzymatic Hydrolysate (FEH) on the Morris water maze test in mice with scopolamine-induced memory and learning impairment.
Figure 1: Effect of Fibroin Enzymatic Hydrolysate (FEH) on the Morris water maze test in mice with scopolamine-induced memory and learning impairment.
(A) Escape latency in sec. (B) Time spent in target quadrant in sec. (C) Time spent in target quadrant in sec. Mice were divided into the following treatment groups: normal (placebo) control, scopolamine control, DO positive control, and scopolamine plus FEH. Values are presented as means ± SD. Non-identical superscript letters indicate significant differences at P
Scopolamine administration decreased the step-through latencies in the passive avoidance test (Figure 2A) and spontaneous alteration in the Y-maze test (Figure 2B), indicating decreased memory and learning. These effects were reversed by the oral administration of FEH for three weeks. The differences in the effects of the three dosages of FEH were not statistically significant. DO was shown to be effective as a positive control in reversing scopolamine -induced effects with respect to the passive avoidance (Figure 2A) and Y maze (Figure 2B) tests.
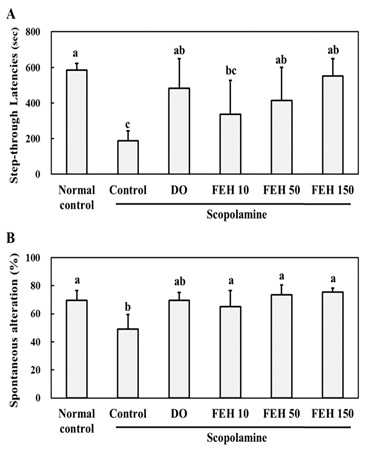 Figure 2: Effect of Fibroin Enzymatic Hydrolysate (FEH) on the (A) passive avoidance test and (B) Y maze test in mice with scopolamine-induced memory and learning impairment.
Figure 2: Effect of Fibroin Enzymatic Hydrolysate (FEH) on the (A) passive avoidance test and (B) Y maze test in mice with scopolamine-induced memory and learning impairment.
Mice were divided into the following treatment groups: normal (placebo) control, scopolamine control, DO positive control and scopolamine plus FEH. Values are presented as means ± SD. Non-identical superscript letters indicate significant differences at P<0.05.
In order to provide information regarding the mechanism(s) of action of FEH, the effects of FEH on scopolamine-induced ACh reduction and inflammation in the brain hippocampus were determined. Scopolamine-injected mice displayed significantly decreased Ach (Figure 3A), increased AChE (Figure 3A) and decreased BDNF (Figure 3C) levels in brain tissue. Oral administrations of FEH as well as the positive control DO significantly enhanced Ach (Figure 3A), decreased AChE (Figure 3B) and increased BNDF (Figure 3C) levels in the hippocampal tissue (P < 0.05). The small differences that were noted in the beneficial effects of the three dosages of FEH were not significantly different from one another.
Compared to the normal placebo control group, scopolamine injection resulted in significant increases in the levels of the inflammatory cytokines TNF-α, IL-6 and IL-1β in the brain hippocampus (Figure 3). However, oral administrations of FEH significantly reduced the levels of TNF-α (Figure 3D), IL-6 (Figure 3E) and IL-1β (Figure 3F) (P < 0.05). Again, the small differences in the anti-inflammatory effects of the three different doses of FEH were not significant. The positive control DO also decreased the levels of the cytokines in the scopolamine-treated mice. The above results indicate a role for Ach and inflammatory processes in scopolamine-induced memory and learning, and that FEH is capable of reversing these effects of scopolamine.
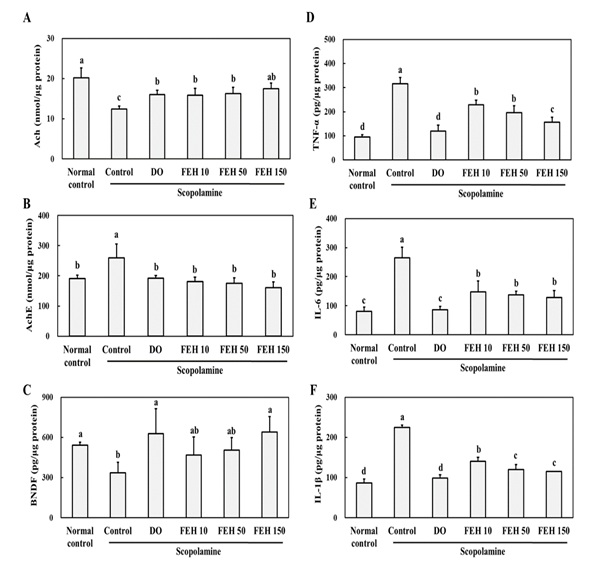 Figure 3: Effect of Fibroin Enzymatic Hydrolysate (FEH) on: (A) Ach, (B) AChE, (C) BDNF, (D) TNF-α, (E) IL-6 and (F) IL-1β in the hippocampal brain tissues of mice with scopolamine-induced memory and learning impairment.
Figure 3: Effect of Fibroin Enzymatic Hydrolysate (FEH) on: (A) Ach, (B) AChE, (C) BDNF, (D) TNF-α, (E) IL-6 and (F) IL-1β in the hippocampal brain tissues of mice with scopolamine-induced memory and learning impairment.
Mice were divided into the following treatment groups: normal (placebo) control, scopolamine control, DO positive control, and scopolamine plus FEH. Values are presented as means ± SD. Non-identical superscript letters indicate significant differences at P<0.05.
To further elucidate the possible protective molecular mechanisms of FEH, protein levels of the PI3K/AKT/mTOR/PSD9 pathway were assessed by western blotting (Figure 4A). Scopolamine administration resulted in inhibition of the p-PI3K/p-AKT/mTOR/PSD95 pathway in the brain (Figure A-E). However, oral administrations of FEH and the positive control DO enhanced expression of the proteins p-PI3K (Figure 4B), p-AKT (Figure 4C), mTOR (Figure 4D) and PSD95 (Figure 4E) in scopolamine-treated mice (P < 0.05). Dose dependent effects of FEH were observed for each of these proteins.
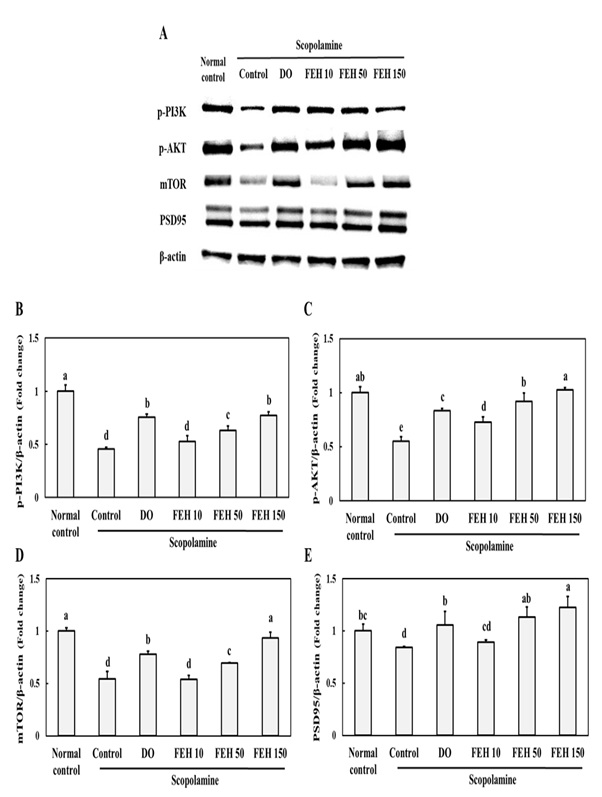 Figure 4: Effect of Fibroin Enzymatic Hydrolysate (FEH) on (B) p-PI3K, (C) p-AKT, (D) mTOR and (E) PSD95 expressions of in the hippocampal brain tissues of mice with scopolamine-induced memory and learning impairment.
Figure 4: Effect of Fibroin Enzymatic Hydrolysate (FEH) on (B) p-PI3K, (C) p-AKT, (D) mTOR and (E) PSD95 expressions of in the hippocampal brain tissues of mice with scopolamine-induced memory and learning impairment.
Mice were divided into the following treatment groups: normal (placebo) control, scopolamine control, DO positive control, and scopolamine plus FEH. Values are presented as means ± SD. Non-identical superscript letters indicate significant differences at P
The protein expression of p-ERK, Ca MKII, CREB, and BDNF in the brain hippocampus of mice with scopolamine-induced memory and learning impairments was investigated. ERK and Ca MKII are involved in CREB and BDNF expression which is required for memory formation [23] Scopolamine injection inhibited protein expression of p-ERK, Ca MKII and p-CREB (Figure 5A-D). However, oral administration of the FEH as well as the positive control DO resulted in significant increases in protein expression of p-ERK (Figure 5B), Ca MKII (Figure 5C) and p-CREB (Figure 5D)in scopolamine-treated mice (P < 0.05).
CREB and BDNF expression were also assessed in the brain hippocampus of mice Immunohistochemical (IHC) (Figure 6). Oral administration of FEH as well as the positive control DOES enhanced CREB and BDNF expression in the brains of mice with scopolamine-induced memory and learning impairments, whereas scopolamine alone suppressed CREB and BDNF expression (Figure 6).
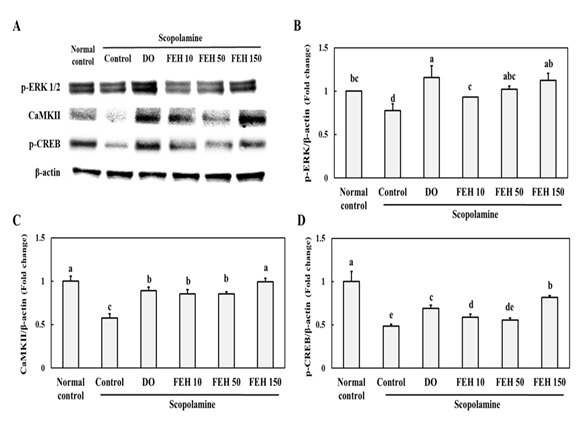 Figure 5: Effect of Fibroin Enzymatic Hydrolysate (FEH) on (B) p-ERK, (C) Ca MKII and (D) p-CREB expressions in the hippocampal brain tissues of mice with scopolamine-induced memory and learning impairment.
Figure 5: Effect of Fibroin Enzymatic Hydrolysate (FEH) on (B) p-ERK, (C) Ca MKII and (D) p-CREB expressions in the hippocampal brain tissues of mice with scopolamine-induced memory and learning impairment.
Mice were divided into: normal (placebo) control, scopolamine control, DOES positive control, and scopolamine plus FEH treatment groups. Values are presented as means ± SD. Non-identical superscript letters indicate significant differences at P
Discussion
In this study, the effects of FEH administration to mice with scopolamine-induced memory and learning impairments were investigated. Scopolamine is a muscarinic ACh receptor antagonist which can induce memory and learning impairment, in part, because of its ability to cross the blood brain barrier. It has therefore been extensively used in research for producing a model of memory and learning impairment in animals [24,25].
Scopolamine treatment of mice increased the escape latency times in the Morris water maze test (Figure 1), decreased step-through latencies in the passive avoidance test (Figure 2B) and decreased spontaneous alteration in the Y-maze test (Figure 2B), thus demonstrating the induction of memory and learning impairment. In the groups of animals treated orally with FEH, the scopolamine-induced memory and learning impairment effects were inhibited, similar to treatment with the positive control drug Donepezil (DO).
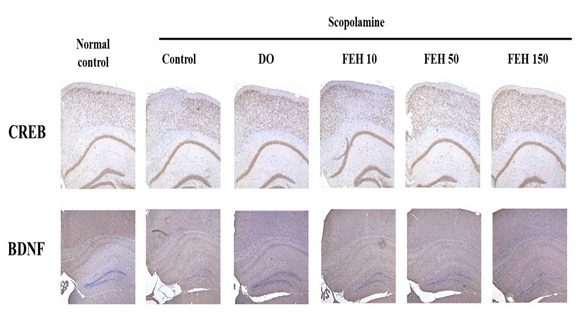 Figure 6: Effect of Fibroin Enzymatic Hydrolysate (FEH) on CREB and BDNF expressions in brain hippocampus of mice with scopolamine-induced memory and learning impairment.
Figure 6: Effect of Fibroin Enzymatic Hydrolysate (FEH) on CREB and BDNF expressions in brain hippocampus of mice with scopolamine-induced memory and learning impairment.
Mice were divided into the following treatment groups: normal (placebo) control, scopolamine control, DO positive control, and scopolamine plus FEH.
DO is a cholinesterase inhibitor that prevents the progression of memory impairment and is used in the treatment of dementia, including Alzheimer's disease [18]. However, DO have common and serious side effects including diarrhea, muscle cramps, insomnia, fatigue, cardio toxicity, and gastrointestinal irritation and nephrotoxicity [18]. As a consequence, medicinal herbs or foods have been assessed for their beneficial effects on memory and learning impairment without side effects [26].
Previous studies have suggested that the levels of ACh and BDNF may be biomarkers for memory and learning impairment [23]. The concentrations of circulating ACh and BDNF did not change in scopolamine-induced memory and learning impaired mice (data not shown). However, the levels of ACh and BDNF in brain hippocampus were decreased by scopolamine. FEH treatment increased both ACh and BDNF hippocampal levels of mice with scopolamine-induced memory and learning impairments.
BDNF is implicated in memory and learning abilities because it is required for neuronal differentiation and survival. BDNF stimulates the activation of distinct signaling pathways involving specific kinases such as p-PI3K/p-AKT, ERK, and Ca MKII, leading to translation initiation by phosphorylation and activation of CREB in the nucleus [27-30]. Robinet et al., [30] have shown that BDNF treatment induced the PI3K/Akt/mTOR pathway and regulated translational activation in primary cultures of mouse cortical neurons. ACh and BDNF signaling eventually cause phosphorylation of CREB, which leads to the expression of BDNF [27-30].
The data in the current study showed that scopolamine induction suppressed the p-PI3K/p-AKT/mTOR/PSD95 pathway during BDNF reduction in the brain hippocampus. In addition, protein expression levels of p-ERK, Ca MKII, and p-CREB were decreased by scopolamine in the brain (Figure 4). However, FEH treatment stimulated the p-PI3K/p-AKT/mTOR/PSD95 pathway and expression of p-ERK, Ca MKII, and p-CREB (Figure 4). Thus, the results suggest that FEH inhibited memory and learning impairment through p-CREB-mediated BDNF expression via the stimulation of the p-PI3K/p-AKT/mTOR/PSD95 pathway and activation of ERK and Ca MKII.
Cui et al., [31] showed that silk peptide inhibited production of IL-1β and IL-6 in lip polysaccharide-stimulated macrophages, and had anti-inflammatory effects. Results from this study demonstrated that FEH treatment inhibited production of IL-1β, TNF-α and IL-6 in the hippocampal brain tissues of mice with scopolamine-induced memory and learning impairments. Jawaid et al., [32] observed that scopolamine promoted inflammation by expression of pro-inflammatory mediators and neurotoxic cytokines in the cerebral tissue. Thus, the data indicate that silk peptide (FEH) acts as an anti-inflammatory substance in the brain and can prevent memory and learning impairment [15,33].
Conclusion
The results of this study demonstrated that oral administration of silk fibroin enzymatic hydrolysate FEH has beneficial memory and learning enhancing effects as demonstrated by three experimental methods. This effect was associated with the expressions of p-CREB and BDNF via stimulation of the p-PI3K/p-AKT/mTOR/PSD95 pathway and activation of ERK and CaMKII, thus providing information regarding the mechanism(s) of action. These data support the use of FEH for the treatment and possible prevention of memory and learning impairment, and agree with the results of a human study which showed that daily doses of over 280 mg FEH enhanced memory in healthy adults with an average age of 55 years.
Conflicts of Interest
KNJ is an employee of NI & Pharm Inc. All other authors declare no conflicts of interest.
Funding
This work was funded by a grant from Brain On Inc., Republic of Korea.
References
- Sweatt JD (2009) Experience-dependent epigenetic modifications in the central nervous system. Biol Psych 65: 191-197.
- Xia MQ, Hyman BT (1999) Chemokines/chemokine receptors in the central nervous system and Alzheimer's disease. J Neurovirol 5: 32-41.
- Pal K, Mukadam N, Petersen I, Cooper C (2018) Mild cognitive impairment and progression to dementia in people with diabetes, prediabetes and metabolic syndrome: a systematic review and meta-analysis. Soc Psych Psych Epidemiol 53: 1149-1160.
- Doll CA, Broadie K (2016) Neuron class-specific requirements for fragile X mental retardation protein in critical period development of calcium signaling in learning and memory circuitry. Neurobiol Dis 89: 76-87.
- Kempadoo KA, Mosharov EV, Choi SJ, Sulzer D, Kandel ER (2016) Dopamine release from the locus coeruleus to the dorsal hippocampus promotes spatial learning and memory. Proc National Acad Sci USA 113: 14835-14840.
- Ferreira-Vieira TH, Guimaraes IM, Silva FR, Ribeiro FM (2016) Alzheimer's disease: targeting the cholinergic system. CurrNeuropharmacol 14: 101-115.
- Haque A, Polcyn R, Matzelle D, Banik NL (2018) New insights into the role of neuron-specific enolase in neuro-Inflammation, neurodegeneration, and neuroprotection. Brain Sci 8:
- Huang C, Irwin MG, Wong GTC, Chang RCC (2018) Evidence of the impact of systemic inflammation on neuroinflammation from a non-bacterial endotoxin animal model. J Neuroinflam 15: 147.
- Agnihotri A, Aruoma OI (2020) Alzheimer’s disease and Parkinson’s disease: A nutritional toxicology perspective of the impact of oxidative stress, mitochondrial dysfunction, nutrigenomics and environmental factors. J Amer Coll Nutr 39: 16-27.
- Rajagopal S, Sangam SR, Singh S, Joginapally VR (2016) Modulatory effects of dietary amino acids on neurodegenerative diseases. Adv Neurobiol 12: 401-414.
- Kim DW, Hwang HS, Kim DS, Sheen SH, Heo DH, et al. (2012) Enhancement of anti-inflammatory activity of PEP-1-FK506 binding protein by silk fibroin peptide. J Microbiol Biotechnol 22: 494-500.
- Jang SH, Oh MS, Baek HI, Ha KC, Lee JY, et al. (2018) Oral Administration of Silk Peptide Enhances the Maturation and Cytolytic Activity of Natural Killer Cells. Immune Network 18: 37.
- Sofia S, McCarthy MB, Gronowicz G, Kaplan DL (2001) Functionalized silk-based biomaterials for bone formation. J Biomed Mat Res 54: 139-148.
- Kim K, Park S, Yoo HK, Lee JY, Jung H, et al.. (2009) Brain Factor-7 extracted from Bombyx mori enhances cognition and attention in normal children. J Med Food 12: 643-648.
- Kang YK, Lee BY, Bucci LR, Stohs SJ (2018) Effect of a fibroin enzymatic hydrolysate on memory improvement: A placebo-controlled, double-blind study. Nutrients 10: 233.
- Drevets WC, Zarate CA Jr, Furey ML (2013) Antidepressant effects of the muscarinic cholinergic receptor antagonist scopolamine: a review. Biol Psych 73: 1156-1163.
- Yun J, Lee J, Cha SY, Maeng S, Noh JK, et al. (2020) Beneficial and mechanistic effects of a fibroin enzymatic hydrolysate on memory impairment induced by amyloid beta in mice. J Alt Compl Integr Med 6: 127.
- Yeo JH, Lee K, Kweon HJY, Woo S, Han O, et al. (2004) Cognitive ability enhancement effects in rats by mori fibroin by enzymatic hydrolysis. Korean J Sericultural Entomol Sci 46: 23-27.
- Shin CY, Kim HS, Cha KH, Won DH, Lee JY, et al. (2018) The effects of donepezil, an acetylcholinesterase inhibitor, on impaired learning and memory in rodents. Biomol Therap (Seoul) 26: 274-28.
- More SV, Kumar H, Cho DY, Yun YS, Choi DK (2016) Toxin-induced experimental models of learning and memory. Int J Mol Sci 2016 17: 1447.
- Higaki A, Mogi M, Iwanami J, Min L-J, Bai H-Y, et al. (2018) Predicting outcome of Morris water maze test in vascular dementia mouse model with deep learning. PLoS ONE 13: 0191708.
- Malikowsha N, Salat K, Podkowa A (2017) Comparison of pro-amnesic efficacy of scopolamine, biperiden, and phencyclidine by using passive avoidance task in CD-1 mice. J Pharmacol Toxicol Meth 86: 76-80.
- Kumar H, More SV, Han SD, Choi JY, Choi DK (2012) Promising therapeutics with natural bioactive compounds for improving learning and memory—a review of randomized trials. Molecules 17: 10503-10539.
- Kraeuter AK, Guest PC, Sarnyai Z (2019) The Y-Maze for Assessment of Spatial Working and Reference Memory in Mice. Meth Mol Biol 1916: 105-111.
- Albert MS, Blacker D (2006) Mild cognitive impairment and dementia. Ann Rev Clin Psychol 2: 379-388.
- More SV, Kumar H, Cho DY, Yun YS, Choi DK (2016) Toxin-induced experimental models of learning and memory impairment. Int J Mol Sci 17: 1447.
- Yamada K, Mizuno M, Nabeshima T (2002) Role for brain-derived neurotrophic factor in learning and memory. Life Sci 70: 735-744.
- Martini F, Regis LM, Goncalves RS, Pregardier KL, Wayne NC (2020) Strength exercise suppresses STZ-induced spatial memory impairment and modulates BDNF/ERK-CAM/CREB signaling pathway in the hippocampus of mice. Cell Biochem Funct 38: 213-221.
- Wan D, Xue L, Zhu H, Luo Y (2013) Catalpol Induces Neuroprotection and prevents memory dysfunction through the cholinergic system and BDNF. Evid Based Compl Alt Med 2013: 134852.
- Mullen LM, Pak KK, Chavez E, Kondo K, Brand Y, et al. (2012) Ras/p38 and PI3K/Akt but not Mek/Erk signaling mediate BDNF-induced neurite formation on neonatal cochlear spiral ganglion explants. Brain Res 1430: 5-34.
- Robinet C, Pellerin L (2010) Brain-derived neurotrophic factor enhances the expression of the monocarboxylate transporter 2 through translational activation in mouse cultured cortical neurons. J Cereb Blood Flow Metab 30: 286-298.
- Cui X, Wen J, Zhao X, Chen X, Shao Z, et al. (2013) A pilot study of macrophage responses to silk fibroin particles. J Biomed Mat Res A 101: 1511-1517.
- Jawaid T, Shakya AK, Siddiqui HH, Kamal M (2014) Evaluation of Cucurbita maxima extract against scopolamine-induced amnesia in rats: implication of tumor necrosis factor alpha. Z Naturforsch C J Biosci 69: 407-417.
Citation: Yun J, Lee J, Cha S-Y, Maeng S, Noh JK, et al. (2021) Mechanistic and Efficacy Study on Effects of Fibroin Enzymatic Hydrolysate (FEH) on Memory and Learning Impairments Induced by Scopolamine in Mice. J Altern Complement Integr Med 7: 160.
Copyright: © 2021 Jeongmoon Yun, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.

