
Menthol Facilitates Acquisition and Progression of Nicotine Intake in Adolescent Rats
*Corresponding Author(s):
Xiu LiuDepartment Of Pathology, University Of Mississippi Medical Center, Jackson, Mississippi, United States
Tel:+1 6019842875,
Email:xliu@umc.edu
Abstract
Our previous animal research work showed an enhancing effect of menthol on reinforcement by nicotine. Given that increasing number of the youth experiments smoking with menthol cigarettes, the issue of whether menthol facilitates the acquisition and progression of nicotine consumption in adolescents deserves experimental attention. Thus, the present study was designed using a rat model of nicotine self-administration to examine effects of menthol on the acquisition and progression of nicotine-taking behavior. Male Sprague-Dawley rats at their late adolescent age were tested in daily 1-h sessions for pressing a lever under a Fixed-Ratio (FR) 1 schedule to intravenously self-administer nicotine at 15µg/kg/infusion. The progression of nicotine self-administration under a FR5 schedule was tested in a different sets of rats. Five min prior to the test sessions, rats received an intraperitoneal administration of menthol at 0, 0.75 and 2.5mg/kg. Pretreatment with menthol facilitated the acquisition of lever press responses for nicotine self-administration. After acquired nicotine self-administration, pretreatment with menthol facilitated responses under a FR5 schedule. These data demonstrate a facilitative effect of menthol on the initiation and progression of self-administration of nicotine in adolescent rats. The results provides an empirical evidence for menthol’s roles in tobacco smoking and nicotine addiction.
Keywords
Adolescent; Acquisition; Menthol; Nicotine; Rats; Self-administration
INTRODUCTION
In the United States, tobacco smoking is a leading cause of death, accounting for the loss of 480,000 lives each year. According to a recent CDC report [1], every day more than 3,200 youth aged 18 years or younger smoke their first cigarette and 2,100 young people become daily cigarette smokers. The prevalence rates of smoking are 7.2% in middle and 20.2% in high school, accounting for a total young smokers being about 4 millions. Approximately 90% of these smokers progresses from occasional to daily smoking, which occurs by age 26. Although the great efforts over the past several decades since the first Surgeon General report in 1964 to recognize the addictive nature of nicotine have led to the overall significant reduction of tobacco smoking, the use of mentholated tobacco products appears to be an alarmingly growing problem, in particular among the younger population [2-4].
Increasing epidemiological surveys and clinical observations have shown a significant impact of mentholated tobacco products on perpetuation of tobacco use, increasing the initiation of smoking, promoting progression to regular smoking and decreasing the rates of smoking cessation success, particularly in young people [2,5-10]. In adolescents and young adults, majority of smokers started their smoking with mentholated tobacco products. Approximately 73% of high school students and 56% of middle school students who used tobacco products in the past 30 days reported smoking menthol cigarettes [1]. Young menthol cigarette smokers, compared to nonmenthol cigarette users, are prone to progress to nicotine dependent, demonstrated by several measurements of nicotine dependence, such as shorter time to smoke first cigarette after awake in the morning, higher level of smoking craving and lower rates of successfully quitting smoking [11-18]. In spite of those epidemiological information suggesting a menthol-smoking association, however, the issue of whether menthol facilitates the initiation and progression of smoking behavior has never directly been examined in human subjects due to ethical reason. Similarly, even in animal research field the issue has received little experimental attention.
Our laboratory, using animal models of nicotine addiction, recently demonstrated significant effects of menthol on the ongoing nicotine-taking as well as perseverance of and relapse to nicotine-seeking behavior [19,20]. Here, this study was designed to investigate whether and how menthol influences the acquisition and progression of nicotine self-administration in adolescent rats. Specifically, nicotine self-administration paradigms were used for examining effects of menthol on the acquisition of nicotine taking and progression to stable nicotine intake under high behavioral demands.
METHODS
Subjects
Male Sprague-Dawley rats were used (Charles River, Portage, MI, USA). Animals arrived in the lab at about postnatal day 30 (body weight: 126-150g). Test sessions were performed at late adolescent age, i.e., postnatal days 48-54 for test 1 and 55-59 for tests 2 and 3 (see below). The animals were individually housed in a humidity- and temperature-controlled (21-22°C) colony room on a reverse light/dark cycle (lights on at 8:00 PM, lights off at 8:00 AM). The first week was allowed for vivarium habituation and handling. The animals had unlimited access to water and food, except in the lever-press training period of 3 days (see below) during which time a food-restriction regimen (20g chow/day) was in effect. The training and experimental sessions were conducted during the dark phase at the same time each day (9:00 AM-3:00 PM). All of the experimental procedures were performed in accordance with the National Institutes of Health Guide for the Care and Use of Laboratory Animals and were approved by the University of Mississippi Medical Center Institutional Animal Care and Use Committee.
Drugs
Nicotine hydrogen tart rate salt (Sigma-Aldrich, St. Louis, MO, USA) was dissolved in physiological saline. The nicotine solution pH was adjusted to 7.0±0.4 with sodium hydroxide. (-)-menthol (cyclohexanol-5-methyl-2-[1-methylethyl) was dissolved in Dimethylsulfoxide (DMSO) and diluted with deionized water to a final DMSO concentration being at 50% (V/V).
Self-administration apparatus
The experimental sessions were conducted in standard operant conditioning chambers (Med Associates, St. Albans, VT, USA). The chambers were equipped with two retractable response levers on one side panel, a 28-V white light above each lever and a red house light on the opposite side panel. Between the two levers was a food pellet receptacle. Intravenous nicotine injections were dispensed by a syringe pump (model PHM100-10 rpm, Med Associates). Experimental events and data collection were automatically controlled by an interfaced computer and software (Med-PC version IV, Med Associates).
Lever-press training
Since there are individual differences in the ability for learning lever-press responses and the first contact with a lever or/and learning to press it are to a larger extent accidental, it was necessary to eliminate this confounding factor for examining the rates of acquiring self-administration of nicotine. To do so, rats were trained to learn the lever-press responding for food pellet reward. Following one day food-restriction, the rats were placed in the experimental chambers and the training sessions began with introduction of a lever. Responses on the lever was rewarded with the delivery of a food pellet (45mg) on a Fixed-Ration (FR) 1 schedule. The training was completed once rats earned 10 food pellets. This lever would not function as the active lever for later-on nicotine self-administration test.
Intravenous catheterization surgery
Rats were anesthetized with isoflurane (1-3% in 95% O2 and 5% CO2) and an indwelling catheter was inserted in the right external jugular vein. The animals were allowed at least 7 days to recover from surgery. During the recovery period, the catheters were flushed daily with 0.1ml of sterile saline that contained heparin (30U/ml) and gentamicin (40mg/ml) to maintain catheter patency and prevent infection. Thereafter, the catheters were flushed with heparinized saline before and after the experimental sessions. On weekends when no experimental session was performed, the catheters were flushed once a day.
Test 1: Effects of menthol on the acquisition of nicotine self-administration
After recovering from intravenous catheterization surgery, the test sessions for examining acquisition of nicotine self-administration were conducted daily Monday through Friday with weekend off. In the daily 1-h sessions, the rats were placed in the operant conditioning chambers and connected to the intravenous drug infusion system. The sessions began with extension of two levers and illumination of the red house light. The lever used for the above mentioned food training was made inactive, i.e., responses on it did not lead to nicotine delivery. Another lever was introduced for the first time and responding on this lever was reinforced by an intravenous infusion of nicotine at 15µg/kg/infusion under a FR1 schedule. Therefore, the rates for rats to respond on this active lever measured the acquisition of nicotine self-administration. The intravenous nicotine infusions were delivered in a volume of about 0.1ml over approximately 1-s, depending on the rat’s body weight. Each nicotine infusion was accompanied by the presentation of auditory/visual stimuli that consisted of a 5-s tone and 20-s illumination of the light above the active lever. The stimuli signaled a 20-s timeout period following each nicotine infusion, during which time responses were recorded but not reinforced.
The animals were divided into three groups (n=10/group). To test effects of menthol on the acquisition of nicotine self-administration, five min prior to the seven daily test sessions the animals were subjected to an intraperitoneal administration of menthol at a volume of 1 ml/kg. The first group received 0mg/kg menthol (vehicle); the second group got 0.75mg/kg menthol; and the third group was pretreated with 2.5mg/kg menthol. The selection of menthol doses was based on our previous work showing that menthol at 2.5mg/k/g significantly enhanced nicotine reinforcement [19]. The dependent variables were the number of lever responses and nicotine infusions in each testing days.
Test 2: Effects of menthol on motivation for nicotine self-administration
This test was a continuation of the above described test 1. In this test, animals in each individual group continued to receive a pre-session administration of menthol at their respective doses. The use of the same animals and the same menthol dose assignment allowed to make the results comparable across acquisition (test 1) and motivation (test 2). Starting on the following day after completion of test 1, the five daily test sessions were performed under a progressive-ration (PR) schedule of reinforcement with 15µg/kg/infusion nicotine. The PR schedule was modified from the formula 5(0.2 ´ infusion number)-5 [21]. Thus, the response requirements for successive nicotine infusion delivery were 3, 6, 10, 15, 20, 25, 32, 40, 50, 62, and so on. The dependent variables were the breaking point, which was defined as the maximum responses rats made for a nicotine infusion (i.e., the last nicotine delivery rats earned in a session) and the latency for rats to emit their first lever-press response after start of the session.
Test 3: Effects of menthol on the progression of nicotine self-administration
Thirty rats were trained in seven daily 1-h sessions to self-administer 15µg/kg/infusion nicotine under a FR1 schedule without pre-session menthol treatment. Then, these animals were randomly divided into three groups (n=10/group) with similar lever-press responses and nicotine self-administration among groups. The five progression test sessions were conducted with two manipulations. First, the behavioral requirement for nicotine self-administration was increased from FR1 to FR5; Second, five min prior to the sessions, animals were pretreated with an intraperitoneal injection of menthol. The first group got vehicle (0mg/kg menthol), the second group received 0.75mg/kg menthol and the third group took 2.5mg/kg menthol. The progression of nicotine self-administration under the FR5 schedule was measured by the number of lever responses and nicotine infusions in each testing days.
Statistical analyses
The data are expressed as the mean±SEM number of lever responses and nicotine infusions. A two-way repeated measures Analysis of Variance (ANOVA) with menthol dose (group) as the between-subject factor and sessions as the within-subject factor. Significant main effects and interactions were followed by further analyses using a one-way ANOVA. And finally, significant main effects in the ANOVAs were followed by Fisher’s PLSD post hoc test to verify differences among individual means.
RESULTS
Effects of menthol on the acquisition of nicotine self-administration
Figure 1 shows the behavioral profile of the rats acquiring self-administration of nicotine at 15µg/kg/infusion. Pretreatment with menthol at 2.5, compared to 0 (vehicle) and 0.75mg/kg as well, significantly facilitated the acquisition of nicotine self-administration. A two-way ANOVA with repeated measures on the number of responses on the active and nicotine delivering lever revealed a significant effect of group, i.e., menthol dose (F2,27=6.36, p<0.01) and sessions (F6,162=29.57, p<0.0001). There was no significant interaction between group and session (F12,162=0.57, p 0.05). Subsequent analysis of the data collected from each individual group using one-way repeated measure ANOVA showed significant session effect in the vehicle (0mg/kg menthol) (F6,54=8.19, p<0.0001), 0.75 (F6,54=8.45, p<0.0001), and 2.5mg/kg (F6,54=15.07, p<0.0001) groups, indicating that rats in all three groups increased responses on the active lever across sessions, acquiring nicotine self-administration. Analysis at each session levels using one-way ANOVA found significant differences of the 2.5mg/kg group from vehicle group, indicating that menthol facilitated lever responses for nicotine self-administration. Details of the statistical results were presented in figure 1, top panel.
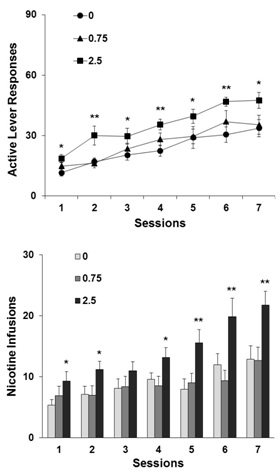 Figure 1: Effect of menthol on the active lever responses (top) and nicotine infusions (below) in the test sessions to examine the acquisition of nicotine self-administration in rats (n=10). Five minute prior to the sessions, animal were subjected to an intraperitoneal injection of menthol (0, 0.75 and 2.5mg/kg). During the sessions, the rats responded on the active lever for nicotine self-administration on an FR1 schedule of reinforcement. Data are expressed as mean±SEM. *p<0.05, **p
Figure 1: Effect of menthol on the active lever responses (top) and nicotine infusions (below) in the test sessions to examine the acquisition of nicotine self-administration in rats (n=10). Five minute prior to the sessions, animal were subjected to an intraperitoneal injection of menthol (0, 0.75 and 2.5mg/kg). During the sessions, the rats responded on the active lever for nicotine self-administration on an FR1 schedule of reinforcement. Data are expressed as mean±SEM. *p<0.05, **p
A similar statistical strategy was applied to the number of nicotine infusions animals self-administered in the seven test sessions. There was significant effect of group (menthol dose) (F2,27=5.58, p<0.01) and sessions (F6,162=25.43, p<0.0001). As in the case of active lever responses described above, there was no significant interaction between the menthol dose and testing sessions (F12,162=0.85, p>0.05). The number of nicotine infusion rats self-administered in each menthol-pretreatment groups significantly (p<0.001) increased across the 7 sessions. Moreover, one-way ANOVA of nicotine infusions at each testing session levels showed significant differences between the 2.5mg/kg and vehicle. Statistical details were presented in figure 1, below panel.
Pretreatment with menthol did not produce a significant effect on the responses rats emitted to press the inactive lever where responses had no consequence. Similar numbers of the inactive lever responses were observed across the three groups (data not shown) so that there was no difference among the three groups (F2,27=0.84, p>0.05).
Effects of menthol on motivation for nicotine self-administration
The mean±SEM number of response latency in seconds and the breaking points was calculated across the five PR test sessions (Figure 2). A one-way ANOVA on the latency revealed a significant effect of menthol dose (F2,27=9.62, p<0.001) with subsequent confirmation of significant difference between 2.5mg/kg and vehicle groups (p<0.01). Similar statistical analysis on the breaking points showed also a significant effect of menthol dose (F2,27 =11.32, p<0.001) and verified significant difference of 2.5mg/kg from vehicle group (p<0.01).
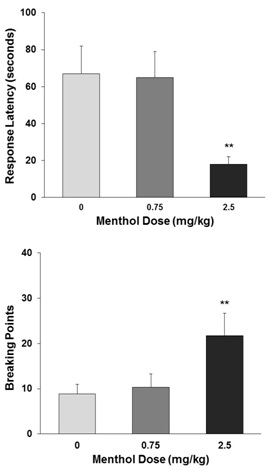 Figure 2: Effect of menthol on the response latency (top) and breaking points (below) in the PR test. Data are expressed as mean±SEM across the five test sessions. **p
Figure 2: Effect of menthol on the response latency (top) and breaking points (below) in the PR test. Data are expressed as mean±SEM across the five test sessions. **p
Effects of menthol on the progression of nicotine self-administration
As shown in figure 3, pretreatment with menthol at 2.5 but not 0.75mg/kg or vehicle significantly facilitated the progression of nicotine self-administration as measured by the quickly increased responses on the active lever under a FR5 schedule of reinforcement. The numbers of active lever responses collected from the five FR5 testing sessions were analyzed using a two-way ANOVA with repeated measures. There was significant effect of group (F2,27=3.62, p<0.05) and sessions (F4,108=34.84, p<0.0001), whereas no significant interaction of group with session was obtained (F8,108=0.56, p>0.05). Subsequent one-way repeated measure ANOVA on the individual groups found significant session effect for vehicle (F4,36=4.66, p<0.01), 0.75 (F4,36=19.90, p<0.0001), and 2.5mg/kg (F4,36=21.82, p<0.0001) groups. Further analysis revealed significant differences of the 2.5mg/kg from vehicle group in the last three test sessions. Details of the statistical results were presented in figure 3, top panel.
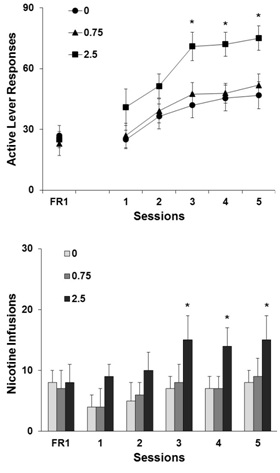 Figure 3: Effect of menthol on the active lever responses (top) and nicotine infusions (below) in the test sessions to examine the progression of nicotine self-administration in rats (n=10). FR1 presents the mean±SEM number across the final 3 sessions on the FR1 schedule. Five minutes prior to the test sessions, animals received an intraperitoneal injection of menthol (0, 0.75 and 2.5mg/kg). During the sessions, the rats responded on the active lever for nicotine self-administration on an FR5 schedule of reinforcement. Data are expressed as mean±SEM. *p<0.05, **p
Figure 3: Effect of menthol on the active lever responses (top) and nicotine infusions (below) in the test sessions to examine the progression of nicotine self-administration in rats (n=10). FR1 presents the mean±SEM number across the final 3 sessions on the FR1 schedule. Five minutes prior to the test sessions, animals received an intraperitoneal injection of menthol (0, 0.75 and 2.5mg/kg). During the sessions, the rats responded on the active lever for nicotine self-administration on an FR5 schedule of reinforcement. Data are expressed as mean±SEM. *p<0.05, **p
The numbers of nicotine infusions animals self-administered under the FR5 schedule in the 5 test sessions were shown in the below panel of figure 3. Significant effect of group (F2,27=4.21, p<0.05) and sessions (F4,108=29.825, p<0.0001) but not group x session interaction (F8,108=0.43, p>0.05) was obtained. Subsequent one-way repeated measure ANOVA on the individual groups found significant session effect for vehicle (F4,36=4.66, p<0.01), 0.75 (F4,36=19.90, p<0.0001), and 2.5mg/kg (F4,36=21.82, p<0.0001) groups. As in the case of active lever responses, the number of nicotine infusions was significantly (p<0.05) higher in the 2.5mg/kg menthol relative to the vehicle group.
Responses on the inactive lever in the 5 test sessions remained unchanged after pretreatment with menthol (data not shown). Statistical analysis showed no significant effect of groups (F2,27=0.67, p>0.05).
DISCUSSION
This study demonstrated that menthol facilitated the acquisition of nicotine self-administration behavior in adolescent rats. After establishing nicotine self-administration behavior under a FR1 schedule, menthol increased lever-press responses under a FR5 schedule, higher behavioral demanding condition, indicating that menthol promoted progression of nicotine self-administration. Moreover, pretreatment with menthol resulted in shorter latency for starting nicotine self-administration responses and higher breaking points under a PR schedule test condition, indicating an enhanced motivation for nicotine self-administration.
The finding that menthol produced a facilitating effect on the acquisition of nicotine self-administration presents an empirical support for clinical observations suggesting that menthol cigarettes might promote smoking experimentation and initiation in adolescents and young adults [1]. The present results are in line with other recent studies. In adolescent rats, menthol enhanced nicotine-induced locomotor sensitization measured in open field test and functional connectivity monitored using functional magnetic resonant imaging scan in brain regions involved in rewarding and addiction process such as ventral tegmental area and striatum [22]. In a nicotine-induced Conditioned Place Preference (CPP) test, mice treated with nicotine plus menthol showed greater CPP compared to their counterparts treated with nicotine alone, indicating an enhanced nicotine reward by menthol [23]. In adolescent smokers, menthol significantly enhanced the positive reinforcing effects of nicotine in e-cigarettes so that menthol e-cigarette users showed higher levels of liking and wanting of the menthol-containing e-cigarettes compared to the ones without menthol content [24]. It is acknowledged, however, that the present finding is at odds with a recent report showing that menthol did not produce an effect in adolescent mice although in adults it increased nicotine consumption measured using a home cage and single bottle forced drinking paradigm [25]. Obviously, there are several factors possibly contributing to the discrepancy, which includes mice versus rats, operantly self-administering nicotine versus home cage forced nicotine-containing fluid drinking, systemic menthol administration versus ingesting menthol along with nicotine, 1-h limited nicotine availability versus 24-h free access to nicotine solution containing menthol.
The significantly higher breaking points for nicotine self-administration observed after menthol pretreatment indicate the enhanced motivation of rats for procuring nicotine as a result of increased reinforcing actions of nicotine. In the PR test, the numbers of active lever responses and corresponding nicotine infusions observed in the vehicle-pretreated rats matched with a previous report using similar PR schedule in adolescent rats [26], indicating the validity of the procedure for measuring nicotine reinforcing efficacy and rats’ motivation to self-administer nicotine. The increased breaking points obtained in the rats pretreated with 2.5 mg/kg menthol were consistent with our previous report showing that menthol pretreatment made adult rats emit much more lever press response under a PR schedule, i.e., an elevated breaking points [19], indicating the enhanced motivation for nicotine self-administration. The legitimate explanation for the menthol-enhanced motivation resides in the increased efficacy of nicotine reinforcement. Our previous work [19], showed that the inverted “U” shaped dose-response curve of nicotine to support self-administration behavior shifted to the left after menthol pretreatment. And relative to other doses, self-administering nicotine at 15µg/kg/infusion (a dose on the ascending limb of the curve) was increased the most by menthol pretreatment.
The finding that the latency for rats to start lever press responses for nicotine self-administration was significantly shortened after menthol pretreatment indicates the heightened eagerness for nicotine intake. This finding keeps in line with clinical observations showing that menthol cigarette smokers including adolescents, compared to nonmenthol users, smoked their first cigarette of the day much sooner, such as being within 5 min after getting up in the morning [7,11,27,28]. In addition, a shorter latency for smokers to start smoking is thought of as a measurement of physical dependence.
After increasing the cost for obtaining nicotine self-administration from FR1 to FR5 schedule, rats showed a quicker increase in their lever press responses after pretreatment with menthol relative to vehicle controls. Moreover, menthol-treated rats self-administered under the FR5 schedule higher level of nicotine compared to vehicle counterparts. These findings match with clinical observations showing the heightened progression of menthol smokers. For instance, young people in the USA who start smoking menthol cigarettes are at greater risk of progression to regular smoking and nicotine dependence than their counterparts starting smoking with non-menthol cigarettes [16]. Similarly, a Canadian study reported that students at 9-12 grades who smoked menthol cigarettes not only consumed more cigarettes but also showed stronger intention to continue smoking compared with nonmenthol smokers [29].
Menthol has been found to interact with dopamine reuptake and nicotinic acetylcholine receptors (nAChRs). In in vitro electrophysiological studies, menthol can directly change neuronal firing activities via modulating the α4β2 and α7 subtypes of the nAChRs [30,31]. Co-administration of menthol and nicotine increased expression of the β2-containing nAChRs in adolescent rat brain in an additive manner, higher than either drug alone [32]. Neural imaging study in human smokers showed a significant greater upregulation of nAChRs in the brain of menthol cigarette smokers compared to nonmenthol cigarette smokers [33]. In addition, Umezu and Morita [34], reported that menthol directly interacted with dopamine transporters to change dopamine neurotransmission in rodent brains. Our recent intracranial micordialysis study showed that menthol enhanced nicotine-induced dopamine release in rat nucleus accumbens [35]. On the other hand, although menthol produces topical cooling actions via the activation of Transit Receptor Potential (TRP) M8 ion channels [36-42], the effects of menthol on the neuronal firing activities in brain slices of adolescent rodents were not blocked by antagonists of the TRPM8 and TRPA1 [43,44]. In addition, our previous work showed that an inability of a TRPM8 antagonist RQ-00203078 did not alter menthol effect on nicotine-seeking behavior [20]. Taken together, it is proposed that the direct interactions of menthol with nAChRs and dopamine neurotransmission may underlie the promoting actions of menthol on the acquisition and progression of nicotine self-administration observed in the present work.
ACKNOWLEDGEMENT
This work was supported by the National Institute on Drug Abuse and Food and Drug Administration Center for Tobacco Products (R01DA037277 to X. Liu). The funding source had no other role other than financial support. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health or the Food and Drug Administration.
REFERENCES
- CDC (2017) Tobacco use among middle and high school students - United States, 2011- Morbidity and Mortality Weekly Report 66: 597-603.
- Giovino GA, Villanti AC, Mowery PD, Sevilimedu V, Niaura RS, et al. (2015) Differential trends in cigarette smoking in the USA: Is menthol slowing progress? Tob Control 24: 28-37.
- SAMHSA (2009) The NSDUH report: Use of menthol cigarettes. Substance abuse and mental health services administration. Office of Appied Studies, Rockville, USA.
- USDHHS (2014) The health consequences of smoking-50 years of progress: A report of the surgeon general. U.S. Department of Health and Human Services, Centers for Disease Control and Prevention, Atlanta, USA.
- Delnevo CD, Gundersen DA, Hrywna M, Echeverria SE, Steinberg MB (2011) Smoking-cessation prevalence among U.S. smokers of menthol versus non-menthol cigarettes. Am J Prev Med 41: 357-365.
- Delnevo CD, Villanti AC, Wackowski OA, Gundersen DA, Giovenco DP (2015) The influence of menthol, e-cigarettes and other tobacco products on young adults' self-reported changes in past year smoking. Tob Control 25: 571-574.
- Fagan P, Moolchan ET, Hart A, Rose A, Lawrence D, et al. (2010) Nicotine dependence and quitting behaviors among menthol and non-menthol smokers with similar consumptive patterns. Addiction 105: 55-74.
- Fagan P, Pohkrel P, Herzog T, Pagano I, Vallone D, et al. (2015) Comparisons of three nicotine dependence scales in a multiethnic sample of young adult menthol and non-menthol smokers. Drug and alcohol dependence 149: 203-211.
- Tobacco (2011) Tobacco products scientific advisory committee. Menthol cigarettes and public health: Review of the scientific evidence and recommendations. US Food and Drug Administration, Washington DC, USA.
- Villanti AC, Collins LK, Niaura RS, Gagosian SY, Abrams DB (2017) Menthol cigarettes and the public health standard: A systematic review. BMC Public Health 17:
- Collins CC, Moolchan ET (2006) Shorter time to first cigarette of the day in menthol adolescent cigarette smokers. Addictive Behaviors 31: 1460-1464.
- D'Silva J, Boyle RG, Lien R, Rode P, Okuyemi KS (2012) Cessation outcomes among treatment-seeking menthol and nonmenthol smokers. Am J Prev Med 43: 242-248.
- Hersey JC, Ng SW, Nonnemaker JM, Mowery P, Thomas KY, et al. (2006) Are menthol cigarettes a starter product for youth? Nicotine Tob Res 8: 403-413.
- Hersey JC, Nonnemaker JM, Homsi G (2010) Menthol cigarettes contribute to the appeal and addiction potential of smoking for youth. Nicotine Tob Res 12: 136-146.
- Levy DT, Blackman K, Tauras J, Chaloupka FJ, Villanti AC, et al. (2011) Quit attempts and quit rates among menthol and nonmenthol smokers in the United States. Am J Public Health 101: 1241-1247.
- Nonnemaker J, Hersey J, Homsi G, Busey A, Allen J, et al. (2013) Initiation with menthol cigarettes and youth smoking uptake. Addiction 108: 171-178.
- Smith SS, Fiore MC, Baker TB (2014) Smoking cessation in smokers who smoke menthol and non-menthol cigarettes. Addiction 109: 2107-2117.
- Wackowski O, Delnevo CD (2007) Menthol cigarettes and indicators of tobacco dependence among adolescents. Addictive behaviors 32: 1964-1969.
- Biswas L, Harrison E, Gong Y, Avusula R, Lee J, et al. (2016) Enhancing effect of menthol on nicotine self-administration in rats. Psychopharmacology (Berl) 233: 3417-3427.
- Harrison E, Biswas L, Avusula R, Zhang M, Gong Y, et al. (2017) Effects of menthol and its interaction with nicotine-conditioned cue on nicotine-seeking behavior in rats. Psychopharmacology (Berl) 234: 3443-3453.
- Depoortere RY, Li DH, Lane JD, Emmett-Oglesby MW (1993) Parameters of self-administration of cocaine in rats under a progressive-ratio schedule. Pharmacol Biochem Behav 45: 539-548.
- Thompson MF, Poirier GL, Davila-Garcia MI, Huang W, Tam K, et al. (2017) Menthol enhances nicotine-induced locomotor sensitization and in vivo functional connectivity in adolescence. J Psychopharmacol 32: 332-343.
- Henderson BJ, Wall TR, Henley BM, Kim CH, McKinney S, et al. (2017) Menthol enhances nicotine reward-related behavior by potentiating nicotine-induced changes in nAChR function, nAChR upregulation, and DA neuron excitability. Neuropsychopharmacology 42: 2285-2291.
- Krishnan-Sarin S, Green BG, Kong G, Cavallo DA, Jatlow P, et al. (2017) Studying the interactive effects of menthol and nicotine among youth: An examination using e-cigarettes. Drug Alcohol Depend 180: 193-199.
- Fait BW, Thompson DC, Mose TN, Jatlow P, Jordt SE, et al. (2017) Menthol disrupts nicotine's psychostimulant properties in an age and sex-dependent manner in C57BL/6J mice. Behav Brain Res 334: 72-77.
- Zou S, Funk D, Shram MJ, Le AD (2014) Effects of stressors on the reinforcing efficacy of nicotine in adolescent and adult rats. Psychopharmacology (Berl) 231: 1601-1614.
- Muscat JE, Liu HP, Stellman SD, Richie JP (2012) Menthol smoking in relation to time to first cigarette and cotinine: Results from a community-based study. Regul Toxicol Pharmacol 63: 166-170.
- Rosenbloom J, Rees VW, Reid K, Wong J, Kinnunen T (2012) A cross-sectional study on tobacco use and dependence among women: Does menthol matter? Tob Induc Dis 10:
- Azagba S, Minaker LM, Sharaf MF, Hammond D, Manske S (2014) Smoking intensity and intent to continue smoking among menthol and non-menthol adolescent smokers in Canada. Cancer Causes Control 25: 1093-1099.
- Ashoor A, Nordman JC, Veltri D, Yang KH, Al Kury L, et al. (2013) Menthol binding and inhibition of alpha7-nicotinic acetylcholine receptors. PloS One 8:
- Hans M, Wilhelm M, Swandulla D (2012) Menthol suppresses nicotinic acetylcholine receptor functioning in sensory neurons via allosteric modulation. Chem Senses 37: 463-469.
- Alsharari SD, King JR, Nordman JC, Muldoon PP, Jackson A, et al. (2015) Effects of menthol on nicotine pharmacokinetic, pharmacology and dependence in mice. PloS One 10:
- Brody AL, Mukhin AG, La Charite J, Ta K, Farahi J, et al. (2013) Up-regulation of nicotinic acetylcholine receptors in menthol cigarette smokers. Int J Neuropsychopharmacol 16: 957-966.
- Umezu T, Morita M (2003) Evidence for the involvement of dopamine in ambulation promoted by menthol in mice. J Pharmacol Sci 91: 125-135.
- Zhang M, Harrison E, Biswas L, Tran T, Liu X (2018) Menthol facilitates dopamine-releasing effect of nicotine in rat nucleus accumbens. Pharmacol Biochem Behav 175: 47-52.
- Journigan VB, Zaveri NT (2013) TRPM8 ion channel ligands for new therapeutic applications and as probes to study menthol pharmacology. Life Sci 92: 425-437.
- Keh SM, Facer P, Yehia A, Sandhu G, Saleh HA, et al. (2011) The menthol and cold sensation receptor TRPM8 in normal human nasal mucosa and rhinitis. Rhinology 49: 453-457.
- Liu B, Fan L, Balakrishna S, Sui A, Morris JB, et al. (2013) TRPM8 is the principal mediator of menthol-induced analgesia of acute and inflammatory pain. Pain 154: 2169-2177.
- Liu BY, Lin YJ, Lee HF, Ho CY, Ruan T, et al. (2015) Menthol suppresses laryngeal C-fiber hypersensitivity to cigarette smoke in a rat model of gastroesophageal reflux disease: The role of TRPM8. J Appl Physiol (1985) 118: 635-645.
- McKemy DD, Neuhausser WM, Julius D (2002) Identification of a cold receptor reveals a general role for TRP channels in thermosensation. Nature 416: 52-58.
- Peier AM, Moqrich A, Hergarden AC, Reeve AJ, Andersson DA, et al. (2002) A TRP channel that senses cold stimuli and menthol. Cell 108: 705-715.
- Rath P, Hilton JK, Sisco NJ, Van Horn WD (2015) Implications of Human Transient Receptor Potential Melastatin 8 (TRPM8) channel gating from menthol binding studies of the sensing domain. Biochemistry 55: 114-124.
- Lau BK, Karim S, Goodchild AK, Vaughan CW, Drew GM (2014) Menthol enhances phasic and tonic GABAA receptor-mediated currents in midbrain periaqueductal grey neurons. Br J Pharmacol 171: 2803-2813.
- Pezzoli M, Elhamdani A, Camacho S, Meystre J, Gonzalez SM, et al. (2014) Dampened neural activity and abolition of epileptic-like activity in cortical slices by active ingredients of spices. Sci Rep 4:
Citation: Tran T, Rousselle T, Hobbs B, Biswas L, Harrison E, et al. (2020) Menthol Facilitates Acquisition and Progression of Nicotine Intake in Adolescent Rats. J Addict Addictv Disord 7: 52.
Copyright: © 2020 Thuy Tran, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.

