
Metastatic Cervical Cancer Masquerading As Cavitary Pneumonia
*Corresponding Author(s):
Priyanka MakkarDepartment Of Pulmonary Medicine, Department Of Medicine, Memorial Sloan Kettering Cancer Center, New York, United States
Tel:+1 8184064133,
Email:pri.makkar@gmail.com
Abstract
Diffuse Cystic Lung Disease (DCLD) is an uncommon presentation and has a broad differential diagnosis. The etiology of DCLD can be divided into 3 categories; infectious, inflammatory, and neoplastic. Using the patient’s history, physical exam, laboratory findings and the CT of the chest as a guide, the differential diagnosis can be narrowed. The hypothesized mechanisms for development of malignant cyst are: 1. Excavation or central necrosis, 2. Infiltration of malignant cells into pre-existing bulla, 3. “Ball-Valve” effect caused by atypical cell infiltration leading to small airways distention. We describe a case of a metastatic cervical adenocarcinoma that presented as diffuse cystic and cavitary lung disease 20 years after the primary neoplasm. The less common cervical adenocarcinoma, disease-free interval between the diagnosis of cervical cancer and recurrence; and the radiographic presentation of DCLD with associated GGO make this case presentation unique.
Keywords
Cavitary pneumonia; Cervical adenocarcinoma; Lung cavity; Metastatic cervical cancer
INTRODUCTION
Diffuse Cystic Lung Disease (DCLD) is an uncommon presentation and has a broad differential diagnosis. It’s can be broadly divided into 3 categories; infectious, inflammatory, and neoplastic [1]. Using the patient’s history, physical exam, laboratory findings and imaging as a guide, the differential diagnosis can be narrowed down. Infrequently, metastatic malignancies such as sarcomas, mesenchymal tumours, or lung adenocarcinomas present as DLCD [3-9]. Pulmonary metastases occurs infrequently in patients with cervical cancer with pulmonary nodules being the most common radiographic presentation. We describe a rare case of metastatic cervical adenocarcinoma presenting as cystic lung disease 20 years after the primary neoplasm.
CASE DESCRIPTION
67-year-old woman, never smoker, of Ecuadorian origin with past medical history of hypertension, coronary artery disease, hyperlipidaemia, non-occlusive coronary disease, stage IB cervical adenocarcinoma treated with radical hysterectomy and pelvic radiation 20 years ago, ER+/PR+/HER2- left breast invasive ductal carcinoma treated by lumpectomy and radiation therapy one year ago presented with fever, and cough. Physical exam was notable for temperature of 39.0oC, pulse of 115 beats per minute and oxygen saturation of 90% on room air. Respiratory exam revealed rhonchi in lung bases. A High-Resolution Chest Computed Tomography (HRCT) showed bilateral cysts, nodules, consolidative changes with cavity formation and Ground Glass Opacities (GGO) (Figure 1, Panel A). Prior chest imaging from 2 years ago demonstrated clear lungs. Infectious work-up was negative for urinary Streptococcus pneumoniae, Legionella pneumophila and Histoplasma capsulatum antigens, nasopharyngeal respiratory PCR panel (Adenovirus, Coronavirus, Human Metapneumovirus, Human Rhinovirus, Influenza A and B, Parainfluenza Types 1-4, Respiratory Syncytial Virus, Bordetella pertussis, Chlamydophila pneumoniae, Mycoplasma pneumonia), sputum bacterial/fungal/mycobacterial cultures and ova/parasite exam, serum antibodies against H.capsulatum and Echinococcus granulosus. Patient was treated with ceftriaxone and azithromycin with resolution of fever and cough. At a follow-up visit 6 weeks later; chest CT was repeated and demonstrated improvement in the airspace consolidation with persistence of cystic/cavitary lesions (Figure 1, Panel B). Pulmonary function tests showed mild restrictive lung disease (TLC 3.27L, 77% of predicted) with mildly reduced diffusing capacity (13.18 ml/min/mmHg, 78% of predicted). A thorascopic wedge biopsy of the right lower lobe was performed and pathology was noted to be metastatic adenocarcinoma of endocervical origin (Figure 2) (tumour was diffusely positive for HPV, p16, CK7 and PAX8) and this was noted to be compatible with her known endocervical cancer. She thereafter followed up with gynaecologic oncology, however refused chemotherapy.
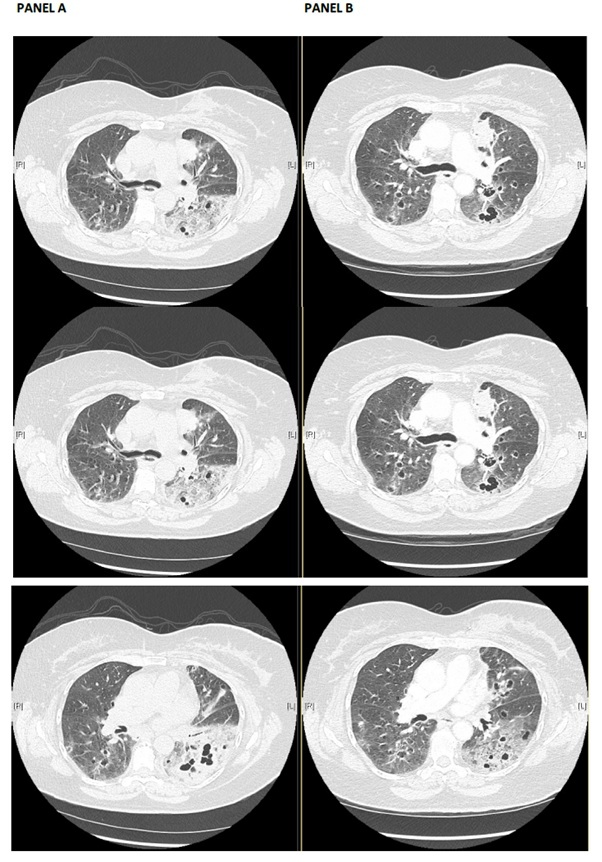 Figure 1: Panel A: Representative images from the CT scan on presentation. Significant findings include Multiple bilateral cavitary/cystic lesions, some containing air-fluid levels, associated with nodules, with basal predominance. The areas of cavitary/cystic changes are associated with patchy consolidations and ground glass opacities, predominantly in the left lower lobe. Panel B: Representative images from CT scan done 6 weeks post completion of antibiotic course shows improvement in the airspace opacity and essentially unchanged bilateral cavitary/cystic lesions.
Figure 1: Panel A: Representative images from the CT scan on presentation. Significant findings include Multiple bilateral cavitary/cystic lesions, some containing air-fluid levels, associated with nodules, with basal predominance. The areas of cavitary/cystic changes are associated with patchy consolidations and ground glass opacities, predominantly in the left lower lobe. Panel B: Representative images from CT scan done 6 weeks post completion of antibiotic course shows improvement in the airspace opacity and essentially unchanged bilateral cavitary/cystic lesions.
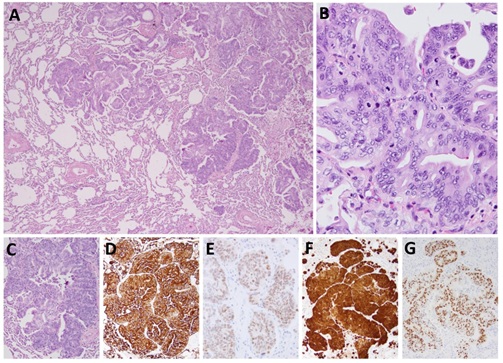 Figure 2: Adenocarcinoma of primary endocervical orgin, metastatic to the lung. (A) Low power view showing multiple tumor islands infiltrating the lung parenchyma. (B) High power view demonstrating the morphological features of endocervical adenocarcinoma, usual type, with cribriform to papillary architecture, pseudostratified columnar epithelium characterized by amphophilic apical cytoplasm, enlarged, elongated, hyoerchromatic nuclei and “floating mitotic figures”. The tumor islands (C) are diffusely positive for CK7 (D), PAX-8 (E) and p16 (F) By immunohistochemistry, while high risk HPV (G) Is diffusely positive by Mrna IN SITU chromogenic hybridization.
Figure 2: Adenocarcinoma of primary endocervical orgin, metastatic to the lung. (A) Low power view showing multiple tumor islands infiltrating the lung parenchyma. (B) High power view demonstrating the morphological features of endocervical adenocarcinoma, usual type, with cribriform to papillary architecture, pseudostratified columnar epithelium characterized by amphophilic apical cytoplasm, enlarged, elongated, hyoerchromatic nuclei and “floating mitotic figures”. The tumor islands (C) are diffusely positive for CK7 (D), PAX-8 (E) and p16 (F) By immunohistochemistry, while high risk HPV (G) Is diffusely positive by Mrna IN SITU chromogenic hybridization.
DISCUSSION
A cyst is a round air space surrounded by a thin (<4mm) epithelial or fibrous wall [1]. Cyst formation can result from airway obstruction with distal airspace dilatation, necrosis of airway walls or protease induced destruction of the lung parenchyma [2]. Cysts need to be distinguished from cavities, centrilobular emphysema, honeycomb change and cystic bronchiectasis. Isolated cysts are commonly seen in otherwise healthy older adults, but their presence in multiple lobes of both lungs defines cystic lung disease. The list of conditions associated with cystic lung disease is long (Table 1). Patient’s history, physical exam, laboratory findings and radiographic characteristics (e.g. presence vs. absence of nodules, GGO’s and consolidations) allows the differential diagnosis list to be narrowed [1].
|
Pulmonary Langerhans cell histiocytosis |
|
Lymphangioleiomyomatosis (with or without tuberous sclerosis) |
|
Lymphoid interstitial pneumonia |
|
Light chain deposition disease |
|
Desquamative interstitial pneumonia / Respiratory bronchiolitis interstitial lung disease |
|
Congenital/genetic (Birt Hogg Dube syndrome, Proteus syndrome, Ehrels-Danlos syndrome, neurofibromatosis, congenital pulmonary airway malformation) |
|
Neoplastic (squamous cell carcinoma, adenocarcinoma, sarcoma, blastoma) |
|
Fungi (Pneumocystis jiroveci pneumonia, coccidioidomycosis) |
|
Parasites (paragonimiasis, echinococcosis) |
|
Hypersensitivity pneumonitis |
|
Amyloidosis |
|
Respiratory papillomatosis |
|
Erdheim Chester disease |
|
Fire-eater’s lung |
|
Hyper IgE syndrome |
|
Lymphoproliferative disease (MALToma, follicular bronchiolitis, lymphomatoid granulomatosis) |
Table 1: Causes and Associations of cystic lung disease in adults.
The most common non-infectious causes of cystic lung disease with accompanying nodules and GGO’s are LIP, LCDD and DIP, while those without HRCT accompaniments are most often LAM and PLHC. LIP is a benign lymphoproliferative disorder occurring usually in the 4th to 6th decades of life with female predominance. It is commonly associated with connective tissue diseases or immune deficiency states. The cysts are randomly distributed but may have basilar and perivascular distribution and are associated with diffuse GGO’s, poorly defined nodules and interlobular septal thickening, sometimes mimicking lymphangitic carcinomatosis. LCDD is a rare condition usually associated with multiple myeloma or macroglobulinemia. Lung involvement includes nodules, lymphadenopathy and cysts. DIP typically occurs in smokers, although CTDs and non-tobacco inhalational exposures can be associated. Small cysts surrounded by GGO’s most prominently in the lower lung zones are characteristic of DIP. Sporadic (without tuberous sclerosis) LAM occurs almost exclusively in women of childbearing age but has been described in post-menopausal women. Cysts are usually numerous, thin walled, surrounded by normal lung parenchyma and distributed throughout the lungs. PLHC is typically seen in smokers between the ages of 20 and 40 and imaging is characterized by nodules earlier in the course that eventually become cysts (sometimes bizarre-shaped) with relative sparing of the lung bases and costophrenic angles.
Pulmonary metastases typically present with one or multiple round, peripherally located, lower zone predominant, nodules of various sizes (hematogenous spread) [3]. Alternatively, thickening of interstitium along septae and bronchovascular bundles is observed with lymphangitic carcinomatosis. Less common radiographic characteristics include cavitation, calcification/ossification, hemorrhage (nodular attenuation with surrounding GGO a.k.a. CT halo sign), air space consolidation, tumor (micro) embolism and endobronchial metastases. Infrequently, malignancies such as squamous cell tumors of the lung or head and neck, sarcomas, mesenchymal tumors, or lung adenocarcinomas present as diffuse cystic lung diseases [4-8]. The mechanism for development of malignant cyst is hypothesized to include excavation or central necrosis of the tumor with the walls of cavities becoming thin from inflation by a ball valve mechanism [5,9]. Cystic manifestations of endocervical carcinoma are distinctly unusual. In a retrospective review of 243 patients with cervical cancer; Tellis et al. reported a 9.1% incidence of metastasis to the lung; 45% of which were in patients with Stage II disease at diagnosis, with the median disease-free time being the longest (39 months) in patients with Stage I disease and the shortest (18 months) with stage III disease. Multiple pulmonary nodules were the most common CT finding, noted in 59% of the patients and none had cystic pulmonary lesions [10]. More recently, a report of cystic lung disease as a presentation of metastatic squamous cervical cancer 7- months after the initial diagnosis was reported [10,11].
In the case we described; the long (20 years) disease-free interval between the diagnosis of cervical cancer and its distinctive radiographic presentation make this case presentation unique. We hypothesize that given that the original tumor was mucinous in nature it followed the growth pattern similar to invasive mucinous carcinoma leading to a pneumonic, cavitary appearance.
CONFLICTS OF INTEREST
None
DISCLOSURES
None
REFERENCES
- Suhail R, Bondalapati P, Vydyula R, Ryu JH, Gupta N, et al. (2016) Cystic lung diseases. Chest 150: 945-965.
- Silva CIS, Marchiori E, Souza AS, Müller NL (2010) Illustrated brazilian consensus of terms and fundamental patterns in chest CT scans. J Bras Pneumol 36: 99-123.
- Seo JB, Im JG, Goo JM, Chung MJ, Kim MY (2001) Atypical pulmonary metastases: Spectrum of radiologic findings. RadioGraphics 21: 403-417.
- Traweek T, Rotter AJ, Swartz W, Azumi N (1990) Cystic pulmonary metastatic sarcoma. Cancer 65: 1805-1811.
- Hasegawa S, Inui K, Kamakari K, Kotoura Y, Suzuki K, et al. (1999) Pulmonary cysts as the sole metastatic manifestation of soft tissue sarcoma: Case report and consideration of the pathogenesis. Chest 116: 263-265.
- Mahadeva R, Stewart S, Wallwork J (1999) Metastatic endometrial stromal sarcoma masquerading as pulmonary lymphangioleiomyomatosis. J Clin Pathol 52: 147-148.
- Zhang J, Zhao YL, Ye MX (2012) Rapidly progressive diffuse cystic lesions as a radiological hallmark of lung adenocarcinoma. J Thorac Oncol 7: 457-458.
- Kushima H, Ishii H, Yokoyama A, Kadota J (2013) Lung adenocarcinoma presenting with diffuse multiloculated cystic lesions. Intern Med 52: 2375.
- Gupta N, Vassallo R, Wikenheiser-Brokamp KA, McCormack FX (2015) Diffuse cystic lung disease. Part II. Am J Respir Crit Care Med 192: 17-29.
- Tellis CJ, Beechler CR (1982) Pulmonary Metastasis of carcinoma of the cervix: A retrospective study. Cancer 49: 1705-1709.
- Diaeddin S, Adeni A (2018) Unusual cystic lung metastasis. BMJ Case Reports 2018: 2018224648.
Citation: Makkar P, Geyer A, Rekhtman N, Vaucher J (2020) Metastatic Cervical Cancer Masquerading As Cavitary Pneumonia. J Pulm Med Respir Res 6: 033.
Copyright: © 2020 Priyanka Makkar, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.

