
Microplastics and Bisphenol A (BPA) in Sediments of Coastal Lagoons of Veracruz, Mexico
*Corresponding Author(s):
Botello AVMarine Pollution Laboratory, Institute Of Marine Sciences And Limnology, UNAM, 04510, Mexico
Tel:+52 5556225765,
Email:gatoponcho2015@gmail.com
Abstract
Due to the importance and effects caused by microplastics and BPA in aquatic systems, the present study analyzed the presence of microplastic particles, their structure and their composition in 16 sediment samples from 4 coastal lagoons of Veracruz State, Gulf of Mexico. The presence of BPA in 7 samples close to the reef system of Veracruz, were also analyzed. These lagoons are important for fisheries and provide sustenance for a large number of families that inhabit them. The microplastic particles were separated from the sediments by sieves and flotation. Its shape and characteristics were analyzed by optical Microscopy, scanning Electron Microscopy (MEB) techniques, were applied for the identification of sample degradation marks, as well as the analysis by Infrared Spectrometry (IR) technique for the identification of polymers that make them up. The results show that in all the lagoons there is the presence of microplastics of different shapes and sizes, as well as of different compositions, the largest number of particles corresponded to the lagoons of Alvarado and Tampamachoco that are important fishing and oyster culture areas.BPA also appeared in all samples and in concentrations ranging from 0.04 to 0.35 ng / g, the highest corresponding to the samples close to the Veracruz reef system. The fact that microplastics were presented in all the study areas, indicates that these areas are highly impacted by the presence of garbage, urban and industrial waste, coming from the various activities that are carried out nearby.
INTRODUCTION
The coastal area of Mexico is within the politically recognized territorial sea, covering an area of approximately 430000 km2 [1]. Within this coastal area is the state of Veracruz, in which there is a great diversity of habitats of high productivity, which are interconnected systems, such as bays, deltas, seagrasses, coral reefs and coastal lagoons and estuaries [2]. These coastal ecosystems have a strong anthropogenic impact, as a consequence of demographic growth trends [2]. The river systems, being the means of transport of the terrestrial material towards the coast and the ocean, are the means of the transport of the garbage generated by the human and industrial activity [3].
The coastal states of the Gulf of Mexico have had a significant urban evolution in a century, as happened in the Veracruz-Boca del Río tourist corridor in Veracruz since by its mega tourism development it is expected that by 2025, 40% of the national population at a distance of less than 100 km from the coast will live under marine and coastal influence. This entails a wide range of toxic wastes [4], which include an urban Solid Waste (MSW), as well as wastewater discharge. This type of contamination is due to its poor disposal, leaks and leaching at its storage sites (inland, warehouses, pipelines, sanitary landfills, among others) or in areas of productive activities, or by accidental spills during transport. Approximately 2% is deposited in rivers and bodies of water [5].
Within the RSU are plastics, which are the set of synthetic or semi-synthetic materials formed from polymers of organic molecules obtained from raw materials such as cellulose, coal, natural gas or oil, among others; which can be deformed to achieve the desired shape through extrusion, molding or spinning [6]. According to Greenpeace [7], the plastics market is dominated by polymers such as polypropylene, Thermoplastic Polyester (PET), Polyvinyl Chloride (PVC), polyethylene, among others. These tend to accumulate being subject to both abiotic factors such as solar radiation, tidal movement, salinity, friction with sand and temperature, as well as biotic factors, such as biofilm formation.
The existence of plastics in coastal systems undergoes the generation of microplastics, which consist of plastic fragments, covering sizes of <10 mm [8], <5 mm [9,10], 2-6 mm [11], <2 mm [12] and <1 mm); they are found in sediments, water column and organisms. In recent years, microplastics have been widely detected in the sea, freshwater, terrestrial environment and organisms [13]. Microplastic contamination is of growing concern and has been listed as the second important scientific topic in the field of environment and ecology [14].
Due to the great importance and concern for this type of contamination, the objective of this study was to determine the presence and evaluation of microplastics present in sediment samples corresponding to the Mandinga, Tampamachoco, Alvarado and Verde lagoon systems, Veracruz, Mexico, applying the techniques of optical Microscopy, scanning Electron Microscopy (MEB) for the identification of degradation marks of the microplastic samples, as well as the analysis by the Infrared Spectrometry (IR) technique for the identification of the polymers that comprise them.
MATERIALS AND METHODS
Study areas
The Lagoon Systems of Mandinga (MLS), Tampamachoco (TLS), Alvarado (ALS) and Laguna Verde (VL) are located in the state of Veracruz [15]. The problems they present in common are contamination by agrochemicals, fertilizers, industrial waste, sewage, mangrove felling. The economic activities are based on the fishing of shrimp, robalo and crab; its problem is due to the increase in erosion and hauling of sediments, environmental damage from fishing vessels; contamination by solid waste and sugar mills [15,16] (Figure 1).
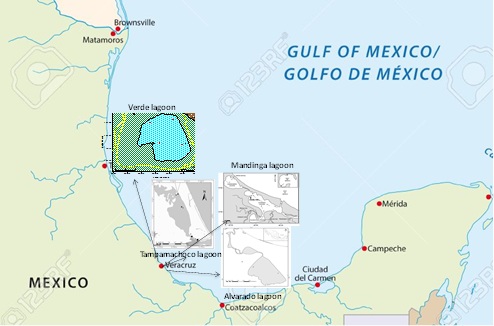 Figure 1: Collection areas and stations of the coastal lagoons of the state of Veracruz.
Figure 1: Collection areas and stations of the coastal lagoons of the state of Veracruz.
Mandinga Lagoon System (MLS): It is located in the state of Veracruz, between 19° 00 'and 19° 06' north latitude and meridians 96° 02 'and 96° 06' west longitude. The lagoon system has a north-south orientation while the nearby coast adopts a northwest-southeast direction, forming the tip of Antón Lizardo. To the northwest, the lagoons are separated from the sea by a barrier of dunes. The lagoon is associated with the Jamapa River, which is born with the thaws of the Orizaba Peak, runs 150 km; It runs from west to east and receives several tributaries of the Huatusco, Cotaxtla, Totolapan rivers, flows into the Gulf of Mexico, in the place known as Boca del Río, near the City of Veracruz and there are approximately 53 suburban towns and rural. It includes the Mandinga lagoon system, which has an approximate length of 20 km and is composed of six interconnected bodies of water: Estero del Conchal, Larga lagoon, Estero de Horcones, Mandinga Chica lagoon (Redonda lagoon), Mandinga estuary and lagoon by Mandinga Grande [17] (Figure 1).
Tampamachoco Lagoon System (TLS): they are located in the Huasteca Region, in the Coastal Plain of the Gulf of Mexico, in the state of Veracruz, ± 10 km west of the city and port of Tuxpan, Municipality of Tuxpan. The Mangroves and wetlands of Tuxpan are located in the lower coastal part and are divided by the Tuxpan River. To the North of the Tuxpan River, the mangroves of the Tampamachoco Lagoon are observed and to the South of it, the mangroves and wetlands associated with the Tumilco and Jácome estuaries. It is located between parallels 20°58 '15' 'to 21°05' north latitude and meridians 97°20'30 '' to 97°24 'west longitude. Coastal wetland at sea level: 0 masl. the Tampamachoco Lagoon has an area of 1,500 ha [18] (Figure 1).
Alvarado Lagoon System (ALS): It is located 66 kms southeast of the port of Veracruz, between geographical coordinates; 18°43’51” and 18°52’40” N; 95° 42’23 ” and 95° 57’25” W [19]. It is limited to the north by the Gulf of Mexico; to the east by the Gulf of Mexico as by the complex system of wetlands of Papaloapan, to the south with the municipalities of Tlacotalpan, Acula, Ixmatlahuacan and Lerdo de Tejada; to the west with the municipalities of Ignacio de la Llave, Tlalixcoyan, Medellin and Boca del Río. It is 17km long parallel to the coastline and with a maximum of 4.5 km. Communication with the sea is done through a 0.4 km wide bar [20,21] (Figure 1).
Verde Lagoon: It is located at a latitude 20° 00 'at 19° 01'48' ' and at a length 96° 29'24 "at 95° 48'36", it has an extension of 3 657 km 2. It is formed by lagoons with conserved vegetation (wetlands), ocean areas, marshes, estuaries, bays the climate is warm subhumid with rains in summer (286-320mm) and the average annual temperature of 21-28°C. It has multiple linearly separated sandy barriers, as well as shapes and bathymetries gently modified by tidal action and non-marine processes, the Mexican counter current prevail and tropical storms, north and red tides occur. There may be seasonal droughts, so salinity varies from brackish to hypersaline and has freshwater contributions by rivers and lagoons [17] (Figure 1).
Sample collection
Surface sediments were collected in a network of four stations for each of them, using a Van Veen stainless steel dredge and an epoxy coating of 4 L capacity; selected according to the different morphologies, fluvial and tidal influence, as well as the villages settled on the margins and their possible discharges for each of the stations and were stored in plastic bags for transport to the laboratory.
Laboratory analysis
The methodology for the identification of microplastics was proposed by Gómez [22], which consists of drying the samples, elimination of organic matter, sieving and separation, using a series of sieves of 8 different pore sizes, the which are recorded in the range of -2.25 µm to 4.00 µm (taking into account the sedimentary residue known as PAN *), as well as an automatic sieve. The sediments already separated were stored in bags marked with both the station number and the sieve number. Subsequently, the flotation separation was performed. The identification was carried out using an optical Microscope, scanning Electron Microscopy (MEB) for the identification of degradation process marks and Infrared Spectrometry (IR) for the characterization of the microplastics and to identify the polymer from which the different microplastics present are formed.
The sediments were dried and sieved through a 250 µm mesh; BPA was obtained by the method proposed by Wang et al. [23]; generally consists of, 5 g of dry sediment is placed in polypropylene centrifuge tubes, 10 mL of methanol HPLC is added to them, orbital shaking (250 rpm/20 min), sonication extraction (20 min), centrifugation (8000 rpm/20 min), solid phase extraction with florisil cartridges and elution with methanol (10 mL); it is taken to dryness under a gentle nitrogen stream. It is derivatized with acetic anhydride, 0.1 M potassium carbonate, hexane HPLC, taken to orbital shaking (250 rpm/20 min), centrifuged (8000 rpm/20 min) and the supernatant is analyzed by GC-MS with a gas chromatograph coupled to an Agilent Technologies (6890) mass spectrometer, with an automatic injector and quadrupole with negative chemical ionization (5973 N) and a DB-35 MS capillary column (60 m×0.25 mm×0.25 μm). The carrier gas was helium grade 5.0 with a 1 ml/min flow rate. Reagent targets and fortified targets were included. The detection and quantification limits were estimated from the signal-to-noise ratio of 3 and 10 at the fortified targets and were 0.03 and 0.10 ngg-1 respectively [24,25]. The recovery rate was 85%.
RESULTS AND DISCUSSION
River systems subjected to untreated sewage discharges are also important sources of plastics contributions that, after degrading, microplastics are formed. In the municipality of Boca del Río, Veracruz. In 2014, the presence of a wastewater discharge point is reported, which corresponds to the Jamapa River, which is the main contribution of freshwater from the Mandinga lagoon. In the municipality of Alvarado (related to the Alvarado lagoon), until 2014 there were 10 points of discharge of wastewater into rivers or streams. The lagoon systems of Alvarado and Mandinga are river-lagoon systems, which have large contributions of rivers, so they are impacted by the content of this wastewater being the last sites of current disposal within the coast. In the municipality of Tuxpan (related to the Tampamachoco lagoon), there are connections between the lagoon and the Tuxpan River, which implies two sources of wastewater entering into the river system. This is observed in the results obtained in this study.
In figure 2 the levels of presence of the different fractions for each of the lagoons are presented and a great variety of fraction sizes is presented and the Alvarado lagoon corresponds to the highest levels of presence in all the fractions, followed by Mandinga Lagoon, Tampamachoco Lagoon and finally Verde Lagoon. In the four lagoons analyzed there is the presence of microplastics in the majority with a high variability of sizes.
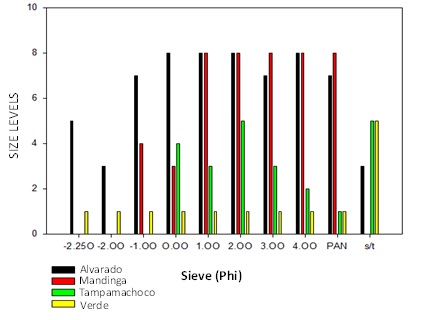 Figure 2: Presence of microplastics in the different sieve sizes in the four study areas.
Figure 2: Presence of microplastics in the different sieve sizes in the four study areas.
From the analysis of the form of particles present and by comparison with reference images, the presence of secondary type microplastics is reported, which consisted of both microfibers and particles (Figure 3), which have different colors, observing a greater amount of fibers than particles for all the lagoons.
 Figure 3: Microplastics in the form of fiber (A and A’) and in the form of particles (B). Both correspond to microplastics of secondary types.
Figure 3: Microplastics in the form of fiber (A and A’) and in the form of particles (B). Both correspond to microplastics of secondary types.
The presence of fragments, to a lesser extent compared to microfibers, is due to the disintegration of macroplastics, which are found within the garbage or urban Solid Waste (MSW), which according to Rosado et al. [26], it is because after the life of the plastic has ended, the plastic becomes part of the urban solid waste (MSW), which due to its mismanagement (which includes collection, transport and final disposal), this material it has become the most frequent exogenous material in the ocean. Acrylic (Figure 4) is used in the manufacture of different utensils such as glasses, spoons, plates, among others, many of which, when discarded, form part of the MSW, so they imply a source of this contribution, material, as well as many other types of plastics.
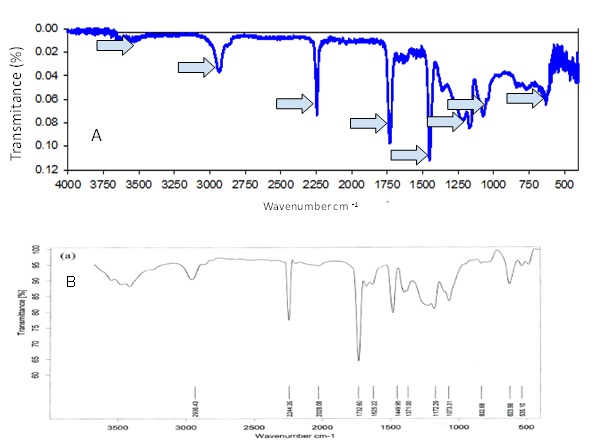 Figure 4: IR spectrum obtained from a microplastic sample from the Tampamachoco lagoon (A). Acrylic IR spectrum recorded by Hassani et al. [27], (B). The matching absorption bands between both spectra are indicated by blue arrows in (A).
Figure 4: IR spectrum obtained from a microplastic sample from the Tampamachoco lagoon (A). Acrylic IR spectrum recorded by Hassani et al. [27], (B). The matching absorption bands between both spectra are indicated by blue arrows in (A).
SEM IMAGES
Scanning Electron Microscopy (SEM) images obtained from different microplastic samples show degradation marks, which appear in different ways: according to Wang et al. [28], are presented as fractures, according to Fries et al. [29], appear as detachments in the fibers and concerning Tollinsky et al. [30], are shown in the form of pores on the surface of the particles, being an example of the latter microplastic particles of the Alvarado lagoon (Figure 5).
The basic process of formation of secondary microplastics within an aquatic system consists in the exposure of a larger plastic to Ultraviolet (UV) radiation, whereby plastics are photodegraded, that is; there is the degradation of the polymer matrix, which leads to the rupture of its bonds [31].
The presence of microplastics in the sediments is due both to their sinking, given by the density of the fragmented materials as well as by the organic activity that occurs on them. According to Setälä et al. [32], benthic organisms are susceptible to denser plastics, including Polyethylene Terephthalate (PET) and polyvinyl chloride, indicating that due to their density; it is expected that these are the polymers found within the sediments in the first instance. On the other hand, the aggregation of organic material, such as seaweed coating, can move the microplastics of lower density suspended towards the sediment, where they are incorporated and concentrated, which increases their sinking rate, coupled with the fact that there is a relationship between abundance of microplastics with organic content and fine fraction. Therefore, microplastics are added in the deposit areas [33]. This justifies the entry of microplastics formed by less dense polymers into the sediments (Figure 5).
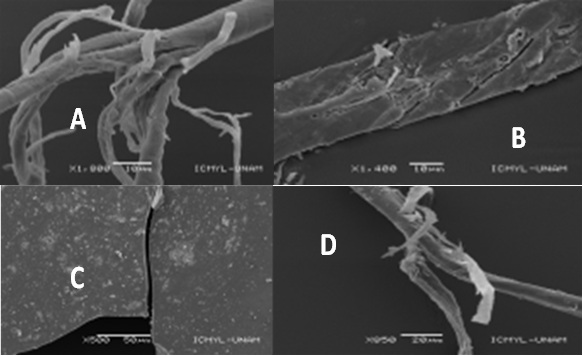 Figure 5: SEM images obtained from microplastic samples from the Lagoons of Alvarado (A): Verde (B): Tampamachoco (C): Mandinga (D): Veracruz, Mexico.
Figure 5: SEM images obtained from microplastic samples from the Lagoons of Alvarado (A): Verde (B): Tampamachoco (C): Mandinga (D): Veracruz, Mexico.
Infra-Red (IR) spectrometry
From the comparison of the IR spectra obtained from the microplastic samples of the Alvarado lagoon with that reported by Al-Sabagh [34], the presence of the polypropylene polymer was deduced. The matching absorption bands between both spectra correspond to those found 3369.31 cm-1, 2920.58 cm-1, 1734.36 cm-1, 1458.48 cm-1 and 1116.67 cm-1 (Figure 6).
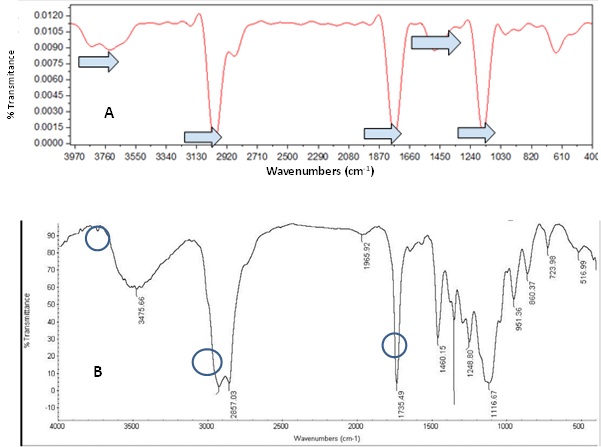 Figure 6: IR spectrum obtained from different microplastic samples from the four study areas (A): IR spectrum reported by Al-Sabagh [34] (B): The coincident absorption bands between both spectra are indicated by arrows blue.
Figure 6: IR spectrum obtained from different microplastic samples from the four study areas (A): IR spectrum reported by Al-Sabagh [34] (B): The coincident absorption bands between both spectra are indicated by arrows blue.
Similarly, the presence of the acrylic polymer is reported, which forms the green microplastic particle used to obtain the IR spectrum, due to the similarity in the absorption bands with the spectrum reported by Hassani et al. [27], which correspond to 2936.43 cm-1, 2244.35 cm-1, 2028.08 cm-1, 1732.5 cm-1, 1449.95 cm-1, 1172.29 cm-1, 1073.31 cm-1, 623.99 cm-1 mainly (Figure 4).
Fiber-shaped microplastics, as well as particles, within the study areas, are also subjected to UV radiation, which according to Carbery et al. [35]. This process is known as oxidative weathering and is caused in the first instance by the incidence of sunlight and by the interaction of physicochemical factors such as salinity, temperature and dissolved oxygen, which enhance the effects of that radiation, thus increasing the degree of degradation, as well as other biotic factors, such as microbial colonization.
This process is confirmed due to the presence of an absent absorption band in the infrared spectra of polypropylene, without effects of environmental alteration, which is around the 1700 cm-1 wave number, which corresponds to the carbonyl group a, present during processes of oxidative degradation [36]. This is consistent with the images of SEM in terms of degradation processes that occur within the lagoons, this being corresponding to the process of photo oxidation by ultraviolet radiation from sunlight, which indicates that they were initially microplastic floating in the superficial part of the waters of the lagoons. The presence of these microplastics in the superficial part of the lagoons makes possible the development of algae that cover them, thus causing the biodegradation process. IR spectra were also found whose greatest coincidence is polyethylene, material from which the fishing nets are manufactured, intense activity that is carried out in the four lagoons, so that these fishing gears generate some impact on the lagoon ecosystems, given the wear of the networks.
By losing its structural integrity, due to its degradation, plastics are increasingly susceptible to fragmentation resulting from abrasion, wave action, temperature and turbulence. As coastal lagoons are defined as hydrodynamic barriers, the movement of water is a factor that can cause that given that structural weakness there is fragmentation of different types of particles [1].
BPA
Table 1 shows the results obtained from the Alvarado lagoon and the Veracruzano reef system, for the sediments of the Alvarado lagoon the concentrations obtained range from 0.04 to 0.18 ng/g of BPA, while the samples of the Reef system its concentrations are higher and range from 0.23 to 0.35 ng/g. The above data show lower concentrations than those determined by Wang et.al. [23], whose concentration ranges from 0.32 to 27.30 ng/g in sediments of Lake Taihu in China. Recently Borges et al. [37], report concentrations of BPA ranging from 18.29 to 21.72 ng/g in sediments of the Ria and Costa de Campeche, Mexico. It should be noted that these results are among the first to appear in Mexican coastal systems and show that this compound is widely distributed in various environmental matrices (sediments, water and organisms) and that its importance lays in the high toxicity that BPA already shows. It has been considered as a disruptor of the endocrine system and produces carcinogenic effects when metabolized by hydroxylation and subsequent oxidation forms an orthoquinone that can establish covalent bonds with DNA and develop mutagenic effects that could be the initiators of prostate and breast cancer.
Therefore, it is imperative to increase the number of analysis sites and determine them in organisms of fishery and commercial importance.
|
Alvarado Lagoon System (ALS) |
BPA Concentration (ng/g) |
|
Station 7A |
0.09 |
|
Station 7B |
0.18 |
|
Station7C |
0.06 |
|
Station7D |
0.12 |
|
Station 7E |
0.04 |
|
Veracruzano Reef (Veracruz) |
|
|
Station1A |
0.35 |
|
Station1B |
0.23 |
Table 1: BPA concentrations in surface sediments of two coastal systems in Veracruz.
CONCLUSION
The presence of microplastics implies a problem of contamination of great importance and impact both within the coastal lagoons and for aquatic ecosystems in general. The lagoon systems of Alvarado and Mandinga were the ones that presented the majority of microplastics of different sizes, due in large part to these lagoons; they have the favourable environmental conditions to fragment plastics to very small sizes.
The presence of the polypropylene polymer shows that clothing made from synthetic materials is one of the most important and abundant sources of microplastics and greater risk to aquatic ecosystems. In addition to the above, the presence of acrylic polymer shows the risk that exists in the increase in the use of plastic in everyday life, many of which are of single-use, coupled with its poor management to prevent them from ending up in the coastal areas.
The investigations carried out and consulted for the comparison of the results obtained do not rule out the presence of bioaccumulation and biomagnifications processes within the lagoons that were the subject of this study, so it can be ensured that these processes are possible.
REFERENCES
- Lara-Lara JR (2008) Los ecosistemas costeros, insulares y epicontinentales. Capital natural de México 1: 109-134.
- Caso M, Pisanty I, Ezcurra E (2004) Ezcurra. Diagnóstico ambiental del Golfo de México. Instituto Nacional de Ecologia 1: 28-29.
- Toledo A (2014) Caracterización Ambiental del Golfo de México: Marco Conceptual. En Botello et al. Golfo de México, Contaminación e Impacto Ambiental: Diagnóstico y Tendencias. Tercera Edición. UAC, UNAM-ICMyl, CINVESTAV-Unidad Mérida.
- Diaz M (2012) Conformado de materiales plasticos. Departamento de Ingenieria. Pg no: 1-86.
- Greenpeace (2019) Datos sobre la producción de plásticos. Obtenido el 19 de junio de. Amsterdam, Netherlands.
- Graham ER, Thompson JT (2009) Deposit- and suspension-feeding sea cucumbers (Echinodermata) ingest plastic fragments. J Exp Mar Biol Ecol 368: 22-29.
- Barnes DKA, Galgani F, Thompson R. Barlaz M (2009) Accumulation and fragmentation of plastic debris in global environments. Philosophical transactions of the Royal Society B: Biological Sciencies 364: 1526: 1985-1998.
- Betts KS (2008) New thinking on flame retardants. Environ Health Perspect 116: 210-2103.
- Derraik JGB (2002) The pollution of the marine environment by plastic debris: A review. Mar Pollut Bull 44: 842-852.
- Ryan PG, Moore CJ, van Franeker JA, Moloney CL (2009) Monitoring the abundance of plastic debris in the marine environment. Philosophical Transactions of the Royal Society B: Biological Sciences 364: 1999-2012.
- Eriksen M, Lebreton LC, Carson HS, Thiel M, Moore CJ, et al. (2014) Plastic pollution in the world's oceans: More than 5 trillion plastic pieces weighing over 250,000 tons afloat at sea. PloS One 9: 111913.
- Horton AA, Svendsen C, Williams RJ, Spurgeon DJ, Lahive E (2017) Large microplastic particles in sediments of tributaries of the River Thames, UK - Abundance, sources and methods for effective quantification. Mar Pollut Bull 114: 218-226.
- Comisión Nacional para el Conocimiento y Uso de la Biodiversidad (1998) Regiones Prioritarias Marinas de México. Comisión Nacional para el Conocimiento y Uso de la Biodiversidad, Mexico City, Mexico. Pg no: 198.
- Amaya PG, Villanueva S, Botello AV (2005) Metales en tres lagunas costeras del estado de Veracruz. In: Botello AV, von OstenJR, Bouchot GG,Hernández CA (eds.). Golfo de México Contaminación e Impacto Ambiental: Diagnóstico y Tendencias, 2nd Edición. Univ Autón de Campeche Univ Nal Autón, de México, Instituto Nacional de Ecología. Pg no: 696: 361-372.
- Comisión Nacional para el Conocimiento y Uso de la Biodiversidad (2009) Cuarto Informe Nacional de México al Convenio sobre Diversidad Biológica (CDB). Comisión Nacional para el Conocimiento y Uso de la Biodiversidad y Secretaría de Medio Ambiente y Recursos Naturales. México D.F.
- Botello AV, de la Lanza G, Villanueva SF (2016) Laguna de Tampamachoco, Veracruz, México. Características y diagnóstico ambiental. 2009-2012. Editorial académica española. Verlag/Editorial. Pg no: 300.
- Instituto Nacional de Estadística y Geografía (INEGI). Marco geoestadístico nacional. Localidades geoestadísticas municipales. Alvarado. 2016.
- SEMAR (2015) Alvarado, Veracruz; Consultado en línea el.
- Botello AV, de la Lanza G y Villanueva SF (2017) Monografía ambiental del Sistema Lagunar de Alvarado (SLA), Veracruz, México LM editors 120 p.
- Hernandez G, Romana I (2016) Efecto de los microplásticos de polivinil cloruro (pvc) y del fluoranteno en Eupolymniarullieri e Isognomonalatus, dos espcies del macrobentos del Caribe mexicano. Universidad Nacional Autónoma de México, Ciudad de México, México.
- Wang Z, Wagner J, Ghosal S, Bedi G, Wall S (2017) SEM/EDS and optical microscopy analyses of microplastics in ocean trawl and fish guts. Sci Total Environ 603-604: 616-626.
- Liao C, Liu F, Moon HB, Yamashita N, Yun S, et al. (2012) Bisphenol analogues in sediments from industrialized areas in the United States, Japan, and Korea: Spatial and temporal distributions. Environ Sci Technol 46: 11558-11565.
- Fang S, Chen X, Zhao S, Zhang Y, Jiang W, et al. (2014) Trophic magnification and isomer fractionation of perfluoroalkyl substances in the food web of Taihu lake, China. Environ Sci Technol 48: 2173-2182.
- Rosado-Piña N, Vazquez M, Álvarez A, Beltrán JC, Ojeda S (2018) Caracterización de microplásticos y muestreo de residuos sólidos urbanos de la playa de Tuxpan, Veracruz. Los residuos como recurso. Tuxpan, Veracruz: Sociedad Mexicana de Ciencia y Tecnología Aplicada a Residuos.
- Hassani S, Abdouss M, Mousavi A, Haji A (2012) Study of ion adsorption on micro and new nano acrylic fibers modified by ethanolamine. 5th Texteh international conference.
- Wang Q, Zhu L, Chen M, Ma X, Wang X, et al. (2017) Simultaneously determination of bisphenol A and its alternatives in sediment by ultrasound-assisted and solid phase extractions followed by derivatization using GC-MS. Chemosphere 169: 709-715.
- Fries E, Dekiff JH, Willmeyer J, Nuelle MT, Ebert M, et al. (2013) Identification of polymer types and additives in marine microplastic particles using pyrolysis-GC/MS and scanning electron microscopy. Environ Sci Process Impacts 15: 1949-1956.
- M Tolinski (2015) Ultraviolet light protection and stabilization. In: M Tolinski (eds) Addives for polyolefins: Getting the most out of polypropylene, polyethylene and TPO. Elsevier, Amsterdam, Netherlands.
- Cole ML, Halsband C, Galloway T (2011) Microplastics as contaminants in the marine environment: A review. Marine Pollution Bulletin 62: 2588-2597.
- Setâlâ O, Lehtiniemi M, Coppock R, Cole M (2018) Microplastics in marine food webs. In: E Zeng (eds) Microplastic contamination in aquatic environments. Elsevier, Amsterdam, Netherlands. Pg no: 339-363.
- Everaert G, Van Cauwenberghe L, De Rijcke M, Koelmans AA, Mees J, et al. (2018) Risk assessment of microplastic in the ocean: Modeling approach and first conclusion. Environmental Pollution 242: 1930- 1938.
- Hassan AM, Dosouky S, Nasser N, Elsharaky E, Abdelhamid M (2015) Preparation of some thermal stable polymers basedondiesters of polyethylene and polypropyleneoxides macro monomers to use as surfactants at high temperature and pressure. Egyptian Journal of Petroleum 25: 355-366.
- Carbery M, O' Connor W, Thavamani P (2018) Trophic transfer of microplastics and mixed contaminants in the marine food web and implications for human health. Environ Int 115: 400-409.
- Longo C, Savaris M, Mára Z, Nichele R, Coulon-Grisa AM (2011) Degradation study of polypropylene (PP) and Biorientedpolypropylene (BOPP) in the environment. Material Research 4.
- Borges MM, Dzul RC, Rendon von Osten J (2019) Occurrence and seasonal distribution of microplastic and phthalates in sediments from the urban channel of the Ria and coast of Campeche, Mexico. Science of the Total Environment 672: 97-105.
Citation: Botello AV, Villanueva SF, Leon D, de la Lanza G, Ponce G (2020) Microplastics and Bisphenol A (BPA) in Sediments of Coastal Lagoons of Veracruz, Mexico. J Toxicol Cur Res 4: 015.
Copyright: © 2020 Botello AV, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.

