
Modeling of Tobacco Smoking and Smoke as Well as Resin Microparticles Residue in Respiratory Tract
*Corresponding Author(s):
Ariel BMSt. Petersburg City State Pathologists Bureau, Uchebny Per, Russian Federation
Tel:+7 8125136098,
Email:arielboris@rambler.ru
Abstract
Aim
Aim of this study is the smoking process modeling with visualizing of the smoke microparticles' residue on the walls of the digestive and respiratory tracts.
Methods
The study was performed on patients with tuberculosis and chronic non-infectious lung diseases. All the patients underwent bronchoscopy. Powdered tantalum microparticles < 1μm in size were used as markers of tobacco smoke microparticles' residue. The tantalum powder was administered orally and intratracheally. Tantalum microparticles deposited in the respiratory tract were observed using the chest X-ray examination immediately after the aspiration and 4 h after it.
Results
With the majority of patients (64.3%), 95% of microparticles were deposited in the mouth, the oropharyngeal area, the pharynx, the pyriform sinuses and the larynx just after the aspiration, and only 5% of them reached the lower respiratory system.
Conclusion
We investigated the factors that contribute to the deposition of microparticles in the bronchia. Such deposition can lead to bronchial obstruction and further penetration of microparticles into the alveoli. Three degrees of optical density of smoke microparticles deposited on the mucosa were determined which characterize the layer thickness, deposition mechanisms (impaction, sedimentation, diffusion), and its precise localization.
Keywords
Smoking modeling; The mouth; The pharynx; The larynx; The trachea; The bronchia; Tantalum powder
INTRODUCTION
Tobacco smoking is a major social-economic problem [1]. From a biological point of view, this is a complex pathophysiological process, its features depend on a number of physical factors (aerodynamic, temperature, etc.), as well as on the specific smoking regime, primarily on the number of cigarettes smoked, the depth and frequency of puffs, smoking experience, etc.
The physical and chemical composition of tobacco smoke is of vital importance, i.e. those smokes represent an aerosol mixture of gases, liquids and solid microparticles (products of tobacco combustion) of 76 metals, including those carcinogenic and toxic (radioactive polonium 210, cadmium, lead, etc.).
However, these metals, numerous in the smoke microparticles, are present in them only in small quantities and therefore do not appear on X-ray images of the respiratory tract even when the X-rays pictures are made with smokers of long experience, who have accumulated many layers of metals on the mucosa. This is an obvious obstacle when studying the pathophysiology of smoking, especially at its initial stages, and is only partially compensated by data from numerous studies involving volunteers, results of animal experiments, including those in vitro, and mathematical modeling of smoking [2].
The results of histological examination of the smokers' respiratory tract show that they are subject to massive pathogenic impact of smoke. In this regard, they can be considered a priori as a kind of locus morbi, or a mosaic picture of structural changes of varying severity, intensity and duration in different parts of the respiratory system.
Obviously, the decisive role in the progression of structural and functional disorders caused by smoking belongs to the very initial changes resulting from the precipitation of tobacco smoke microparticles on the mucosa and their instantaneous effect upon the epithelium [3].
It is these early changes that remain practically unexplored, and represent the tabula rasa. Most likely, this could be explained by the lack of specific markers and smoking simulation devices that would model the precipitation of smoke microparticles on the surface of the respiratory tract mucosa, their moving and removal.
Obviously, the visual inspection as well as endoscopic and radiological examinations are an insufficient answer to this important problem. Meanwhile, in recent works on bronchiography that use the biologically inert tantalum powder as a radiopaque with optical density 5 times higher than that of iodine-containing contrasting substances [4], the surface of the respiratory tract mucosa was proved to be an information field which depicts distinctly all individual details of the mucosal microrelief (such as expanded excretory ducts, interfold spaces, exocrine gland cysts, and such like) as well as areas of different optical density representing the thickness of the microparticles layer (resp. concentration), their localization, area, and boundaries.
Consequently, there is every reason to believe that when simulating the smoking process with a special device which introduces defined doses of tantalum microparticles similar in size to solid microparticles of tobacco smoke in concentrations approximating those observed in bronchiography, we obtain a model that allows us to study the deposition of smoke microparticles in the respiratory tract.
The aim of this study is to simulate smoking with visualization of microparticles of smoke deposited on the walls of the initial part of the digestive and respiratory tract when using a cigarette simulating device and tantalum powder as a marker.
MATERIALS AND METHODS
Patients (n=28) aged 26-58 with various lung pathologies (tuberculosis, chronic non-contagious diseases) were examined. Those smokers with the > 10 years were 17 (60.7%) patients, the remaining 11 (39.3%) were non-smokers. The bronchiofiberscopy revealed injuries in a form of diffuse (n = 20) and drainage (n = 8) endobronchitis. Local anesthesia was performed as the conventional procedure. The following facilities were used:
- • A pilot model of the powder inhaler PAI-2 simulating a cigarette (RF license No. 2053801)[1] synchronized with respiratory rate and operating at the aspiration phase (Figure 1 and table 1).
- • A vial for powder that could be inserted into the smoking modeling device.
- • A compressor of the portable aerosol apparatus PAI-2.
RF license No. 2053801. MPK A 61 B/00. A device for inhaling powdery preparations. A. A. Krishtafovich, S. Ya. Ryabinkin, S. Ya. Zhukov. Publ. 10.02.96. Bull. No. 46.
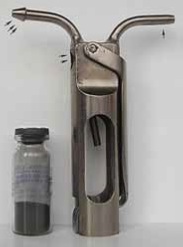
Figure 1: A device for smoking modeling. On the right: a "mouthpiece" (one arrow), a valve for dosage insufflation of microparticle aerosol (two arrows), and connection to an aerosol inhaler PAI2 (three arrows). On the left: A reservoir for tantalum powder.
|
Section examined |
Number of patients, n |
|
Oral cavity |
4 |
|
Oropharynx / Oropharyngeal area |
4 |
|
Pharynx |
4 |
|
Pyriform sinuses |
3 |
|
Larynx |
3 |
|
Trachea and bronchia |
6 |
Table 1: A number of investigations of the mouth and the upper digestive as well as respiratory tract.
As a tobacco smoke microparticles marker the monodisperse tantalum microparticles < 1 μm in size were used. This size was close to that of solid tobacco smoke microparticles. The effect of one cigarette smoked in 12-14 puffs on combustion for 5-6 minutes was taken as a measure of smoke microparticles precipitation on the surface of mucosa.
Powder was introduced into the trachea by oral injection through a catheter. Areas of the mucosa with multilayered accumulation of tantalum microparticles detected on the monitor were exhibited on radiographs immediately after spraying and 4 h after it. To reduce radiation exposure, X-ray filming was accomplished selectively, the number of radiographs being 2-3 in each patient. Table 1 shows the number of patients whose mouth, oropharyngeal area and other organs, including small bronchia and bronchioles, were examined after tantalum spraying. No complications associated with the procedure have been observed. The obtained information on the condition of the respiratory tract walls was further used for diagnostic purposes.
A prerequisite for this work were the physical and aerodynamical principles according to which all solid particles in tobacco smoke including metal ones which are similar in their chemical and physical properties and in size are moving in complex air ducts in the same direction and at the same velocity, precipitating on the surface of the respiratory tract. In course of smoking modeling, we studied the mechanisms of the generation and motion of aerogenic microparticles flow in the airways and compared them with those observed in a real smoking process. To produce a microparticles flow when performing smoking simulation, we closed the valve of the device with a finger and the air jet generated in the vial activated tantalum powder forming a concentrated aerosol which moved at a high speed through the spraying nozzle. When the patient inhales, tantalum microparticles mix with the air flow at the nozzle outlet, and microparticles of the resulting air-powder aerosol are moving, clearly, as fast as the air flow does (approximately 30 l/min.) and gradually precipitate in the upper parts of the digestive tract and in the airways.
Presumably, all inhaled microparticles without exception must be deposited in the respiratory tract providing high optical density, that is reflected on the radiograms together with a clear picture of tantalum microparticles arrangement on the surface of the respiratory tracts mucosa.
It is well known that, in contrast to simulated smoking, in the process of real smoking the flow of aerogenic smoke microparticles starts at the moment of inhaling by puffing in the cone of a burning cigarette. About 20% of microparticles going through a cigarette body are held down by the filter. 20-25% of these microparticles coming through the filter mix with the air flow and continue their motion at the speed of the flow, just as it happens in simulated smoking.
Between the inhales, about 55 to 65% of smoke microparticles form a side stream and escape into the environment. It can be seen, that despite the differences in the mechanics of flow formation and quantitative indices of their sedimentation, the speed of microparticles movement in the flows and the path of their sedimentation in the respiratory tract are the same during imitation and real smoking depending on the depth of inhaling.
Thus, it is quite natural to believe that the image of tantalum microparticles in the smoking simulation, obtained through X-rays of the initial segments of the digestive and respiratory tracts, can be regarded as a radiological model of aerogenic tobacco smoke microparticles deposition.
RESULTS AND DISCUSSION
The radiographs of 18 patients (64.3% of total) taken immediately after spraying showed that some 95% of microparticles precipitated on the mucosa of the mouth, the oropharyngeal area, the pharynx, the larynx and the pyriform sinuses, and only 5% of them reached by transit the lower respiratory ways. In all probability, a high percentage of microparticles held down in the upper sections of the digestive tract is due, above all, to their complicated anatomical structure characterized by bends, dilatations and constrictions which create optimal conditions for detaining maximal quantities of microparticles. As for smoking modeling, it becomes evident that with every cigarette smoked by a person with long smoking experience the same 95% of solid tobacco smoke particles and resin remain deposited in the mouth, the pharynx, the larynx, the trachea and bronchia.
Microparticles enter at high speed the oral cavity when lips are closed and jaws half-locked. It is apparent that in such circumstances the amount of suspended and deposited tobacco smoke microparticles as well as their concentration per unit of the mucosal area increase many-fold with every inhale of smoke. It is well known that in the real process of smoking, the clouds of smoke consisting of suspended microparticles emerge during the exhale.
The process of the microparticles' residue takes place step-by-step in a subconscious puffing mode when such particles collide uncontrolled with the lips, teeth, gums and the anterior part of the tongue, thus damaging mechanically the epithelium of the mouth, dental enamel and clogging the excretory ducts of salivary glands (Figure 2). At the sites where gingival mucosa comes into contact with that of the cheeks, we detected areas of various shape and size exhibiting high optical density. These are multilayer agglomerates of microparticles where pathogenic impact of smoke on the mucosa must be a lot more severe than in the adjacent sites with lesser quantities of deposited microparticles. These high optical density fields are the risk areas for the development of one or other type of pathology. Regarding the individuals that have never smoked, it is not difficult to imagine that after the very first inhale of tobacco smoke a quick absorption of smoke toxins begins in the above-mentioned places causing instant giddiness, nausea and other symptoms of a brain vessels spasm.
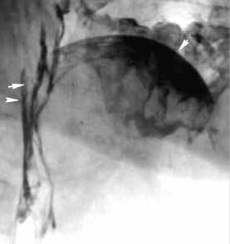 Figure 2: Lateral head and neck X-ray. Multilayer deposition of microparticles, which appears in form of linear and curved shadows, is seen on the tongue (one arrow) and lateral walls of the pharynx and oropharyngeal area) (two arrows)
Figure 2: Lateral head and neck X-ray. Multilayer deposition of microparticles, which appears in form of linear and curved shadows, is seen on the tongue (one arrow) and lateral walls of the pharynx and oropharyngeal area) (two arrows)
Microparticles penetrate to the oropharyngeal area through a crevice space between the tongue, the hard and the soft palate. Colliding inertially with posterior and lateral walls at an angle of 90°, they form straight and twisted multilayer streams (width of 3-5 mm) going down the pharynx (Figure 2). Microparticles get to the pyriform sinuses in two ways: from the pharynx inertially and from the larynx by way of clearance. There, they create an another risk area: the multilayer depot of high optical density (Figure 3). It is clear that with frequent and prolonged smoking pyriform sinuses have no time to clear themselves from smoke microparticles fully by swallowing them and in their way they accumulate, last and gather there for a long time providing a cumulative pathogenic effect on tissues.
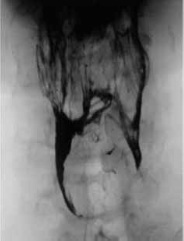 Figure 3: Pharyngogram, the anteroposterior view. The pharynx and the pyriform sinuses are seen. Multilayer deposition of the microparticles is found.
Figure 3: Pharyngogram, the anteroposterior view. The pharynx and the pyriform sinuses are seen. Multilayer deposition of the microparticles is found.
In the hourglass-shaped larynx microparticles are deposited chaotically by turbulent whirlwinds on top of each other with multilayer accumulation on the vocal cords and in the voice slot. Location and prevalence of risk areas in the upper parts of the digestive tract and the larynx are shown in figure 4.
 Figure 4: Location and prevalence of risk areas in the mouth, the oropharyngeal area, the pharynx, the pyriform sinuses and the larynx.
Figure 4: Location and prevalence of risk areas in the mouth, the oropharyngeal area, the pharynx, the pyriform sinuses and the larynx.
As it was found in course of the investigation, approximately 5% of the tobacco smoke microparticles penetrate the lower airways with each cigarette. It is safe to assume, that when a person smokes, say, 20 cigarettes a day, the depth of microparticles' penetration into the bronchia, bronchioles, etc., as well as their number come up to 100 percent. The following factors make that possible:
- • Straightforward direction of particles' path;
- • The dehydration effect of the air currents on mucosa;
- • Paralyzing effect of tobacco smoke upon ciliated epithelium;
- • Sedimentation of microparticles on the membranous parts of the tracheal and bronchial walls;
- • Concentration of microparticles suspended in the air of the respiratory tract which increases with every inhale of smoke; microparticles precipitation in layers;
- • Reduced contact of microparticles with mucosal surface and gliding upon it.
In the trachea and bronchia microparticles are deposited on the walls of cartilaginous semi-rings colliding by inertia at an angle of 45° and creating risk zones (Table 2). In the membranous areas and in the interfold spaces their deposition occurs through sedimentation (Figure 5) while at the bifurcation sites of the trachea and bronchia the process goes on by way of inertial collision. It was observed that the clearance of suspended microparticles and the velocity of their transit in the cranial direction decrease unavoidably (60-65 times as compared with the larynx) with decreasing caliber of the bronchia. This, in turn, leads to the thickening of the microparticles layer on the walls of the bronchia to the extent that their lumen becomes obturated (Figure 6). The diameter of clogged bronchia may be as great as 3-4 mm, which is distinctly seen in the bronchiograms. In other words, at this level the phase of microparticles deposition gives way to the phase of their accumulation and prolonged retention. Subsequent diffusion of microparticles in the alveoli causes polysegmental (lobar) reduction of the lungs venting function and respiratory insufficiency. How well this process is pronounced and how long it persists depends, in all probability, on the number of cigarettes smoked daily, the length of smoking experience and the caliber of the clogged bronchia [5].
|
Deposition thickness |
Qualitative and quantitative characteristics of deposition |
Location |
|
|
Degree |
Optical density |
||
|
I |
Low |
Thin layer of smoke microparticles on smooth surface. Sedimentation |
Membranous part of the trachea and the bronchia |
|
II |
Medium |
Multilayer deposition of microparticles colliding with the wall and changing direction of their movement. Impaction |
The mouth, the pharynx, the larynx, tracheal and bronchial cartilages |
|
III |
High |
Deposition, retention and accumulation of microparticles. Impaction and sedimentation. |
Pyriform sinuses, small bronchia and bronchioles |
Table 2: Morpho-functional changes in different parts of the digestive and respiratory tracts mucosa in accordance to the microparticle layer thickness.
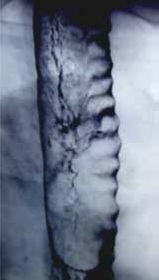 Figure 5. Tracheogram, the lateral view. Multilayer deposition of microparticles on the tracheal cartilages via impaction; on the membranous wall of the trachea and in the subglottis via sedimentation.
Figure 5. Tracheogram, the lateral view. Multilayer deposition of microparticles on the tracheal cartilages via impaction; on the membranous wall of the trachea and in the subglottis via sedimentation.
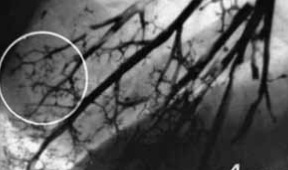 Figure 6: Bronchogram of the right lung (a fragment). Small bronchia and bronchioles are filled with tantalum microparticles.
Figure 6: Bronchogram of the right lung (a fragment). Small bronchia and bronchioles are filled with tantalum microparticles.
The investigation of aerodynamics of tantalum microparticles deposition on the walls of the initial departments of the digestive and respiratory tract revealed regional differences in the mechanisms of deposition (impaction, sedimentation or diffusion) resulting from anatomical and topographical peculiarities and aerodynamic laws operating in complicated air ducts. Thus, the precipitation of microparticles in the mouth, the oropharyngeal area, the pharynx, the pyriform sinuses and the larynx went on via impaction (inertial collision); in the trachea and large bronchia - via sedimentation (or impaction at the points of bifurcation); in small bronchia and bronchioles sedimentation was characterized by multilayer deposition and accumulation of microparticles on the walls, as well as constriction and clogging of the lumen, while diffusion occurred in the alveoli.
All the above-mentioned indicates that the upper parts of the digestive tract, where up to 95% of inhaled tobacco smoke microparticles are deposited, serve as a highly efficient organ protecting respiratory zones of the lungs from the harmful influence of pathogenic smoke components. However, with the very first cigarette smoked the multilayer accumulation of many thousands of millions of smoke microparticles injure the epithelium, paralyze clearance and clog the excretory ducts of salivary glands. All this predisposes to the appearance and propagation of inflammatory diseases. Moreover, it can be said with confidence that long-lasting and frequent smoking converts the upper parts of the digestive tract into a permanent storage of pathogenic products of tobacco smoke, infection agents and such likes.
Sections of different optical density (resp. the thickness of the layers) were seen on X-ray images indicating the mechanisms of microparticles deposition at different levels of the upper parts of the digestive and respiratory tracts. It is obviously true that morphological and functional changes in the mucosa fall into three groups according to their progression stage (Table 2).
Thus, differences in tantalum microparticles deposition in the upper sections of the digestive tract, the pharynx, the trachea and the bronchia are ruled predominantly by aerodynamic laws as applied to complicated airways. This reflects the degree of severity of morpho-functional changes in the mucous membrane in the areas of risk. Since composition and physical properties of tantalum microparticles are similar to those of solid particles of tobacco smoke and resin, our data may be considered as an illustrative model of the smoking process. Histological studies [6] and investigations with tantalum powder spraying showed that a lot of tiny gland output ducts opens on the mucosal surface of the upper parts of the digestive tract, trachea and large bronchia [7]. Obviously, as a result of prolonged smoking, the ostiums of these ducts become blocked with many billions of tobacco smoke and resin microparticles, which leads to the accumulation of the gland secret and emergence of closed and open retention cysts of endocrine glands. Their dimensions vary from the ones unseen by an ordinary eye to those 10 mm in size [8]. Hence, there is a good reason to believe that dilated excretory ducts and open cysts in the airways walls which are filled with tobacco smoke and resin microparticles serve as an anatomical foothold for the development of tumors, inflammation, and other pathological processes. In addition, 5% of tobacco smoke microparticles penetrating into the lower parts of the respiratory tract, create with every cigarette smoked a necessary and sufficient condition for both their deposition on the walls of the trachea, large and medium-size bronchia as well as accumulation in small bronchia, bronchioles and alveoli. In their turn, these zones represent areas of high risk for development of various pathologies and irreversible structural changes that remain for a long time a source of autointoxication and autoinfection even after a person quits smoking.
Modeling is one of the universal methods of cognition in all branches of natural sciences and medicine is of no exception. Generally, a model can represent any investigated process in several ways, so it is highly important to know beyond any doubt which of them would serve the best and which of the modeling techniques should be chosen. Clearly, the method exhibiting the closest analogy between the model and the process studied would be most preferable. Cicero eloquently formulates this paradigm in one of his works: «Inter visa vera aut falsa ad animi assensum nihil interest». (The mind does not have to choose if the choice is between truth and fiction).
As our investigation shows, the radiological model of smoking resembles very closely a real smoking process in many ways. It allows to trace gradually all stages of tantalum microparticles distribution in the initial parts of the digestive and respiratory tracts and their propagation through the whole length of the organs. Hence, it is evident that tobacco smoke microparticles are distributed and deposited just like those of tantalum, viz. they pass some parts and are held down due to natural anatomical barriers in others. Obviously, in those sections where tantalum particles accumulate in excess, the concentration of tobacco smoke solid components and, respectively, the thickness of the layer are also increased. When this is the case, the areas of multilayer tantalum accumulation are the zones of increased risk of inflammatory, tumoral or other pathological processes.
Neither endoscopy nor traditional radiological and radionuclide methods are able to elucidate these problems as clearly and convincingly as the radiological model does. As for mathematical models, including the latest experiments in silico, one cannot in fact consider them faultless. They reconstruct a whole process as some function with a limited number of arguments but they are inept to take into account the total diversity of specific features characterizing such manifold pathophysiological process as smoking.
One of the most important sideline effects of the undertaken tantalum spraying was the visualization of enlarged excretory ducts of exocrine glands on the walls of the trachea and large bronchia. Due to smoking, their orifices become plugged, which leads to the secret accumulation and formation of gland retention cysts. It is quite possible that both pathogens and tobacco smoke particles are captured in the output ducts and open cysts. Thus, to a greater or lesser extent, the protective function of the digestive and respiratory tracts is realized.
At the same time, we would like to emphasize that this medal also has the reverse side. It is known to which undesirable consequences a long retaining of antigens in tissues leads. This is one of the most important conditions for the development of autoimmune pathology, which is so widespread nowadays. The question of the exact location of antigen depots in case of autoimmune diseases remains open. It is unlikely that antigens are deposited in the organs of the mononuclear phagocyte system (resp. reticuloendothelial system), although this view is shared by a number of authors. Most likely, all things being equal, antigens are fully utilized in macrophages and other cells of the reticuloendothelial system. This being the case the question arises: where are the products that retain the antigenicity in toto deposited? The answer is, almost undoubtedly, the above-mentioned dilated excretory ducts and open cysts of exocrine glands in the upper parts of the digestive and the respiratory tracts. At present, this hypothesis can neither be confirmed nor refuted; however, it should be emphasized that it suggests one of the most promising directions for future inquiry.
Thus, simulation of smoking with a powder inhaler instead of a cigarette and tantalum powder used as a marker gave an authentic picture of deposition of tobacco smoke microparticles in the upper parts of the digestive and respiratory tracts. In other words, we offer a demonstrative radiological model of smoking that can be used for studying the physiological processes that occur when tobacco smoke microparticles interact with the mucosa of the upper sections of the digestive and respiratory tracts.
CONCLUSION
In course of current investigation, a roentgenological model of smoking was elaborated in which tantalum microparticles were used as markers and a special-purpose device was worked out for simulating the process of cigarette smoking. It was shown through usage of this model that physiological mechanisms of interaction of solid tobacco smoke microparticles and mucosa as well as their deposition are determined both by anatomical peculiarities of the upper parts of the digestive and respiratory tracts and by aerodynamic laws as applied to complex airways.
Using the proposed model of smoking for examining the mucosa of the upper parts of the digestive and respiratory tracts, we detected areas with degree III of optical density of the deposit (the thickness of the layer) that characterize the localization, expansion and distinctness of morpho-functional disturbances. Areas with optical density of degrees II and III can be recognized as hazardous zones and serve as a foothold for development of inflammatory processes and oncological diseases.
It's been established that 95% of microparticles contained in the tobacco smoke are deposited in the upper parts of the digestive tract and only 5% of them reach the lower respiratory ducts. When small and smallest bronchia as well as terminal respiratory bronchioles (usually in the lower lungs lobes) are filled with microparticles, there is a danger of regional reduction of lungs ventilating function and development of autoinfection, autointoxication and irreversible morphological changes in the respiratory lung tissue.
CONFLICT OF INTERESTS
The authors declare no conflict of interest. This study was not sponsored.
REFERENCES
- Arzhenovskiy SV (2006) Social and economic determinants of smoking in Russia. Kvantil 1: 81-100.
- Brodskaya TA, Nevzorova VY, Gel'tser BI, et al. (2009) Experimental modeling of smoking-related chronic obstructive lung diseases with vascular dysfunction. Bulletin SO RAMN 135: 60-65.
- Mestecky J, Lamm ME, McGhee JR, Bienenstock J, Mayer L, et al. (2005) Mucosal immunology. Elsevier Science Inc, San Diego, USA.
- Krishtafovich AA, Puchkova TV (2003) Functional activity of bronchial mucosa in tuberculosis and other pulmonary diseases. Problemy tuberkuleza i drugikh zabolevaniy legkikh 10: 17-20.
- Tager IB, Speize FE (1976) Risk estimates for chronic bronchitis in smokers: A study of male-female differences. Am Rev Respir Dis 113: 619-625.
- Sapin MR, Nikityuk VB, Shadlinskiy DB, Movsumov TN (2001) Small glands of the digestive and respiratory systems. Elista, Dzhangar.
- Krishtafovich AA, Ariel BM (2018) Exocrine gland cysts in tracheal and bronchial walls. Lung Breath J 2: 99-102.
- Arcavi L, Benovitz NL (2004) Cigarette smoking and infection. Arch Intern Med 164: 2206-2216.
Citation: Krishtafovich AA, Ariel BM (2020) Modeling of Tobacco Smoking and Smoke as Well as Resin Microparticles Residue in Respiratory Tract. J Pulm Med Respir Res 6: 044.
Copyright: © 2020 Krishtafovich AA, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.

