
Noonan Syndrome with Plastic Bronchitis in an Adult
*Corresponding Author(s):
Rupak SinglaDepartment Of Tuberculosis And Respiratory Diseases, National Institute Of TB And Respiratory Diseases, New Delhi, India
Email:drrupaksingla@yahoo.com
Abstract
Noonan syndrome is an autosomal dominant disease with low incidence. The incidence of Plastic bronchitis is not well defined. In Noonan syndrome, various lymphatic abnormalities have been described. Due to these abnormalities Plastic bronchitis may develop in the patients of Noonan syndrome. In the past, very few paediatric cases of Noonan syndrome with Plastic bronchitis with cardiovascular abnormalities that underwent Fontan operation had been reported. Till date, no adult case of Noonan syndrome with Plastic bronchitis has been reported. We are reporting a case of Noonan syndrome with Plastic bronchitis in an adult without any cardiovascular abnormality. Features like short stature, hypertelorism, widely spaced nipples and bilateral cryptorchidism were present in our case. The features of Noonan syndrome were absent in the siblings. The other associated findings in our case were right azygous lobe and laxity of abdominal wall musculature.
INTRODUCTION
Noonan syndrome is an autosomal dominant disease. The incidence of Noonan syndrome is reported to be between 1 in 1000 and 1 in 2500 live births [1,2]. Approximately half of patients are sporadic cases and represent new mutations. In rest 50 percentage, it is familial disorder consistent with autosomal dominant inheritance having variable expression. Missense mutation in gene PTPN11 (on chromosome 12q24) accounts for half of cases of Noonan syndrome [3]. Predominance of maternal transmission is noted in familial cases. This has been thought to be due to infertility in affected males which may be related to cryptorchidism. For this mild/subtle phenotype needs to be searched in parent of affected person. The incidence of Plastic bronchitis is not well defined. Various lymphatic abnormalities have been observed in the patients of Noonan syndrome including pulmonary and intestinal lymphangiectasia and lymphoedema [4]. Due to the lymphangitic abnormalities, plastic bronchitis may happen in these patients [5]. Few paediatric cases were reported of Noonan syndrome with plastic bronchitis in the past. They were also having cardiovascular abnormalities requiring Fontan operation [6,7]. We are reporting first case of Noonan syndrome in an adult patient who presented to us with plastic bronchitis without any cardiovascular abnormality.
CASE REPORT
A 36-year-old male, teacher, non-smoker, came to the hospital, with the complaints of progressive shortness of breath and cough with expectoration for two years. The shortness of breath and cough got relieved after expelling out the expectorant. The expectorant was white in colour with membranes with branching (Figure 1). The patient underwent right side inguinal hernia repair along with right side orchidopexy 3 years back. He started walking at the age of 6 years (motor development delay). He was infertile. There was no history of same complaints in other siblings. There was no history of atopy, recurrent childhood infections, swelling in the limbs, skin changes, and other systemic complaints.
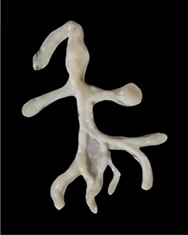 Figure 1: Expectorant with branching.
Figure 1: Expectorant with branching.
On examination the patient was having short stature (148 cm), short neck, microcephaly, hypertelorism, prominent nasolabial fold, high arched palate, widely spaced nipples, broad thorax, protuberant abdomen and cubitus valgus (Figures 2 to 5).
 Figure 2: Hypertelorism.
Figure 2: Hypertelorism.
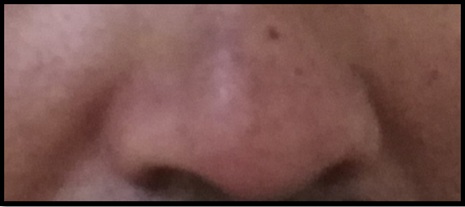 Figure 3: Prominent nasolabial fold.
Figure 3: Prominent nasolabial fold.
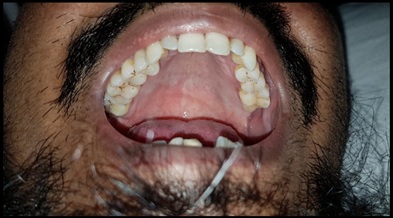 Figure 4: High arched palate.
Figure 4: High arched palate.
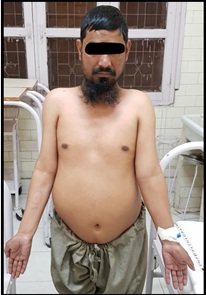
Figure 5: Widely spaced nipples with cubitus valgus.
He had oxygen saturation 92% at room air, pulse rate 120/min, blood pressure 110/70 mm Hg and respiratory rate 24/min. Grade 2 clubbing was present in the fingers. Respiratory system examination revealed low lying sternum with trachea central with bilateral chest symmetrical and bilateral air entry present without any wheeze and crepitations. On his abdomen examination, cough impulse was present in bilateral flanks (Supplementary Material 1). A firm swelling palpable in left inguinal canal (probably undescended testis). Cardiovascular system examination was within normal limit. Higher mental functions were intact. On Musculoskeletal examination, left scapular muscles atrophy was seen with decreased bulk and power (Figure 6). Chest X Ray was within normal limits (Figure 7).
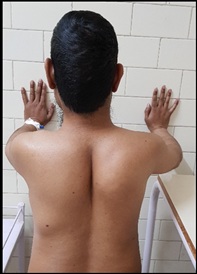 Figure 6: Left scapular muscular atrophy.
Figure 6: Left scapular muscular atrophy. 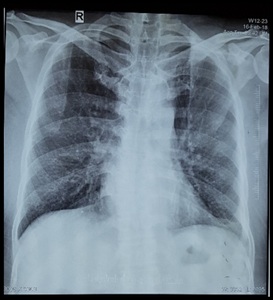 Figure 7: Chest X-Ray was within normal limits.
Figure 7: Chest X-Ray was within normal limits.
On Routine Investigations, haemoglobin was 12.3gm/dl, absolute eosinophil count was 250/mm3, liver and kidney function testing was normal. Peripheral blood smear was normal. Serum Total IgE was within normal limits. Serum precipitin for Aspergillus was negative.
Sputum for pyogenic and fungal culture were negative. Sweat Chloride level was 32 meq/L (Normal value
Electrocardiogram was normal. 2-Dimensional Echocardiography didn’t reveal any valvular defect, any motion abnormality or any septal defect.
Pulmonary function testing was suggestive of mild obstruction, moderate restriction and moderately reduced diffusion capacity -: Ratio of forced expiratory volume in 1 second to forced vital capacity, 65%; Forced expiratory volume in 1 second, 1.33 litres (49% of predicted); Forced Vital capacity, 2.07 litres (62% of predicted); Total lung capacity, 3.03 litres (60% of the predicted value); and diffusing capacity of the lungs by single breath for carbon monoxide, 12.2 ml/min per mm Hg (49% of predicted).
Arterial blood analysis at room air, revealed a partial pressure of oxygen of 67 mm Hg, a partial pressure of carbon dioxide of 33 mm Hg, standard bicarbonate 27.0 meq/L and apH of 7.47.
Histopathological examination of expectorant revealed acellular eosinophilic material and scattered inflammatory cells.
Computed tomography of chest and abdomen were done to look for other congenital abnormality. CECT Chest revealed right azygous lobe (Figure 8). CECT Abdomen (Figure 9) revealed thin and lax lateral abdominal wall on both sides. Oval heterogenous density in left iliac fossa extending up to deep inguinal ring with another tubular structure seen in left inguinal canal (funicular type of spermatic cord hydrocele with undescended testis).
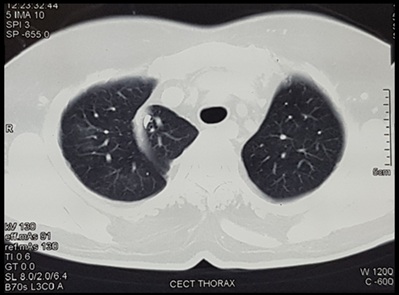 Figure 8: CECT Chest suggestive of Right Azygous lobe.
Figure 8: CECT Chest suggestive of Right Azygous lobe.
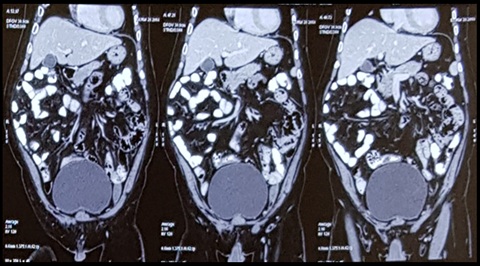 Figure 9: CECT Abdomen suggestive of thin and lax abdominal wall defects with left heterogenous opacity with tubular structure (funicular hydrocele with undescended testis).
Figure 9: CECT Abdomen suggestive of thin and lax abdominal wall defects with left heterogenous opacity with tubular structure (funicular hydrocele with undescended testis).
Fibreoptic Bronchoscopy (FOB) was within normal limit without any intraluminal mucous and secretions. It may be noted that FOB was done at a time when the patient had already expectorated the secretions as mentioned above (Figure 1).
Based on diagnostic criteria proposed by Van der Burg et al [8], as mentioned in table 1, diagnosis of Noonan syndrome was made. Our Patient had typical facial dysmorphology (1A), height <3rd centile (3A), broad thorax (4B), developmental delay (6B), cryptorchidism (6B) and therefore, diagnosis of Noonan syndrome were made (1A+3A). Genetic testing could not be done in our patient due to sparse resources.
|
Feature |
A = Major |
B = Minor |
|
1 Facial |
Typical face dysmorphology |
Suggestive face dysmorphology |
|
2 Cardiac |
Pulmonary valve stenosis, HOCM and/or ECG typical of NS |
Other defect |
|
3 Height |
||
|
4 Chest wall |
Pectus carinatum/excavatum |
Broad thorax |
|
5 Family history |
First degree relative with definite NS |
First degree relative with suggestive NS |
|
6 Other |
Mental retardation, cryptorchidism and lymphatic dysplasia |
One of mental retardation, cryptorchidism, lymphatic dysplasia |
Table 1: Diagnostic criteria of Noonan Syndrome proposed by Van der Burg et al [8]. Noonan syndrome is considered present if the patient has typical facial dysmorphology plus one feature from categories 2A through 6A or two categories from features 2B through 6B or has suggestive facial dysmorphology plus two features from categories 2A through 6A or three features from categories 2B through 6B.
The histopathological examination of the expectorant revealed acellular eosinophilic material with inflammatory cells and the classical branching of the expectorant which confirms the diagnosis of Plastic bronchitis. The patient was advised inhaled bronchodilators and inhaled steroids. Patient symptomatically improved and is under follow up.
DISCUSSION
Plastic bronchitis is a rare disease characterized by the formation of large gelatinous or rigid airway casts. The casts are generally associated with cyanotic congenital heart disease, acute chest syndrome associated with sickle cell disease and diffuse bronchial hypersecretory disorders, such as asthma, cystic fibrosis, allergic bronchopulmonary aspergillosis, bacterial or viral respiratory infection [6,9,10]. The cause of Plastic bronchitis may be attributed to various lymphatic abnormalities [11-13]. All these common causes have been excluded based on investigation reports mentioned in the case report. Restrictive pattern with reduced diffusion capacity of lung has been described in plastic bronchitis earlier also [14].
This patient had clinical features suggestive of Noonan syndrome as per diagnostic criteria [8]. Noonan syndrome was first recognized as a unique entity in 1963 when Noonan and Ehmke described a series of patients with unusual facies and multiple malformations, including congenital heart disease [15]. Noonan syndrome is a genetic multisystem disorder characterised by distinctive facial features, developmental delay, learning difficulties, short stature, congenital heart disease, renal anomalies, lymphatic malformations and bleeding difficulties [16]. The diagnosis of Noonan syndrome is made on distinct clinical features [17,18]. Lymphatic abnormality seems to be inherent in patients with Noonan Syndrome. About 20% of Noonan Syndrome patients may have lymphatic vessel dysplasia, hypoplasia or aplasia [19]. This may predispose the patients to develop plastic bronchitis [5].
Brogn et al [6], published a case series of 42 children with Plastic bronchitis. Among them 22 children had cardiovascular abnormality. 15 children had undergone some cardiac surgeries. Among 15 children, 10 had developed plastic bronchitis after Fontan operation. However, 7 children out of 22 were found to have plastic bronchitis prior to any cardiac surgery. In this case series, they also described a case of 3-year-old child with Noonan Syndrome who developed plastic bronchitis after right ventricular outflow tract resection. This study indicates that Plastic bronchitis may be associated with cardiovascular abnormality and cardiac surgical procedures. Noonan Syndrome with cardiovascular abnormalities, has been described in an adult also [17]. However, our patient of Noonan syndrome is an adult without any cardiovascular abnormality.
Sheetal et al. [19], also reported a case of 15-year-old male with Noonan syndrome who developed plastic bronchitis after bilateral orchidopexy.
The present case is probably the first case of Plastic bronchitis in Noonan syndrome in an adult. He didn’t have any cardio vascular abnormality. Other features like azygous lobe on the right side and abdominal wall musculature defect were additionally present in our patient. In adult patients presenting with branching expectoration suggestive of Plastic bronchitis, features of Noonan syndrome should also be explored.
CONFLICTS OF INTEREST
All authors declare that there is no conflict of interest regarding the publication of this paper.
REFERENCES
- Allanson JE (1987) Noonan syndrome. J Med Genet 24: 9-13.
- Sharland M, Burch M, McKenna WM, Paton MA (1992) A clinical study of Noonan syndrome. Arch Dis Child 67: 178-183.
- Gelb BD, Tartaglia M (2006) Noonan syndrome and related disorders: Dysregulated RAS-mitogen activated protein kinase signal transduction. Hum Mol Genet 15: 220-226.
- Hernandez RJ, Stern AM, Rosenthal A (1980) Pulmonary lymphangiectasis in Noonan syndrome. American Journal of Roentgenology 134: 75-80.
- Brett J (2016) Plastic bronchitis. In: Kliegman RB, Stanton B, Geme JW, Schor NF, Behrman RE (eds.). Nelson textbook of pediatrics Elsevier, Philadelphia, Pennsylvania. Pg no: 2049-2050.
- Brogan TV, Finn LS, Pyskaty Jr DJ, Redding GJ, Ricker D, et al. (2002) Plastic bronchitis in children: A case series and review of the medical literature. Pediatr Pulmonol 34: 482-487.
- Grutter G, Di Carlo D, Gandolfo F, Adorisio R, Alfieri S, et al. (2012) Plastic bronchitis after extracardiac Fontan operation. The Annals of thoracic surgery 94: 860-864.
- van der Burgt I, Berends E, Lommen E, van Beersum S, Hamel B, et al. (1994) Clinical and molecular studies in a large Dutch family with Noonan syndrome. Am J Med Genet 53: 187-191.
- Madsen P, Shah SA, Rubin BK (2005) Plastic bronchitis: New insights and a classification scheme. Paediatric respiratory reviews 6: 292-300.
- Bowen A, Oudjhane KA, Odagiri K, Liston SL, Cumming WA, et al. (1985) Plastic bronchitis: Large, branching, mucoid bronchial casts in children. American journal of roentgenology 144: 371-375.
- Itkin M, McCormack FX (2016) Nonmalignant adult thoracic lymphatic disorders. Clinics in chest medicine 37: 409-420.
- Lam K, Ruoss S (2018) Plastic Bronchitis: A Tale of Lymphatic Anomalies. American Thoracic Society 197: 6733.
- Norton M, Nichols F, Andrews J, Kern R, Kelm D (2018) Cast away: A Case of lymphatic plastic bronchitis with chyloptysis utilizing advanced lymphatic imaging. American Thoracic Society 201: 6439.
- Jett JR, Tazelaar HD, Keim LW, Ingrassia TS (1991) Plastic bronchitis: An old disease revisited. Mayo Clin Proc 66: 305-311.
- Noonan JA, Ehmke DA (1963) Associated noncardiac malformations in children with congenital heart disease. J Pediatr 63: 468-470.
- Roberts AE, Allanson JE, Tartaglia M, Gelb BD (2013) Noonan syndrome. The Lancet 381: 333-342.
- Khan F, Waqar S, Jamal NUA, Saleem A (2018) Noonan Syndrome: A Case Report. Annals of Health Research 4: 82-87.
- Poaty H, Mbika-Cardorelle A, Moukouma C, Mouko A (2017) Clinical diagnosis of Noonan syndrome and brief review of literature. Annals of Medical and Health Sciences Research 7: 76-79.
- Jagtap SR, Iyer HR, Bakhshi RG, Lahoti HN (2015) Post-operative airway obstruction in Noonan syndrome: An unusual presentation. Indian journal of anaesthesia 59: 442.
SUPPLEMENTARY MATERIAL
A video clip showing the cough impulse positivity in bilateral flanks regions has been attached (Supplementary material 1).
Citation: Kumar V, Goswami A, Anand S, Golani DD, Golani M, et al. (2021) Noonan Syndrome with Plastic Bronchitis in an Adult. J Pulm Med Respir Res 7: 058.
Copyright: © 2021 Vikas Kumar, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.

