
Novel Technique of Aesthetic Skin Rejuvenation with Cellular Extracted Compounds Enriched with Tissue-Specific Peptides and Hyaluronic Acid
*Corresponding Author(s):
Volodymyr ChernykhEuropean Wellness Aesthetic Academy, (EU, Asia-Pac), Klosterstrasse 205, Edenkoben, Germany
Email:dr.chernykh@ew-academy.com
Abstract
The structural composition of the skin is dynamic, exhibiting various signs of aging such as the decrease in collagen and elastin content leading to increase in tactile and visual roughness, dryness, wrinkles, fine lines and stiffness as age advances. This study was done to elicit the efficacy and potency of mesotherapy using a cocktail of triple strength nano-sized peptides from the skin, placenta, mesenchyme fortified with collagen and Hyaluronic Acid (HA) in the revitalisation of the skin of the dorsum of the hand. Ten male subjects suffering from mild signs of aging of the skin were treated with the formulation of MF+ SPMC + HA over the dorsum of both hands. The treatment course spanned over eight weeks with once a week application. Baseline assessment was done before the treatment and at the end of the first and second month and 90 days post-therapy, respectively, using photography, ultrasound comparison and biophysical parameters of the skin. The results proved the application of SPMC + HA rendered long-term hydration and improved the viscoelasticity of the treated area. The study concluded that mesotherapy application of MF+ SPMC extracts/peptides in combination with hyaluronic acid could be considered as a safe and effective method for rejuvenation of the skin of the dorsum of hands.
Keywords
Aesthetic medicine; Cell therapy; Cellular extracts; Mesotherapy; Skin rejuvenation; Stem cells
INTRODUCTION
Beauty may lie within the skin’s depth, but there is more to the skin than it appears on the surface. The physiological function of human skin is numerous, and some diseases of the skin might not just be deep [1]. Being the largest organ in the body, the skin is an essential flag of systemic illnesses [2]. The skin is engineered with the complex anatomical composition, which caters numerous vital functions [2].
The pathologies of skin are vast, and aging is one of the most common and inevitable conditions which displays both pathological and cosmetic symptoms (visible and tactile) such as dryness and roughness.These could be explained by collagen and elastin content of the skin, leading to impaired structural integrity and eventually the formation of deep and superficial creases and fine lines [3]. Microscopic analysis of aging skin would reveal common pathological changes which include significant exhaustion of the epidermal HA, flattening of the epidermal-dermal junction, reduced cell turnover rate, decreased melanocytes and their unusual activities [4]. These along with intracellular matrix and collagen dysfunction lead to physically visible conditions including dehydration, loss of skin turgor, skin atrophy, pigmentations, lentigo and keratosis. Skin peptides and HA have been implicated in age-related skin conditions [5-9], well-hydrated and youthful skin retains its turgor, elasticity and suppleness. The water content of the skin is a pivotal factor to regulate and maintain optimal physiological function [10]. The ultimate pathophysiology in aging has been explained by cellular and DNA damage by free radicals as well as mitochondrial dysfunction [11].
Hyaluronic acid
Hyaluronic Acid (HA) is most abundantly found in skin, about 50% of the total body content. Reed RK et al., [12] followed by the vitreous of the eye [13], umbilical cord [14] and the synovial fluid [15]. The HA or hyaluronate is a unique non-sulphated glycosaminoglycan, a relatively large molecule weighing upto 7 million Dalton [16] found commonly in connective, epithelial and neural tissues [14,17]. The repeating polymeric disaccharides of D-glucuronic acid and N-acetyl-D-glucosamine linked by a glucuronidic β (1→3) bond makes HA a unique molecule that forms numerous different types of stable polymers with an array of physiological properties, depending upon the configurations, and shapes, size, salt concentration, pH and associated cations [18]. In human body, HA is produced by the mesenchymal cells, primarily the peripheral connective tissues. The key feature of this molecule is its ability to release the high number of water molecules even at low concentration and high viscosity state [19]. As skin moisturization is vital in achieving skin beauty, commercial skin care products should include active compounds such as hyaluronic acid, which can bind water to attain water retention [20].
The era of cellular extracts
The consumption of placenta for cosmetic and therapeutic purposes is dated. Laboratory trials of placental extracts date back to the 30s by Filatov VP who demonstrated the halt of disease progression and rapid progress of healing by using placental extract [21]. Since the ’60s, women in China and Thailand have been documented to consume placenta from young mothers, which they believed is rich in array of bioactive compounds that are therapeutically beneficial [22]. Subsequently, in the 1970s, the same practice had been found in a small number of women in the United States of America and Mexico [23].
Hagmeier W [24] published excellent results with fetal mesenchyme - even in long-lasting defects, which were not successfully treated by other therapies. Based on his experience in large scalds and burns, F. Schmid developed a fetal tissue ultra filtrate for long-term topical applications, which was prepared by the extraction of fetal connective tissue, fetal skin placenta and adrenal gland. Two multi centric trials were designed to determine the list of indications. The following indications were listed for the fetal cutaneous extract: burns, scalds, irradiation burns on the skin injuries, explosion injuries, keloids, hyperkeratosis, anhidrosis syndromes, ulcus cruris, senile atrophy of the skin, trophic disturbances in circulatory trouble, Lyell syndrome, cosmetic lesions, keloids, sclerodermia [24,25].
Four decades of research and development of cell extracts witnessed a break through in the area. The therapeutic properties of cell extracts were further expanded in the world of aesthetics and anti-ageing and were seen to be widely used in India, Japan, Korea, Thailand and United States of America [26-30]. Subsequent years saw a different group of researchers and clinicians documenting positive therapeutic outcome using various kinds of placental preparations and components [31-34]. These include placental tissue, amniotic and chorionic membranes, amniotic fluid, umbilical cord, placental extracts and lyophilizates and cord blood serum, as well as number of differentiated cells and stem cells administered through different routes such as oral, subcutaneous and intramuscular [35-41].
The biological properties of placental peptides have been studied according to its active component. Instead of using the tissue, researchers have lysed placenta of full-term to obtain the bioactive components, an array of proteins, minerals, amino acids, and steroid hormones [31]. Besides cosmetic reasons, these extracts have been successfully used in the treatment of wounds, non-healing ulcers, and burns. Researchers documented an accelerated rate of reepithelisation and reduced infiltration and pain syndrome [36,42].
The past two decades are marked by invention and vast application of aesthetic formulations of organ-specific extracts from skin, placenta, and mesenchyme enriched with collagen via cold-enzymatic processing with further nano-filtration of biologically active ingredients since 2003. Nano-peptides show high bioavailability, biocompatibility and injectability due to the linear size of the peptides below ten nm and molecular weight below ten kDa. The above mentioned advantages not only increase the biomedical applications of the nano-sized product but provide a unique rejuvenating and revitalising effect of this compelling tissue therapy, which can be applied either via mesotherapy or topically.
Albeit being a novel treatment modality the idea of using a naturally-occurring peptides and cellular extracted compounds rich in growth factors and various biologically active compounds with anti-inflammatory, stimulatory and rejuvenation properties is not new and has being successfully used in aesthetic and therapeutic dermatological and even wound-care practice. Application of cell extracts from skin, placenta, mesenchyme with collagen and elastin in aesthetic dermatology and skin revitalization: evaluation of outcomes in cohort study [43-45].
The new dawn
Aesthetic dermatology has been of significant interest in recent years and it expands beyond the facial skin rejuvenation. The entire human body garnered much attention in this regard, with an increasing concern on the hands, especially the skin of the dorsum. A casual handshake can reveal the state of one’s skin. Rough, wrinkly and dry skin of the dorsum remains a major concern despite the emergence of store-available hand care products. As such, the revolutionary discovery of cellular extracts is promising to address the above mentioned issues.
The application of peptides and HA has been proven to successfully reverse the aging manifestation of the skin [46-48]. This combination works synergistically in halting aging process as well as to stimulate regeneration of the tissue. Various types of skin peptides embedded in a meshwork of hyaluronic network provide an optimal micro-environment for bioactive molecules [49-51]. An important key feature of the peptides in this cocktail is the ability to stimulate regeneration of fibroblasts and keratinocytes as well as collagen, elastin and extracellular matrix synthesis [52,53]. Besides being an excellent hydrating agent, the HA used in this preparation renders the structural integrity of dermal collagen [54]. HA has also been successfully used as a vector for targeted drug delivery, ensuring a more extended bioavailability [55].
OBJECTIVE
The objective of this study was to identify the efficacy, potency and safety of mesotherapy with nano-sized peptides from the skin, placenta and mesenchyme fortified with collagen and HA “SPMC + HA” in treating aging signs of the surface of the dorsum of the hand.
MATERIALS AND METHODS
This case study was performed at the clinical research facilities of the European Wellness centres. A total of 10 male patients with no significant concurrent illness were invited in the study. All 10 underwent eight treatment sessions using the cellular extracts concoction known as “SPMC + HA” (MF+ brand, Germany) and took part in the follow-up session. All patients were Asian; the mean age standard deviation was 48 ± 3.1 years old.
The used formulation “SPMC + HA” by MF+ brand (Germany) contains cells extracts from the skin, placenta, and mesenchyme with addition of pro-collagen peptides blended with HA. These compounds are dispersed in physiological saline solution (pH 7.0).Treatment sessions were performed across eight weeks at weekly interval.
All subjects were asked not to apply any local aesthetic treatments and to refrain from any form of commercial food supplements with rejuvenating and skin firming effects during the treatment. A follow-up visit was scheduled 90 days after the first treatment. A topical anaesthetic cream EMLA (Recipharm Karlskoga AB, Sweden) was applied to the area to be treated, and all patients were administered with injection in seated positions. Tiny amounts of the tested product (approximately 0.03 – 0.04 ml) were injected intradermally after the disinfection with an aqueous solution of chlorhexidine using a 33G needle (Beaumed, Korea) with a papule technique. The spacing between two injections was approximately 0,5 - to 1.0 cm apart; around 1 ml of the product was injected into the skin of the dorsum of both hands per session over an area of about 11x9 cm.
Assessments
Assessments were performed at the baseline, after the one, two months and the 90-day clinical follow-up visit. The next biophysical parameters were evaluated: stratum corneum hydration, measured using a capacitance device (the Corneometer CM825, Courage & Khazaka, Cologne, Germany); skin thickness, investigated ultrasonographically, using the Ultrascan C22 (Courage & Khazaka, Cologne, Germany)); and skin elasticity, using a Cutometer dual MPA 580 (Courage & Khazaka, Cologne, Germany). Three elasticity parameters were assessed: skin extensibility (the maximum extent to which the skin is stretched after applying a standard force, R0), gross elasticity (a measure of the ability of the skin to return to its original position after theforce is off, R2), and viscoelasticity (the ratio of extensibility caused by the skin’s viscous vs elastic components, R6).
The measurements were conducted under air-conditioned conditions (temperature 22.5±1.5°C; relative humidity 50±10%) after an acclimatisation period at least 20 minutes. Digital photographs were taken to evaluate treatment success at the 90-day follow-up treatment using a Canon IOS R camera (Japan). Every patient was photographed at the same distance from the camera using standardized light conditions. Patients also provided written consent for the photographs to be published. Adverse events observed by the investigator or reported by the patient were recorded.
Data were summarized, and analysis of variance was used to compare means. Pair-wise comparisons controlling for the family-wise error rate were done using the Tukey test (p≤.05).
Hydration
Moisturizing increased continuously during the study period. Statistically significant gradual increases from baseline in capacitance values were obtained during the trial period (p
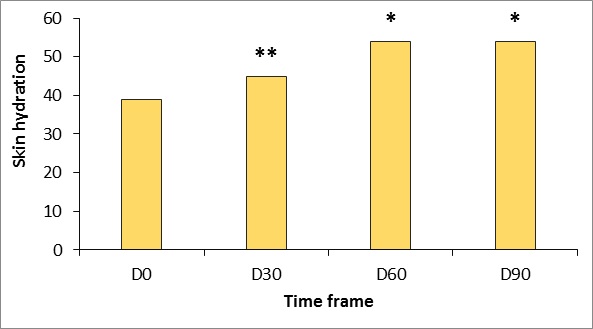 Figure 1: Changes in stratum corneum hydration measured using the Corneometer CM825 (Courage & Khazaka, Germany).
Figure 1: Changes in stratum corneum hydration measured using the Corneometer CM825 (Courage & Khazaka, Germany).
*p
Skin thickness
Ultrasound measurements reflect a continuous increase in dermal thickness within the study period (Figures 2 and 3). Increases over baseline were statistically significant from 30 days after the first treatment (p<.05) and were highly significant (p<.001) at D60 and D90. 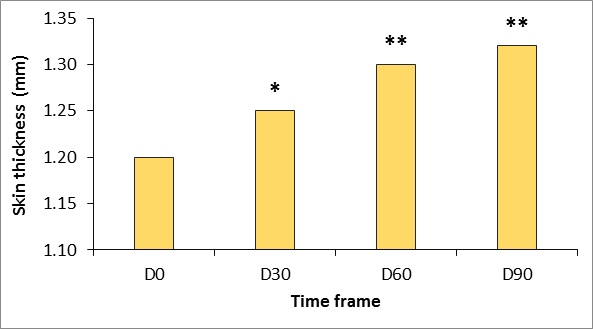
Figure 2: Changes in skin thickness measured using Ultrascan 22 (Courage & Khazaka, Cologne, Germany).
* p
(A) (B)
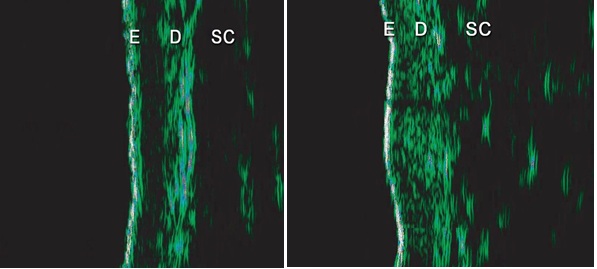
Figure 3: Ultrasound images were taken at (A) baseline (D0) and (B) 90 days after initial treatment (D90) using Ultrascan22, (Courage & Khazaka, Cologne, Germany). E - Epidermal entrance echo; D - Dermis; SC -subcutaneous layer.
The increased echogenicity that was evidenced at the end of the treatment is related to an increased density of dermal collagen fibres resulting from treatment.
Elasticity
Generally there was a good clinical response to the tested product “SPMC + HA” characterized by dramatic improvement of the gross elasticity of the skin: after pressure was removed from the suction device, the skin comes back to its original position much faster after treatment (p
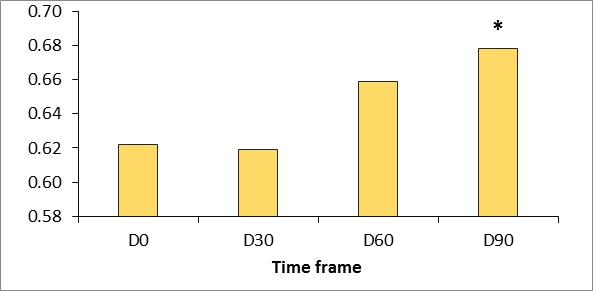 Figure 4: Gross elasticity measured using the Cutometer dual MPA 580 (Courage & Khazaka, Germany).
Figure 4: Gross elasticity measured using the Cutometer dual MPA 580 (Courage & Khazaka, Germany).
*p <0.5.
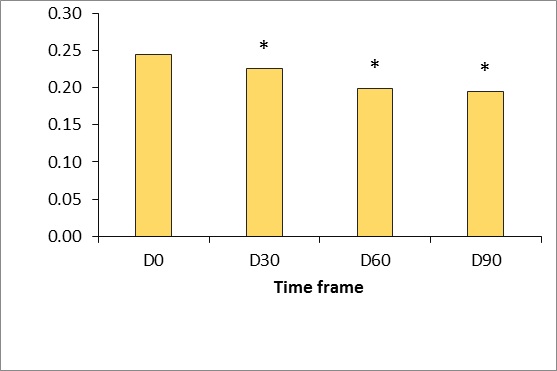
Figure 5: Gross viscoelasticity measured using the Cutometer dual MPA 580 (Courage & Khazaka, Germany).
*p <0.5.
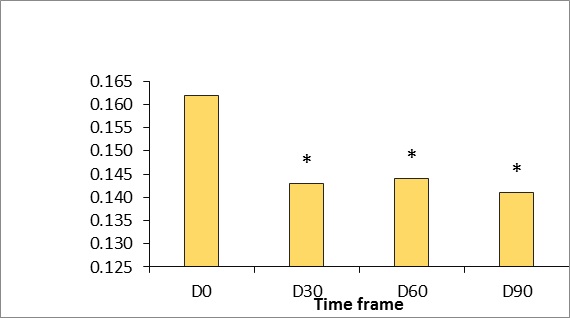 Figure 6: Gross skin extensibility measured using the Cutometer dual MPA 580 (Courage & Khazaka, Germany).
Figure 6: Gross skin extensibility measured using the Cutometer dual MPA 580 (Courage & Khazaka, Germany).
*p <0.5.
Clinical photographic evaluation
Comparisons of photographs taken before and after treatment showed that the skin on the backside of both hands looked much more hydrated, radiant and smoother (Figure 7). 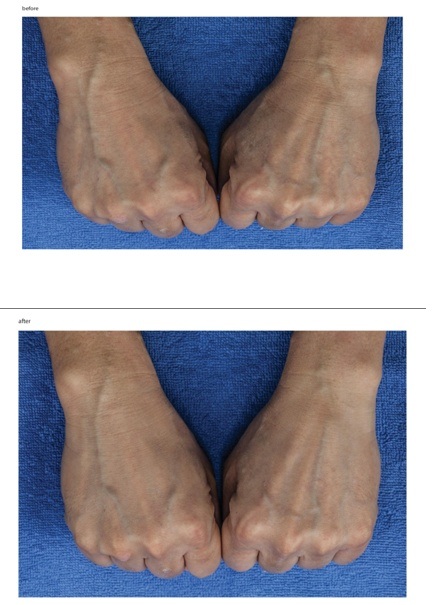
Figure 7: Clinical photographic evaluation before and after treatment.
Adverse events
Product was well tolerated. Unexpected adverse events weren’t reported. All expected treatment-related adverse events, such as pain and discomfort at the injection site, bruising, and the hematoma were transient.
CONCLUSION
Overall the achieved outcomes of the “SPMC+ HA” clinical application were therapeutically and cosmetically laudable. The clinical outcome of wrinkle-free, supple, vitalised as well as rejuvenated and pigment-free skin of the hand’s dorsum is the expected result of this novel strategy. Treatment resulted in a gradual thickening of the dermis, and increased hydration of the horny layer and the enhancement in the gross elasticity of the skin was enhanced. These biophysical changes transformed into visible changes with the decrease in skin wrinkles during the clinical follow-up period.
The tested product “SPMC + HA” (MF+, Germany) provides long-lasting and promising results of mesotherapy. This combination should be made available as one of the primary therapeutic approach in treating aging changes of the skin, taking into consideration the existing guidelines. Further studies to investigate and demonstrate the longer-term effects of “SPMC + HA” for facial rejuvenation with multiple treatment cycles and investigate the efficacy of combining treatment modalities would be of interest.
DISCLAIMER
Authors declare no conflict of interests and no direct commercial benefit from this publication.
REFERENCES`
- Kanitakis J (2002) Anatomy, histology and immunohistochemistry of normal human skin. Eur J Dermatol12: 390-401.
- James WD, Berger TG, Andrews GE, Elston DM (2006). Andrews' diseases of the skin: Clinical dermatology(10th edn), Elsevier Saunders, Philadelphia, USA.
- Glogau RG (2003) Systemic evaluation of the aging face. In: Bolognia JL, Jorizzo JL, Rapini RP. Dermatology,Edinburgh, Mosby, Pg No’s: 2357-2360.
- Grove GL (1989) Physiologic changes in older skin. Clin Geriatr Med 5: 115-125.
- Smith JG Jr, Davidson EA, SAMS WM Jr, Clark RD (1962) Alterations in human dermal connective tissue with age and chronic sun damage. J Invest Dermatol 39: 347-356.
- Lavker RM (1979) Structural alterations in exposed and unexposed aged skin. J Invest Dermatol 73: 559-566.
- Pieraggi MT, Julian M, Bouissou H (1984) Fibroblast changes in cutaneous aging. Virchows Arch A Pathol Anat Histopathol 402: 275-287.
- Marks R (1992) London: Martin Dunitz; Sun-Damaged Skin.
- Lavker RM. Gilchrest BA, Cambridge MA (1995) Blackwell Science; Cutaneous aging chronologic versus photoaging, Photoaging, Pg No’s: 123-135.
- Klokol D, Tulina D, Teppone M, Chan M, Wong M, et al. (2016) Application of cell extracts from skin, placenta, mesenchyme with collagen and elastin in aesthetic dermatology and skin revitalization: evaluation of outcomes in cohort study. Am J Adv Drug Deliv 4.
- Pan SY, Chan MKS, Wong MBF, Klokol D, Chernykh V (2017) Placental therapy: An insight to their biological and therapeutic properties. J Med Therap 1: 1-6.
- Klokol D, Nallenthiran L, Chan MKS, Wong MBF, Chernykh V, et al. (2019) Cell therapy as the main stratagem of anti–aging and regenerative medicine. Europ J Pharm Med Res 6: 295-299.
- Farage MA. Miller KW. Maibach HI (2010) Degenerative changes in aging skin. In: Farage MA, editor; Miller KW, editor; Maibach HI, editor. Textbook of Aging Skin.Berlin/Heidelberg: Springer-Verlag, Pg No’s. 25-35.
- Hamanaka RB, Chandel NS (2009) Mitochondrial reactive oxygen species regulate hypoxic signaling. Curr Opin Cell Biol 21: 894-899.
- Reed RK, Lilja K, Laurent TC (1998) Hyaluronan in the rat with special reference to the skin. Acta Physiol Scand 134: 405-411.
- Meyer K, Palmer JW (1934) The Polysaccharide of the vitreous humor. J Biol Chem 107: 629-634.
- Weissmann B, Meyer K (1954) The structure of hyalobiuronic acid and hyaluronic acid from the umbilical cord. J Am Chem Soc 76: 1753-1757.
- Weissmann B, Meyer K, Sampson P, Linker A (1954) Isolation of oligosaccharides enzymatically produced from hyaluronic acid. J Biol Chem 208: 417-429.
- Hamerman D, Schuster H (1958) Hyaluronate in normal human synovial fluid. J Clin Invest 37: 57-64.
- Fraser JR, Laurent TC, Laurent UB (1997). Hyaluronan: its nature, distribution, functions and turnover. J Intern Med242: 27-33.
- Laurent TC (1970) Structure of hyaluronic acid. In: Balazs, EA, ed. Chemistry and Molecular Biology of the Intercellular Matrix, Academic Press, New York, Pg No. 703.
- Turino GM, Cantor JO (2003) Hyaluronan in respiratory injury and repair. Am J Respir Crit Care Med 167: 1169-1975.
- Olejnik A, Goscianska J, Nowak I (2012) Significance of hyaluronic acid in cosmetic industry and aesthetic medicine. Chemik 66:129-135.
- Filatov VP (1944) Tissue Therapy in Ophthalmology. Am Rev Soviet Med 2: 53-66.
- Ober WB (1979) Notes on placentophagy. Bull N Y Acad Med55: 591-599.
- Selander J, Cantor A, Young SM, Benyshek DC (2013) Human maternal placentophagy: a survey of self-reported motivations and experiences associated with placenta consumption. Ecol Food Nutr52: 93-115.
- Hagmaier W (1978) Erfolgreiche Behandlung von Weichteilverletzungen mit Nekrosen. Eiterungen, osteomyelitischen Vertinderungen und unijberbrackbaren Hautdefekten durch lyophilisiertes Mesenchym (Resistocell). Cytobiol Rev 2: 36-38.
- Schmid F (1983) Cell Therapy: A New Dimension of Medicine. Ott Publishers Thoune, Switzerland, Pg No’s: 336-347.
- Chakraborty PD, Bhattacharyya D (2005) In vitro growth inhibition of microbes by human placental extract. Curr Sci88: 1745-1749.
- Kawakatsu M, Urata Y, Goto S, Ono Y, Li TS (2013) Placental extract protects bone marrow-derived stem/progenitor cells against radiation injury through anti-inflammatory activity. J Rad Res54: 268-276.
- Marleau AM, Mcdonald G, Koropatnick J, Chen C, Koos D (2012) Reduction of Tumorigenicity by Placental Extracts. Anticancer Res32: 1153-1161.
- Park SY, Phark S, Lee M, Lim JY, Sul D (2010) Anti-oxidative and anti-inflammatory activities of placental extracts in benzo[a]pyrene-exposed rats. Placenta31: 873-879.
- Poompruek P, Boonmongkon P, Guadamuz TE (2014) 'For me… it's a miracle': Injecting beauty among kathoeis in a provincial Thai city. Int J Drug Policy25: 798-803.
- Zheng J (2012) Recent Advances in Research on the Human Placenta. BoD – Books on Demand.
- Silini AR, Cargnoni A, Magatti M, Pianta S, Parolini O (2015) The long path of human placenta, and its derivatives, in regenerative medicine. Front Bioeng Biotechnol 3: 162.
- Cole LA (2010) Biological functions of hCG and hCG-related molecules. Reprod Biol Endocrinol 8: 102.
- Riboh JC, Saltzman BM, Yanke AB, Cole BJ (2016) Human amniotic membrane-derived products in sports medicine: basic science, early results, and potential clinical applications. Am J Sports Med 44: 2425–2434.
- Lee KH, Kim TH, Lee WC, Kim SH, Lee SY, et al. (2011) Anti-inflammatory and analgesic effects of human placenta extract. Nat Prod Res 25: 1090-1100.
- Chandanwale A, Langade D, Mohod V, Sinha S, Ramteke A, et al. (2008) Comparative evaluation of human placental extract for its healing potential in surgical wounds after orthopaedic surgery: an open, randomised, comparative study. J Indian Med Assoc106: 405-408.
- Park JY, Lee J, Jeong M, Min S, Kim SY, et al. (2014) Effect of Hominis Placentaon cutaneous wound healing in normal and diabetic mice. Nutr Res Pract 8: 404-409.
- Park SB, Kim KN, Sung E, Lee SY, Shin HC (2016) Human placental extract as a subcutaneous injection is effective in chronic fatigue syndrome: a multi-center, double-blind, randomized, placebo-controlled study. Biol Pharm Bull 39: 674-679.
- Erdem E, Yagmur M, Harbiyeli I, Taylan-Sekeroglu H, Ersoz R (2014) Umbilical cord blood serum therapy for the management of persistent corneal epithelial defects. Int J Ophthalmol7: 807-810.
- Reddy BY, Jow T, Hantash BM (2012) Bioactive oligopeptides in dermatology: Part II. Exp Dermatol 21: 569-575.
- Toole BP (2004) Hyaluronan: from extracellular glue to pericellular cue. Nat Rev Cancer 4: 528-539.
- Chen B, Miller RJ, Dhal PK (2014) Hyaluronic acid-based drug conjugates: state-of-the-art and perspectives. J Biomed Nanotechnol 10: 4-16.
- Cargnoni A, Di Marcello M, Campagnol M, Nassuato C, Albertini A, et al. (2009) Amniotic membrane patching promotes ischemic rat heart repair. Cell Transplantation 18: 1147-1159.
- Malhotra C, Jain AK (2014) Human amniotic membrane transplantation: different modalities of its use in ophthalmology. World J Transplant4: 111-121.
- Shukla VK, Rasheed MA, Kumar M, Gupta SK, Pandey SS (2004) A trial to determine the role of placental extract in the treatment of chronic non-healing wounds. J Wound Care 13: 177-179.
- Rotunda AM, Kolodney MS (2006) Mesotherapy and phosphatidylcholine injections: historical clarification and review. Dermatol Surg 32: 465-480.
- Tosti A, De Padova MP (2007) Atlas of mesotherapy in skin rejuvenation. London: Informa Healthcare, UK.
- El-Domyati MM, Attia SK, Saleh FY, Ahmad HM, Uitto J (2004) Effect of topical tretinoin on photoaged facial skin: a histometric, immunohistochemical and ultrastructural study. J Cosmet Dermatol 3: 191-201.
- Slevin M, Krupinski J, Gaffney J, Matou S, West D (2007) Hyaluronan-mediated angiogenesis in vascular disease: uncovering RHAMM and CD44 receptor signaling pathways. Matrix Biol 26: 58-68.
- Slevin M, Kumar S, Gaffney J (2002) Angiogenic oligosaccharides of hyaluronan induce multiple signaling pathways affecting vascular endothelial cell mitogenic and wound healing responses. J Biol Chem. 277: 41046-41059.
- Turley EA (1989) The role of a cell-associated hyaluronan-binding protein in fibroblast behaviour. Ciba Found Symp 143: 121-133.
- Reddy B, Jow T, Hantash BM (2012) Bioactive oligopeptides in dermatology: Part I. Exp Dermatol 21: 563-568.
Citation: Chernykh V, Nallenthiran L, Yemeliyanova M (2020) Novel Technique of Aesthetic Skin Rejuvenation with Cellular Extracted Compounds Enriched with Tissue-Specific Peptides and Hyaluronic Acid. J Stem Cell Res Dev Ther 6: 032.
Copyright: © 2020 Volodymyr Chernykh, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.

