
Organophosphorus Compound Poisoning: Initial Data Providing Evidence of the Presence of Cholinesterase within the Cerebrospinal Fluid and Early Neurovascular Inflammation
*Corresponding Author(s):
Goulay RDepartment Of Toxicology And Chemical Risks, French Armed Forces Biomedical Research Institute, Bretigny Sur Orge, France
Tel:+33 0178651141,
Email:romain.goulay@chemdef.fr
Abstract
The key role of the Cerebrospinal Fluid (CSF) in solute transport and clearance through the brain makes it an interesting way to deliver new therapeutic agents, by avoiding the complicated passage of the Blood Brain Barrier (BBB). Organophosphorus intoxication may lead to BBB breakdown, neuroinflammation and cellular damage within the brain, initially as a consequence of Cholinesterase Inhibition (ChE). In this pilot study, a swine model of paraoxon intoxication has been used to prove the existence of ChE activity within the CSF and early brain inflammation. This CSF ChE activity is strongly inhibited following exposure to paraoxon. We also provide evidence that despite the lack of brain immune cells activation 6 hours after poisoning, vascular cells adhesion molecule is expressed by endothelial cells. This is the first time that CSF ChE activity is described in a large animal model. The role and origin of ChE in the CSF remains unclear and has to be explored. This finding can be useful to develop new therapeutic strategies to offset post intoxication brain cholinesterase inhibition. Vascular inflammation should be considered and cured to avoid related post intoxication brain disorder.
Keywords
Cerebrospinal fluid; Cholinesterase; Delayed neuropathy; Inflammation; Organophosphorus compound
INTRODUCTION
Organophosphorus Compounds (OP) irreversibly inhibit Cholinesterases (ChE) leading to a cholinergic syndrome, epileptic seizures and seizure-related brain damage, and for some of them organophosphorus-induced delayed neuropathy [1]. Despite the efficacy of existing drugs like ChE reactivators to prevent death, they have difficulties to cross the Blood Brain Barrier (BBB) and they cannot fully prevent damage in the central nervous system [2]. The Cerebrospinal Fluid (CSF) plays a key role in solute transport through the brain and has a major importance for the clearance of toxic molecules and metabolites [3]. This transport system notably depends on the integrity of the subarachnoid/perivascular spaces and the blood brain barrier [4,5] and could be used as a new therapeutic vector for drug accessibility to the CNS in case of OP intoxication. As a prerequisite, we studied the ChE activity within the blood and the CSF in an original large animal model consisting in juvenile male swine intoxicated by Paraoxon (POX), the metabolite of the OP pesticide parathion [6]. We also evaluated the impact of POX intoxication on the cerebral parenchyma inflammation processes, which could lead to early and delayed neurological effects.
MATERIALS AND METHODS
Animals
Animal care procedures were carried out in accordance with those promoted by our institute animal care and use committee in compliance with French and European laws and regulations on laboratory animals. N=12 male Large White/Landrace swine (Sus scrofa scrofa, 24 +/- 2.1kg) were purchased from a local supplier and housed in groups in an approved animal facility at the institute.
Intoxication
Animals received an intravenous injection of ethyl-paraoxon dissolved in 2mL saline solution containing 10% isopropanol (iPrOH), through an ear vein catheter. Intoxicated swine were split in two groups: Short IV injection and Slow 5-min long IV injection. Every intoxicated swine received a different dose as described in table 1.
|
Animal |
Group |
Intoxication |
Clinical Symptoms |
Respiratory Assistance |
Time to Death |
|
1 |
CTRL |
No |
No |
No |
6 h |
|
2 |
CTRL |
No |
No |
No |
6 h |
|
3 |
CTRL |
No |
No |
No |
6 h |
|
4 |
CTRL |
No |
No |
No |
6 h |
|
5 |
CTRL |
No |
No |
No |
6 h |
|
6 |
POX 0.1mg/kg |
0.1mg/kg IV |
Chewing hypersecretion short apnea |
No |
Euthanized 1 h |
|
7 |
POX 0.3mg/kg |
0.3mg/kg IV |
Chewing apnea |
3 to 60 min |
1 h |
|
8 |
POX 1mg/kg |
1mg/kg IV |
Hypersecretion chewing limb tremors apnea |
4 to 30 min |
35 min |
|
9 |
POX 1mg/kg |
5mg/kg IV |
Hypersecretion whole body tremors apnea |
5 to 35 min |
36 min |
|
10 |
POX 5mg/kg |
0.1mg/kg Slow IV |
Limb hyperextension localized tremors |
no |
6 h |
|
11 |
Slow POX 0.1mg/kg |
0.2mg/kg Slow IV |
Fasciculations muscles contraction hypersecretion chewing apnea |
9 min 30 to 60 min |
6 h |
|
12 |
Slow POX 0.2mg/kg |
0.2mg/kg Slow IV |
Fasciculations |
no |
6 h |
Table 1: Animal groups and their major clinical signs from intoxication to the time of death.
Tissue sampling and evaluation of the enzymatic activity
Swine brains were extracted from the skull directly after animal sacrifice, carefully dissected in cold water, and prepared for immunochemistry as described in the supplementary information. Blood and CSF samples were collected at time of sacrifice. ChE, Acetylcholinesterase (AChE) and Butyrylcholinesterase (BChE) activities were measured in whole blood and CSF, in duplicate, according to the protocol described by Ellman and Callaway in 1961, a modified Ellman’s assay [7].
RESULTS AND DISCUSSION
To validate our intoxication protocol, we first checked the blood ChE activity (Figure 1). Our results show that for POX doses from 0.3 to 5mg/kg, total ChE, AChE and BChE are strongly inhibited. Residual enzymatic activity below 20% is associated with prolonged apnea and rapid death of the animal (Table 1; Figure 1). However, for doses of 0.2 and 0.1mg/kg, 60 % of residual BChE activity seems sufficient to preserve the animal from death despite drastic inhibition of the AChE activity (Figure 1; <10% for POX 0.1mg/kg). Despite the AChE predominance on BChE within the blood (4.11x10-4 vs. 6.10x10-5 U.g.dL-1), surviving animals show at least 30% of residual blood ChE activity, originating from the limited inhibition of the BChE (Figure 1). This in accordance with findings in human cases [8].
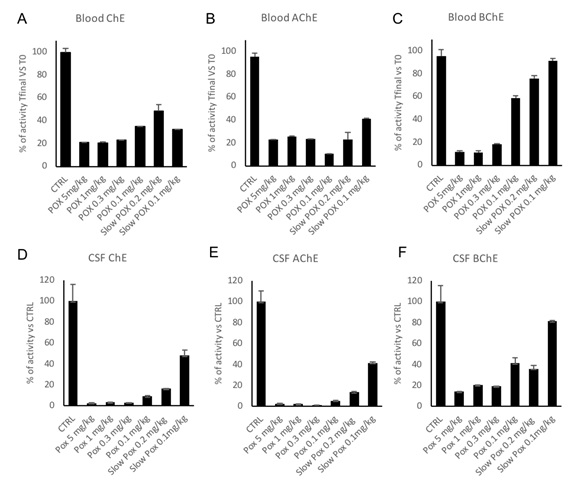 Figure 1: Blood and CSF cholinesterases activity. Figure A, B and C represent respectively the blood total ChE, AChE and BChE activities at the time of death (Tfinal) for each group or single animal. This final enzymatic activity is compared and normalized to the basal enzymatic activity for each animal, evaluated before the intoxication. Figure D, E and F represent respectively the CSF total ChE, AChE and BChE activity normalized on the control group CSF enzymatic activity. Results are presented in average+SEM.
Figure 1: Blood and CSF cholinesterases activity. Figure A, B and C represent respectively the blood total ChE, AChE and BChE activities at the time of death (Tfinal) for each group or single animal. This final enzymatic activity is compared and normalized to the basal enzymatic activity for each animal, evaluated before the intoxication. Figure D, E and F represent respectively the CSF total ChE, AChE and BChE activity normalized on the control group CSF enzymatic activity. Results are presented in average+SEM.
Within the CSF, total ChE activity is completely inhibited in non-surviving animals, due to 98% inhibition of the AChE activity (Figure 1). As described in the blood, BChE activity is minor in comparison with AChE activity within the CSF (3.40x10-5 vs. 2.35x10-4 U.mg-1). In 0.1mg/kg and Slow 0.2mg/kg surviving animals, total ChE are more inhibited in the CSF than in the blood, suggesting that even if the symptoms are less visible, CSF ChE inhibition may lead to effects (Figure 1). Last but not least, the total ChE activity inhibition is modulated by the injected dose of paraoxon in a dose dependent way and by the injection rate. These results support the existence of a significant cholinesterase activity in the CS, that is subject to inhibition by paraoxon. Organophosphorus compounds do penetrate into CSF after peripheral administration [9].
The origin and the molecular form of these cholinesterases and more interestingly, their role in a potential CSF cholinergic system are worth further studies.
Mechanisms that lead from intoxication to early and delayed neurotoxic effects involve neuroinflammation [10]. We thus decided to briefly explore inflammation biomarkers (astrocyte activation, microglial activation and expression of Vascular Cell Adhesion Molecule) in the 3 surviving swine exposed to slow IV to 0.1(n=1) and 0.2(n=2)mg/kg paraoxon.
Quantification of GFAP and IbA1 (Figure 2) does not reveal any difference between control animals and animals exposed to 0.1 or 0.2mg/kg POX at 6 h post intoxication. This may be explained by the absence of seizures, and possibly by a time point too early to detect a significant immune cells activation [11].
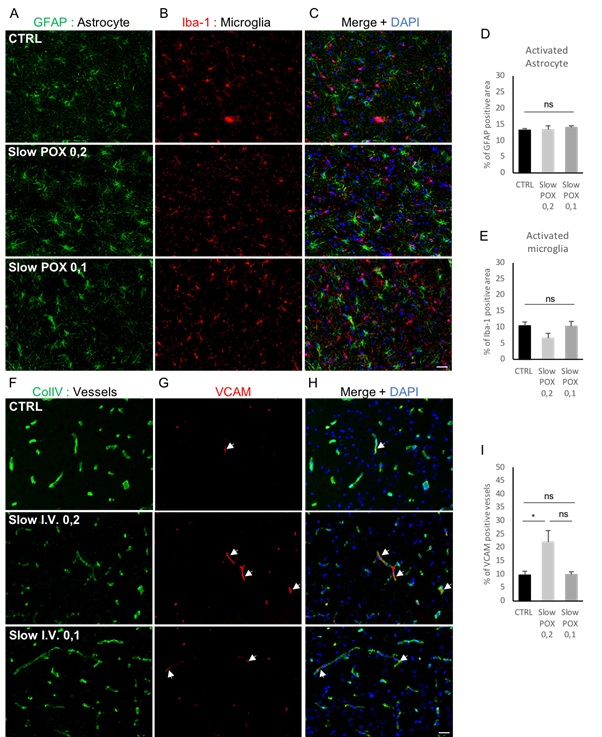 Figure 2: Evaluation of cerebral inflammation biomarkers after a POX intoxication. Astrocyte activation was quantified with an evaluation of the GFAP staining (A-D). Microglial activation was quantified using Iba-1 (B-E). A ColIV staining was performed (F) associated with VCAM (G) to visualize and quantifiy the percentage of VCAM positive vessels (H-I). Inflammatory vessels are indicated with white arrows. Results are expressed as mean+SEM. Scale bar=20μm. *p<0.05, ns p>0.05. Kruskal-Wallis test.
Figure 2: Evaluation of cerebral inflammation biomarkers after a POX intoxication. Astrocyte activation was quantified with an evaluation of the GFAP staining (A-D). Microglial activation was quantified using Iba-1 (B-E). A ColIV staining was performed (F) associated with VCAM (G) to visualize and quantifiy the percentage of VCAM positive vessels (H-I). Inflammatory vessels are indicated with white arrows. Results are expressed as mean+SEM. Scale bar=20μm. *p<0.05, ns p>0.05. Kruskal-Wallis test.
By contrast, Vascular Cells Adhesion Molecules (VCAM) are expressed early by endothelial cells to recruit macrophages from the blood. We observed that endothelial cells are expressing about 2x more VCAM to start macrophages recruitment at 0.2mg/kg POX than the controls (Figure 2, Slow POX 0.2 vs. CTRL, p<0.05). It is worth noting that for the dose of 0.1-mg/kg POX swine showed fewer clinical symptoms (Table 1) than at higher doses and that this was correlated to a lack of neurovascular inflammation, VCAM expression level being not different from that of the CTRL group (Figure 2, Slow POX 0.1 vs. CTRL, p>0.05). The presence of 10% VCAM reactive vessels in the CTRL group may be explained by the inflammation reaction due to the placement of the arterial and venous catheters used for the monitoring. The local inflammation may then spread through the expression of inflammatory molecules as recently described in bypass surgery [12].
Except for organophosphorus induced delayed neuropathy, the long-term effects of OP poisoning stay unclear. Cognitive decline, dementia or neuropathy might be caused by a post poisoning neurotoxicity or myelin impairment as observed in multiple sclerosis [13,14]. Therefore, it would be interesting to study neuronal death, myelinization status and the different factors regulating these processes in this model and their relationship with cholinesterase’s activity.
CONCLUSION
This pilot study has been useful to prove the presence of cholinesterases within determinate if using a nose-to-brain technic or an intrathecal injection to deliver drugs within the CSF can be useful to reactivate cholinesterases and preserve the brain from intoxication effects [15,16]. We have also demonstrated here that early neurovascular inflammation is visible in surviving pigs. Even in the absence of any significant visible CNS involvement in anesthetized swine, these results call for further evaluation of the central inflammatory status following POX or other organophosphorus compound poisoning.
ACKNOWLEDGEMENT
This work used the Imagery platform of IRBA supported by the Service de Santé des Armées. We thank Mr Xavier Butigieg for technical support and The authors declare that they have no competing interest.
REFERENCES
- Costanzi S, Machado JH, Mitchell M (2018) Nerve agents: What they are, how they work, how to counter them. ACS Chem Neurosci 9: 873-8
- Masson P, Nachon F (2017) Cholinesterase reactivators and bioscavengers for pre- and post-exposure treatments of organophosphorus poisoning. J Neurochem 2: 26-
- Jessen NA, Munk AS, Lundgaard I, Nedergaard M (2015) The glymphatic system: A beginner’s guide. Neurochem Res 40: 2583-25
- Goulay R, Flament J, Gauberti M, Naveau M, Pasquet N, et al. (2017) Subarachnoid hemorrhage severely impairs brain parenchymal cerebrospinal fluid circulation in nonhuman primate. Stroke 48: 2301-230
- Ueno M, Chiba Y, Murakami R, Matsumoto K, Kawauchi M, et al. (2016) Blood-brain barrier and blood-cerebrospinal fluid barrier in normal and pathological conditions. Brain Tumor Pathol 33: 89-
- Lorke DE, Nurulain SM, Hasan MY, Ku?a K, Petroianu GA (2019) Oximes as pretreatment before acute exposure to paraoxon. J Appl Toxicol.
- Ellman GL, Callaway E (1961) Erythrocyte cholinesterase-levels in mental patients. Nature 192: 1216.
- Thiermann H, Zilker T, Eyer F, Felgenhauer N, Eyer P, et al. (2009) Moniroting of neuromuscular transmission in organophosphate pesticidepoisoned patients. Toxicol Lett 191: 297-304.
- Göransson-Nyberg A, Fredriksson SA, Karlsson B, Lundström M, Cassel G (1998) Toxicokinetics of soman in cerebrospinal fluid and blood of anaesthetized pigs. Arch Toxicol 52: 459-467.
- Banks CN, Lein PJ (2012) A Review of experimental evidence linking neurotoxic organophosphorus compounds and inflammation. Neurotoxicology 33: 575-584.
- Leon A, Lepouse C (2004) Inflammation et cerveau. Département d'Anesthésie Réanimation. Hopital Robert Debré.
- Vejlstrup A, Møller AM, Nielsen CH, Damgaard D (2019) Release of active peptidylarginine deiminase into the circulation during acute inflammation induced by coronary artery bypass surgery. J Inflamm Res 12: 137-1
- Abou-Donia MB (2003) Organophosphorus ester-induced chronic neurotoxicity. Arch Environ Health 58: 484-4
- Lemus HN, Warrington AE, Rodriguez M (2018) Multiple sclerosis: Mechanisms of disease and strategies for myelin and axonal repair. Neurol Clin 36: 1-
- Sonvico F, Clemntino A, Buttini F, Colombo G, Pescina S, et al. (2018) Surface-modified nanocarriers for nose-to-brain delibery: From bioadhesion to targeting. Pharmaceutics 10: 34.
- Emami A, Tepper J, Short B, Yaksh TL, Bendele AM, et al. (2018) Toxicology evaluation of drugs administered via uncommon routes: Intranasal, intraocular, intrathecal/intraspinal and intra-articular. Int J Toxicol 37: 4-27.
SUPPLEMENTARY INFORMATION
Animal anesthesia and preparation
Before experiment, each animal was pre-medicated with 1mg/kg xylazine and 20mg/kg ketamine IM. Lidocaine was sprayed in the animal’s throat to locally anesthetize before intubation that was performed using a 6.0 endotracheal tube with the cuff covered with Tronotaneâ gel. After intubation, anesthesia was maintained with sevoflurane 2% in a O2 25% - Air mixture, and a venous line was placed into one ear vein to 1) keep the animal hydrated with an 8mL/kg.h saline solution perfusion and 2) inject the POX solution.
A femoral and an external jugular vein catheter (PiCCOâ) were placed to allow arterial blood sampling and cardiac monitoring. Respiratory parameters and temperature were checked with a complete monitoring system (Datex-Ohmedaâ) during at least 7 hours. At maximum 6 hours post intoxication, animals were sacrificed with an IV lethal dose of pentobarbital 180mg/kg.
Respiratory assistance
Basic respiratory assistance was given with the Datex-Ohmedaâ (Inspiratory assistance: 7mmHg and 4mmHg of Positive Expiratory Pressure). For this pilot study, we chose to put intoxicated animals under respiratory assistance after 3 minutes of apnea for 30 minutes or 1-hour depending on animals. We then checked if they were able to breathe on their own without assistance. If they did, animals were kept alive until the end of the experiment. If not, they were sacrificed with a pentobarbital injection as described above.
Samples preparation
Blood samples were collected in lithium heparin tubes at -30, 0, 10, 20, 30, 60, 90, 120, 180, 240, 300- and 360-minutes post intoxication. For non-surviving animals, the last samples were taken before pentobarbital injection, when the animal was still under respiratory assistance. Blood was directly diluted ten times in water for injection and stored at -80°C for enzymatic analysis. Blood enzymatic activity was evaluated in duplicate and reported to the hemoglobin quantity of the sample (U.g.dL-1).
After pentobarbital injection, the CSF was collected via a lumbar puncture and directly frozen and stored at-80°C. For enzymatic activity evaluation, the CSF was centrifuged for 10 minutes at 4°C, 10 000g and only the supernatant was used. CSF enzymatic activity was evaluated in duplicate and reported to the protein amount of
the sample (U.mg-1). For blood and CSF enzymatic measurement, the maximum variation tolerated between duplicates was 10%.
Staining
Brains were extracted from the skull after animal sacrifice, and placed in cold water for injection for dissection. Tissue samples were then stored in a 4% paraformaldehyde solution for 24 hours, then in a 20% sucrose PBS 1X solution for 4 days. Fixed tissues were included in an organic matrix to be stored at -80°C. Cryomicrotome-cut transversal sections (12μm) of frontal cortex were collected on poly-lysine slides and stored at -80°C before processing immunochemistry. After 3x15 min PBS baths, 500μL of a primary antibodies’ solution (PBS Tween 0.3%) was dropped on every slide, in a humid/dark chamber overnight. Primary antibodies were used as followed: GFAP (Glial fibrillary Acidic Protein) dilution 1:500 (Rabbit, DAKO), Col IV (Type IV collagen) dilution 1:500 (Goat, Southern biotec), VCAM (Vascular Cell Adhesion Molecule) dilution 1:500 (Rat, Biosciences) and Iba 1 (Anti Microglia) dilution 1:500 (Rabbit, Biosciences). After 3 PBS baths, 500μL of a secondary fluorescent antibodies’ solution (PBS Tween 0.3%, antibodies dilution 1:500) were deposited during 90 min on every slide in the humid/dark chamber. After 3 PBS baths, every slide was fixed with a DAPI mounting medium.
Statistics
For brain histological evaluation, we used at least 6 pictures per animal per staining. Results were analyzed with the help of PRISMâ software, with a Kruskal-Wallis statistic test.
Citation: Goulay R, Fémy F, Nervo A, Madi M, Knoertzer J, et al. (2019) Organophosphorus Compound Poisoning: Initial Data Providing Evidence of the Presence of Cholinesterase within the Cerebrospinal Fluid and Early Neurovascular Inflammation. J Toxicol Cur Res 3: 012.
Copyright: © 2019 Goulay R, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.

