
Osteoporosis School in Primary Health Care-A Pilot Study
*Corresponding Author(s):
Ann-Charlotte Grahn KronhedRehabvast Vadstena, Local Health Care Services In The West Of Östergötland, Division Of Physiotherapy, Department Of Medical And Health Sciences, Linköping University, Linköping, Sweden
Tel:+46 101048158,
Email:lotta.grahn-kronhed@telia.com
Abstract
An osteoporosis school was introduced as a pilot study in a Swedish primary health care center. Women aged 50 years and over with healed fragility fractures were asked to participate. The aim was to increase awareness amongst the participants and to advise them about life-style and fall prevention strategies to prevent a secondary fragility fracture. The osteoporosis school was scheduled to once a week for eight weeks and included theory and exercise training sessions. Eleven women with mean age 74 years participated. Clinical tests such as back straightening test and balance tests were performed, pain was estimated by the visual analogue scale, and health-related quality of life was measured by the SF-36 questionnaire. Straightening of the back improved 0.6 cm (p<0.05), worst estimated pain decreased from 5.8 to 4.0 (p<0.05), and the SF-36 bodily pain domain improved (p<0.05). The participants completed an average of 7 out of 8 sessions. The exercise training sessions were the most appreciated part of the program.
Keywords
INTRODUCTION
BACKGROUND
A recent review indicated that it is unclear whether patient education is beneficial and whether it has a significant and clinically relevant impact on osteoporosis management results, and therefore requires further investigation [12].
A pilot study with an Osteoporosis school was introduced at Vadstena Primary Health Care Centre (PHCC), Sweden, focusing middle-aged and elderly women with a healed fragility fracture. The aim of the osteoporosis school was to deliver disease-specific information to persons at risk of having a secondary fracture and to advise them about the importance of a healthy life-style and adequate physical activity, and also about ergonomic principles and fall prevention measures to prevent a secondary fracture.
MATERIALS AND METHODS
Participants
Methods
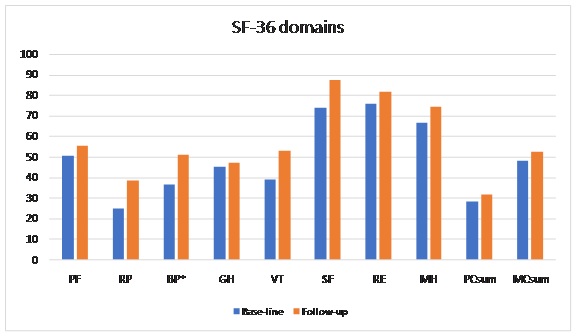
The participants completed the generic Short Form 36 (SF-36, version I) for assessment of their HRQL. The questionnaire has good reliability and validity [17-20]. The SF-36 version 1 compromises 36 items with two to six response options according to an ordinal scale. Eight health domains were assessed: Physical Function (PF), Role Physical (RP), Bodily Pain (BP), General Health (GH), Vitality (VT), Social Function (SF), Role Emotional (RE), and Mental Health (MH) [17,21]. Two summary scores were calculated from these eight domains: Physical Component Summary (PCS) and Mental Component Summary (MCS) indexes using previously established methods [21]. The SF-36 items were coded, scored and summarized to derive the eight domains. The scores were transformed into a 0-100 scale. Zero indicated the worst possible HRQL and 100 the best [18]. Present pain and the worst pain were estimated by a Visual Analogue Scale (VAS) from 0-10, where 0 indicated no pain and 10 the worst possible pain [22,23].
Intervention program
- 1. The disease osteoporosis and its consequences – a public health problem
- 2. Clinical risk factors for the probability of an osteoporotic fracture (FRAX), diagnosis and treatment
- 3. Anatomy of the skeleton and bone structure, and also the balance system
- 4. Ergonomic aspects of performing daily living activities, and also adequate aid and support (orthoses)
- 5. Self-management with nutritional, physical activity and fall prevention behaviour
- 6. Mindfulness
At the end of the intervention period the participants were offered physical activity on prescription (in Swedish FaR®) to improve their physical activity habits [35], and they were also introduced to individual or group training program with sequence training equipment at the PHCC gymnasium [36,37].
Statistical methods
RESULTS
Participants and drop-outs, attendance
Group characteristic
Drugs, fractures and FRAX-values
Back straightening, chair rising, balance performance, handgrip, pain, and health-related quality of life
Significance level p<0.05 is presented as *
| Base-line | Follow-up | |||
| Clinical tests | Mean (SD) | Mean (SD) | n | p |
| Back straightening (C7-wall) (cm) | 6.7 (1.5) | 6.1 (1.8) | 11 | 0.018 |
| Handgrip dominant (kg) | 19.4 (7.5) | 19.8 (7.0) | 11 | 0.307 |
| Handgrip non-dominant (kg) | 17.6 (7.8) | 17.6 (6.7) | 11 | 0.859 |
| Sharpened Romberg, eyes open (s) | 23.2 (11.2) | 25.2 (9.0) | 11 | 0.225 |
| Sharpened Romberg, eyes closed (s) | 12.4 (13) | 11.7 (12.6) | 11 | 0.917 |
| Right leg, eyes open (s) | 12.3 (11.9) | 13.3 (12.3) | 10 | 0.401 |
| Left leg, eyes open (s) | 14.4 (14.3) | 12.2 (10.6) | 10 | 0.646 |
| Right leg, closed eyes (s) | 2.5 (4.5) | 2.6 (5.6) | 10 | 0.654 |
| Left leg, closed eyes (s) | 1.4 (2.3) | 1.4 (2.3) | 10 | 1 |
| Walking forwards (steps) | 10.4 (6.9) | 11.5 (5.9) | 11 | 0.068 |
| Walking backwards (steps) | 8.5 (6.9) | 9.5 (6.2) | 11 | 0.106 |
| Present pain (VAS) | 4.7 (2.4) | 2.8 (2.7) | 11 | 0.069 |
| Worst pain (VAS) | 5.8 (3.5) | 4.0 (3.4) | 10 | 0.043 |
After the intervention period three women joined a strength training group and one woman joined a balance training group at the PHCC. Other participants continued their physical activity by regular walks and home training exercises.
DISCUSSION
The osteoporosis school was appreciated amongst the women in specific the exercise training sessions, which they told us were the best part of the school. A qualitative study previously confirmed that professionally supervised specific back exercise training may bring benefits to everyday life, increase well-being and quality of life in elderly women with vertebral fractures [40]. Supervised exercise training sessions for bone health and balance performance could be recommended to persons suffering from osteoporosis [23,27,30,41-43]. The back-straightening ability was improved in the present study, which may mean better back muscle function [27]. Estimated pain decreased amongst the participants, which is in accordance with training studies designed for elderly women with osteoporosis and fractures with exercise sessions 45-60 minutes 2-3 times per week for 2.5-6 months [23,41-43]. The improvement in the SF-36 bodily pain domain agrees with a training study for four months [41]. A tendency towards improved balance performance was found in tandem walking forwards in spite of the few scheduled training sessions. In a training study for women with osteoporotic vertebral compression balance performance was improved after exercising two times a week for three months [42]. Five women in the present Osteoporosis school did not manage to stand on one leg with the eyes open for 10 seconds or more, which may mean about 2.6 times increased hip fracture risk according to a study with Swedish elderly women [44]. Mean values for hand grip strength (19.6 kg in the dominant and 17.6 kg in the non-dominant hand) amongst the participants in the present study corresponded to normative mean values in American women aged 75 years and over [14].
Personal guidance, encouragement, and advice on adequate physical activity are very important to improve bone health, muscle function and balance in elderly women with osteoporosis and fragility fractures [45-46]. The international physical activity guidelines for public health recommend at least 150 min of moderate intensity distributed amongst 5-7 days a week, such as brisk walks for all adults [47,48]. However, elderly women with osteoporotic related fractures should not be forced to walk too fast due to an increased risk of falls [49]. There is a lack of well-designed studies evaluating the effect of education and physical exercise training in women with established osteoporosis [50,51]. osteoporosis and fracture may have a profound impact on physical function and everyday activity. Thus, healthcare professionals and persons with osteoporosis would benefit from more information on how treatments impact patients’ physical function and everyday activity, to optimise treatment decisions and to improve compliance and persistence with treatment to prevent future fractures [52].
CONCLUSION
ACKNOWLEDGEMENT
REFERENCES
- Reginster JY, Burlet N (2006) Osteoporosis: a still increasing prevalence. Bone 38: 4-9.
- [No authors listed] (1991) Consensus development conference: prophylaxis and treatment of osteoporosis. Am J Med 90: 107-110.
- [No authors listed] (1994) Assessment of fracture risk and its application to screening for postmenopausal osteoporosis. World Health Organ Tech Rep Ser 843: 2: 2-25.
- Kanis JA, Johnell O, De Laet C, Jonsson B, Oden A, et al. (2002) International variations in hip fracture probabilities: implications for risk assessment. J Bone Miner Res 17: 1237-1244.
- Svedbom A, Hernlund E, Ivergård M, Compston J, Cooper C, et al. (2013) osteoporosis in the European Union: a compendium of country-specific reports. Arch Osteoporos 8: 137.
- Kanis JA, Odén A, Johnell O, Jönsson B, de Laet C, et al. (2001) The burden of osteoporotic fractures: a method for setting intervention thresholds. Osteoporos Int 12: 417-427.
- Hernlund E, Svedbom A, Ivergård M, Compston J, Cooper C, et al. (2013) Osteoporosis in the European Union: medical management, epidemiology and economic burden. Arch Osteoporos 8: 136.
- Kanis JA (2008) Assessment of osteoporosis at the primary health care level. World Health Organization Collaborating Centre for Metabolic Bone Diseases, University of Sheffield, London, UK.
- Mundy G (1998) Bone remodeling and mechanisms of bone loss in osteoporosis. In: Meunier PJ (ed.). Osteoporosis: diagnosis and management, Taylor & Francis, London, UK. Pg no: 17-35.
- Xu W, Perera S, Medich D, Fiorito G, Wagner J, et al. (2011) Height loss, vertebral fractures, and the misclassification of osteoporosis. Bone 48: 307-311.
- Siminoski K, Warshawski RS, Jen H, Lee K (2006) The accuracy of historical height loss for the detection of vertebral fractures in postmenopausal women. Osteoporos Int 17: 290-296.
- Morfeld JC, Vennedey V, Müller D, Pieper D, Stock S (2017) Patient education in osteoporosis prevention: a systematic review focusing on methodological quality of randomised controlled trial. Osteoporos Int 28: 1779-1803.
- Antonelli-Incalzi R, Pedone C, Cesari M, Di lorio A, Bandinelli S, et al. (2007) Relationship between the occiput-wall distance and physical performance in the elderly: a cross-sectional study. Aging Clin Exp Res 19 : 207-212.
- Mathiowetz V, Kashman N, Volland G, Weber K, Dowe M, et al. (1985) Grip and pinch strength: normative data for adults. Arch Phys Med Rehabil 66: 69-74.
- Grahn Kronhed AC, Möller C, Olsson B, Möller M (2001) The effect of short-term balance training on community-dwelling older adults. JAPA 9: 19-31.
- Albertsson DM, Mellström D, Petersson C, Eggertsen R (2007) Validation of a 4-item score predicting hip fracture and mortality risk among elderly women. Ann Fam Med 5: 48-56.
- Ware JE, Sherbourne CD (1992) The MOS 36-Item Short-Form Health Survey (SF-36), I: conceptual framework and item selection. Med Care 30: 473-481.
- Sullivan M, Karlsson J, Ware JE Jr. (1995) The Swedish SF-36 Health Survey – I. Evaluation of data quality, scaling assumptions, reliability and construct validity across general populations in Sweden. Soc Sci Med 41: 1349-1358.
- Hallberg I, Rosenqvist AM, Kartous L, Löfman O, Wahlström O, et al. (2004) Health-related quality of life after osteoporotic fractures. Osteoporos Int 15: 834-841.
- Coons SJ, Rao S, Keininger DL, Hays RD (2000) A comparative review of generic quality-of-life instruments. Pharmacoeconomics 17: 13-35.
- Persson LO, Karlsson J, Bengtsson C, Steen B, Sullivan M (1998) The Swedish SF-36 Health Survey II. Evaluation of clinical validity: Results from population studies of elderly and women in Gothenburg. J Clin Epidemiol 51: 1095-1103.
- Andersson S, Arnér S (1991) Acute pain. Studentlitteratur. Lund: Benzon Pharma AB.
- Malmros B, Mortensen L, Jensen MB, Charles P (1998) Positive effects of physiotherapy on chronic pain and performance in osteoporosis. Osteoporos Int 8: 215-221.
- Sinaki M (2012) Exercise for patients with osteoporosis: management of vertebral compression fractures and trunk strengthening for fall prevention. PM & R 4: 882-888.
- Chien M, Yang RS, Tsauo JY (2005) Home-based trunk-strengthening exercise for osteoporotic and osteopenic postmenopausal women without fracture -a pilot study. Clin Rehabil 19: 28-36.
- Hongo M, Itoi E, Sinaki M, Miyakoshi N, Shimada Y, et al. (2007) Effect of low-intensity back exercise on quality of life and back extensor strength in patients with osteoporosis: a randomized controlled trial. Osteoporos Int 18: 1389-1395.
- Bergström I, Bergström K, Grahn Kronhed AC, Karlsson S, Brinck J (2011) Back extensor training increases muscle strength in postmenopausal women with osteoporosis, kyphosis and vertebral fractures. Adv Physiother 13: 110-117.
- Paolucci T, Morone G, Iosa M, Grasso MR, Buzi E, et al. (2014) Efficacy of group-adapted physical exercises in reducing back pain in women with postmenopausal osteoporosis. Aging Clin Exp Res 26: 395-402.
- Ball J, Cagle P, Johnson BE, Lucasey C, Lukert BP (2009) Spinal extension exercises prevent natural progression of kyphosis. Osteoporos Int 20: 481-489.
- Halvarsson A, Franzén E, Ståhle A (2015) Balance training with multi-task exercises improves fall-related self-efficacy, gait, balance performance and physical function in older adults with osteoporosis: a randomized controlled trial. Clin Rehabil 29: 365-375.
- Gillespie L, Robertson MC, Gillespie WJ, Sherrington C, Gates S, et al. (2012) Interventions for preventing falls in older people living in the community. Cochrane Database Syst Rev CD007146.
- Karlsson MK, Magnusson H, von Schewelov T, Rosengren BE (2013) Prevention of falls in the elderly--a review. Osteoporos Int 24: 747-762.
- Papaioannou A, Adachi JD, Winegard K, Ferko N, Parkinson W, et al. (2003) Efficacy of home-based exercise for improving quality of life among elderly women with symptomatic osteoporosis-related fractures. Osteoporos Int 14: 677-682.
- Sinaki M, Mikkelsen BA (1984) Postmenopausal spinal osteoporosis: Flexion versus extension exercises. Arch Phys Med Rehabil 65: 593-596.
- Leijon ME, Bendtsen P, Nilsen P, Festin K, Ståhle A (2009) Does a physical activity referral scheme improve the physical activity among routine primary health care patients? Scand J Med Sci Sports 19: 627-636.
- Grahn Kronhed AC, Möller M (1998) Effects of physical exercise on bone mass, balance skill and aerobic capacity in women and men with low bone mineral density, after one year of training - a prospective study. Scand J Med Sci Sports 8: 290-298.
- Grahn Kronhed AC, Salminen H (2017) Long-term effects of a ten-year osteoporosis intervention program in a Swedish population - a cross-sectional study. Prev Med Rep 5: 295-300.
- Javaid MK, Kyer C, Mitchell PJ, Chana J, Moss C, et al. (2015) Effective secondary fracture prevention: implementation of a global benchmarking of clinical quality using the IOF Capture the Fracture® Best Practice Framework tool. Osteoporos Int 26: 2573-2578.
- Perry SB, Downey PA (2012) Fracture risk and prevention: a multidimensional approach. Phys Ther 92: 164-178.
- Qvist N, Bergström I, Grahn Kronhed AC, Karlsson S, Forss A (2011) Empowering the fragile body: experiences of a back muscle group training program in postmenopausal women with vertebral fractures. A qualitative interview study. Adv Physiother 13: 63-70.
- Grahn Kronhed AC, Hallberg I, Ödkvist L, Möller M (2009) Effect of training on health-related quality of life, pain and falls in osteoporotic women. Adv Physiother 3: 154-165.
- Bergland A, Thorsen H, Kåresen R (2011) Effect of exercise on mobility, balance, and health-related quality of life in osteoporotic women with a history of vertebral fracture: a randomized, controlled trial. Osteoporos Int 22: 1863-1871.
- Gold D, Shipp K, Pieper C, Duncan P, Martinez S, et al. (2004) Group treatment improves trunk strength and psychological status in older women with vertebral fractures: Results of a randomized clinical trial. J Am Geriatr Soc 52: 1471-1478.
- Lundin H, Sääf M, Strender LE, Nyren S, Johansson SE, et al. (2014) One-leg standing time and hip-fracture prediction. Osteoporos Int 25: 1305-1311.
- Dohrn IM, Ståhle A, Roaldsen KS (2016) "You Have to Keep Moving, Be Active": Perceptions and Experiences of Habitual Physical Activity in Older Women With Osteoporosis. Phys Ther 96: 361-370.
- Halvarsson A, Ståhle A, Halén C, Skavberg Roaldsen K (2016) “Better safe than sorry”: a qualitative content analysis of participant’s perspectives of fall-related concerns and balance in older women with osteoporosis after balance training. Disabil Rehabil 38: 796-802.
- [No authors listed] (2009) Physical Activity Guidelines Advisory Committee report, 2008. To the Secretary of Health and Human Services. Part A: executive summary. Nutr Rev 67: 114-120.
- FYSS (2017) Fysisk aktivitet i sjukdomsprevention och sjukdomsbehandling. Läkartidningen förlag AB.
- Ebrahim S, Thompson PW, Baskaran V, Evans K (1997) Randomized placebo-controlled trial of brisk walking in the prevention of postmenopausal osteoporosis. Age Ageing 26: 253-260.
- Giangregorio LM, MacIntyre NJ, Thabane L, Skidmore CJ, Papaioannou A (2013) Exercise for improving outcomes after osteoporotic vertebral fracture. The Cochrane Library Issue.
- Giangregorio LM, Papaioannou A, MacIntyre NJ, Ashe MC, Heinonen A, et al. (2014) Too fit to fracture: exercise recommendations for individuals with osteoporosis or osteoporotic vertebral fracture. Osteoporos Int 25: 821-835.
- target="_blank"Kerr C, Bottomley C, Shingler S, Giangregorio L, et al. (2017) The importance of physical function to people with osteoporosis. Osteoporos Int 28: 1597-1607.
Citation: Grahn Kronhed AC (2017) Osteoporosis School in Primary Health Care-A Pilot Study. J Orthop Res Physiother 3: 033
Copyright: © 2017 Ann-Charlotte Grahn Kronhed, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.

