
Oxidative Stress and Histopathological Alteration of the Liver of Oreochromis niloticus Induced by Sodium Dodecyl Sulphate
*Corresponding Author(s):
Ikpesu TODepartment Of Biology, Federal University Otuoke, Nigeria
Tel:+234803231141,
Email:ikpesuto@fuotuoke.edu.ng
Abstract
The chronic effects of sodium dodecyl sulphate (SDS) inducing oxidative stress and liver histopathology in Oreochromis niloticus after sub-lethal exposure was investigated. This fish was examined 30 days after exposure to the range of the surfactant concentrations (2.5, 5.0, 7.5, and 10 mg/L) that have been reported in the field. The self-bred fish used in the experiment met all applicable standards and laws. Following standard procedures, blood was taken from the caudal region using insulin syringe. The glutathione - S transferase (GST) activities and glucose profiles were spectrophotometrically measured on days 2, 6, 9, 16, 23, and 30, while histopathology was examined on days 2 and 30 using hematoxylin and eosin staining techniques. The surfactant altered fish GST erythrocyte activity and glucose content by increasing the GST activity and glucose level throughout the experiment at all concentrations. Compared to control fish, the liver of the fish exposed to the surfactant had histological changes such as hepatocyte rupture, sinusoid dilation and degeneration, and necrosis. The Induced GST, the blood glucose, and severity of altered liver were dose- and time-dependent. This study demonstrates how SDS poisoning can be harmful to human health and life. Authorities must implement strict mitigation measures to stop ongoing pollution of the aquatic environments because anthropogenic factors have been implicated as the primary causes of surfactant exposure
Keywords
Sodium dodecyl sulphate, oxidative stress, liver histopathology, Oreochromis niloticus
Introduction
Surfactants play an important role in detergents and cosmetics, and in terms of sales volume, anionic surfactants are the most important. According to acute and long-term research, anionic and nonionic surfactants have low toxicity when taken orally, however certain cationic are moderately hazardous. The compatibility of local applications with skin and mucous membranes is greatly reliant on concentration. Surfactant influence on biological systems can be described mostly by the surfactant's physicochemical features and pharmacological activity.
Sodium dodecyl sulfate (SDS) is a chemical compound that can be synthesized or obtained naturally. The production of this surfactant involves the combination of lauryl alcohol obtained from either petroleum or plant sources with sulfur trioxide, resulting in the formation of hydrogen lauryl sulfate. This compound is then neutralized using sodium carbonate, leading to the formation of sodium lauryl sulfate [1]. The existence of this substance in the environment is mostly attributed to its occurrence in complex home and industrial effluents, as well as its direct release from some applications such as oil dispersants and insecticides.
The surfactant is a common anionic surfactant found in larger concentrations in industrial goods like engine degreasers, floor cleaners, and automobile exterior cleaners having surface-active properties that make it useful in cleaning products, personal care products, and cosmetics. It's also an effective surfactant that's used in any work that requires the removal of greasy stains and residues; It's used in hand soap, toothpaste, shampoos, shaving creams, and bubble bath formulations at lower concentrations because of its capacity to create a foam (lather), surfactant capabilities, and thickening effect.
It is also used as a delivery aid in pharmaceuticals and biochemical studies, and electrophoresis in a variety of industrial manufacturing processes [2]. This surfactant is also a major component of dispersants, which have been a source of contention in oil-spill cleanup procedures for many years [3].
Because of their widespread use, there is rising concern about how these substances interact with the environment. Domestic and industrial effluents containing non-biodegradable surfactants continue to infiltrate into natural waters, wreaking havoc on the aquatic environment [4]. SDS is in high demand among the different detergents available due to its inexpensive cost and excellent foaming properties. It is found in household items, industrial products, laboratories, and pharmaceutical preparations. This detergent's biodegradability is frequently cited to excuse its excessive use and disposal.
Despite its exotic status, tilapia is a popular freshwater fish for farming in Nigeria. It's possible that tilapia is less vulnerable to the effects of precipitated contaminants than other fish are because they spend so much time floating near the surface. Similarly, when selecting an indicator species to evaluate the biological effects of pollutants and their impact, it is important to take into account the organism's persistence at a significant population level, pre-exposure history, ecological relevance, sensitivity to contaminants, physiology, and habitats. [5]. Oreochromis niloticus (Cichlidae), also known as the Nile tilapia, is a common study organism in ecotoxicology. This fish's biochemical responses to hazardous substances have been well documented [6].
This surfactant is sometimes ignored as a safe and environmentally friendly alternative to petroleum-based surfactants. Many studies conducted by various scientists have raised concerns about the detergent's environmental impact, including its effect on marine invertebrate embryos and larvae [7], inhibition of bivalves' filter-feeding habits, and mussel suspension feeding [8]. The same is true for animals such as mice and humans, though to a lesser extent, as well as aquatic creatures such as fish, and microorganisms like yeasts and bacteria [9]. But there hasn't been much research done on the effects on oxidative and histopathological consequences on tissues.
Materials and methods
Quality of Water: Daily measurements of sodium dodecyl sulphate SDS concentrations and water quality parameters were taken for each treatment throughout the chronic SDS exposure of O.niloticus. The azo method was utilized to determine nitrite concentrations, whereas a glass electrode was employed to determine water temperature and pH (Thermo Orion, Beverly, MA, USA). The determination of both the total hardness and total alkalinity was accomplished via titration. The concentration of dissolved oxygen was ascertained using the Winkler method.
Pre-Analytical Stage: The fish experiments were conducted in accordance with relevant regulations and legislation. The O. niloticus used as a test organism in this study were monitored and observed from the initial egg stage until they reached the designated investigative stage, which occurred after a period of five months. The process of spawning was conducted within clay ponds with the objective of producing fry of high genetic purity.
The fish were cultivated throughout the months of April and May 2022 in a meticulously constructed shallow pond. The breeding population consisted of one male for every five females. A total of five shallow ponds were created for this purpose. The breeding stocks utilized in the experiment comprised of five males and twenty-five females. During the course of the experiment, the breeding stocks were provided with a diet consisting of fodder that was rich in protein and vitamins. The temperature within the experimental environment was carefully controlled to fall within the optimal range of 25-29oC. Additionally, the aquaria were subjected to a daily illumination period of 12-14 hours, achieved by suspending a 100-W fluorescent bulb at a distance of 50 cm above the water surface. To maintain a suitable environment for spawning, the aquaria were cleaned every 4-5 days.
It was observed that the male begins the mating procedure by choosing the healthiest female and then herding the other females to the opposite end of the tank. It's possible that spawning and fertilization could take up to two hours. The female quickly leaves the tank's nesting chamber, her mouth firmly clamped over the fertilized eggs. Female fish don't eat when incubating their eggs and again once the fry have hatched because their mouths are full of the eggs and later the fry's yolk sac. When the female leaves the nest, the male quickly chooses another female and begins mating with her, starting the cycle all over again.
After the completion of yolk sac absorption, the fry was meticulously transferred to a rearing pond for the purpose of maturation. The fry exhibits independent feeding behavior, assume piscine morphology, and attain a length ranging from 1 to 2 centimeters. A fingerling can be defined as a juvenile fish that has reached a length of approximately 10-15 cm, which is comparable to the size of a human finger. The fingerling stage of the fry is typically attained during a time frame of 45 to 60 days. The fry and fingerlings were provided with a specially formulated diet consisting of a 1:1 ratio of soybean powder or finely powdered cake and rice bran, which was administered to them 4-5 times daily. After a period of around 2-3 weeks following the introduction of fish into the nursery pond, a netting process is conducted to remove the fish. The selected fish, known as advanced fries, are then transferred to a separate rearing pond where they undergo further development and growth as fingerlings.
A simulated aquatic environment was created within a greenhouse structure, designed to replicate the natural habitat of the fish. The maintenance routine involved daily cleaning of the greenhouse. Fifteen earthen ponds were constructed using clayey loam soil, with each pond having dimensions of 27 1/4 x 24 1/8 x 29 12 inches. A total of ten fingerlings were allocated to each of the final ponds for a duration of 12 weeks. These fingerlings were provided with a diet consisting of finely powdered cake and rice bran, which was administered three times each day. The aquaria were cleaned using a manual pumping apparatus to siphon the stale water, which was then refilled twice weekly. By the conclusion of the twelfth week, no instances of mortality were observed.
Analytical Stage: After the twelfth week, the final ponds and their corresponding repetitions were subjected to various doses of sodium dodecyl sulfate (2.5, 5.0, 7.5, and 10 mg/L) as well as a control group for a period of 30 days. During the experimental, the fish in both the control and experimental groups were administered a diet equivalent to 3% of their body weight, which was given twice daily. The water and toxicants were supplied in their whole every 24 hours, and the earthen ponds were diligently maintained to provide the highest possible level of cleanliness.
The physicochemical parameters of the water were measured on a daily basis throughout the duration of the experiment. After the completion of each experimental period, a fish is retrieved from each pond, transported to the laboratory in a container with sufficient air, and promptly anesthetized with MS222 (Ethyl 3-aminobenzoate methanesulfonate salt, Sigma).
The biochemical profiles were assessed on days two, nine, sixteen, twenty-three, and thirty, while the liver pathological abnormalities were analyzed in the laboratory on days two and thirty. The fish that were not utilized were released back into the main reservoir.
Blood samples were obtained from the caudal vein of each fish, located behind the spine, using the approach described by Congleton and LaVoie [10]. The blood sample was obtained and thereafter subjected to centrifugation using anticoagulant-free centrifuge tubes to obtain the serum. The serum was then preserved at a temperature of -80°C until it was ready for analysis.
Following the blood collection, a fish from each treatment group was promptly dissected. The liver was then removed and subsequently preserved in a 10% phosphate-buffered formalin solution
Glutathione-S-Transferase Activities Measurement: In a heparin vial, blood was taken and centrifuged for five minutes at 3,000 rpm at 4°C. Before investigation, the top yellow plasma layer was pipetted into a vial without disturbing the white buffy layer and stored on ice (- 4°C).
The DetectX® Glutathione S-Transferase Fluorescent Activity Kit was used to assess GST activity in plasma samples. The kit employs a non-fluorescent molecule as a substrate for the GST enzyme, which binds to glutathione covalently to form a fluorescent product. The sample is combined with the Detection Reagent and GST in the endpoint mode in order to produce a fluorescent result. The product is subsequently quantified at a wavelength of 460nm utilizing a fluorescent plate reader, with excitation taking place at 390nm. The present kit employs a GST standard curve for the purpose of evaluating and examining the activity of GST. The standard curve was subjected to a four-parameter curve analysis.
Assessment of Blood Glucose Levels: A commercial kit based on the glucose-oxidase reaction was used to colorimetrically evaluate the serum glucose (Glucox 500-Doles Reagents, Brazil). Glucose Colorimetric Detection Kit provides a quantitative analysis of glucose in a variety of samples. Each sample of the induced fish serum was compared to a beta-D-glucose standard against which an assay curve was generated. After combining the colorimetric substrate with the horseradish peroxidase, and initiating the reaction with glucose oxidase, the serum was added. There was a 30-minutes incubation period at room temperature for the reaction. The glucose oxidase mixes with glucose to form hydrogen peroxide, which reacts with the colorimetric substrate to produce a pink-colored result. At 560 nm, the pink product was read
Histomorphology: The Gewaily and Abumandour [11] histological examination approach was followed. The tissue samples were preserved for 24 hours in a 10% neutral buffered formaldehyde solution after being cut into 0.5 cm3 slices. After being dehydrated in increasing concentrations of alcohol, the samples were cleaned with xylene and embedded in paraffin wax. The slices were cut to 5 m thickness on a Leica rotatory microtome (RM 20352035; Leica Microsystems, Wetzlar, Germany) and stained with hematoxylin and eosin. Using a BX50/BXFLA microscope (Olympus, Tokyo, Japan), the tissue slices were analyzed.
Statistical Analysis Gewaily and Abumandour: The basic statistics of the measured parameters were examined using appropriate analysis. One-way ANOVA was used to assess the patterns of variance caused by SDS treatments. The Tukey-HSD test was used to compare the means of the treated parameters and the control parameters. SPSS, version 10 (SPSS, 1998) software was used.
Results
The GST activity in the erythrocytes of C.gariepinus induced with various concentrations of SDS revealed times and concentrations dependent increase. The activity of the enzyme in the control group was considerably unaffected, with the range (33.10 -33.70) mg/ml). On day 2nd to day 30th, GST activity at 2.5mg/l ranges (35.20 – 55.43) mg/ml, with only day 23rd and 30th varies significantly (p < 0.05) when compared with the control. At 5.00mg/l, the range of the enzyme activity on day 2nd to 30th was (35.89 – 57.12) mg/ml, and also varies significantly (p < 0.05) when compared with the control. At 7.50mg/l, the range was (36.20 - 6320) mg/ml), and highly significance (p < 0.01) when compared with the control. At the highest exposure of 10mg/l, the enzyme activity varies significantly (p < 0.05, p < 0.01) between various treatments and the control on day 9th to 30th, and it ranges between (37.00 – 65.00) mg/ml (Figure 1).
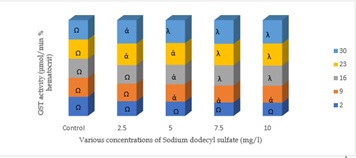 Figure 1: GST activities in the erythrocytes of C.gariepinus exposed to sublethal concentrations of Sodium dodecyl sulfate; A different symbol between the control and treatment indicate significant differences ∝ (p < 0.05); λ(p < 0.01)
Figure 1: GST activities in the erythrocytes of C.gariepinus exposed to sublethal concentrations of Sodium dodecyl sulfate; A different symbol between the control and treatment indicate significant differences ∝ (p < 0.05); λ(p < 0.01)
The glucose level increases with the increase in the concentration of the toxicant on day 2, and were significant between the control and various treatments (p < 0.05) except with the lower concentration of 2.5mg/L (Figure 2). The glucose level decreases after day 2, in the order day 9 < 16 < 23 < 30. At the higher concentration of 6mg/L, on day 16, 23 and 30, the glucose level were highly significances (p < 0.1) when compared with the control. 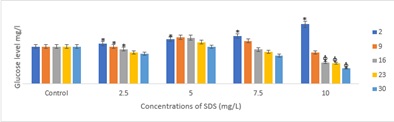
Figure 2: Glucose level in O.niloticus exposed to a different concentrations of SDS. Data presented as mean ± SE. Symbol above bars indicate significant differences between the control and the treatments * (p < 0.05), φ (p < 0.01)
Histopathological Examination of the Liver: Histological examination of the liver of the control fish revealed no abnormalities. Conversely, as illustrated in Figure 3A, the hepatic parenchyma displayed a uniform clustering of hepatocytes in the vicinity of the circulatory system (sinusoids).
The histological structure of the liver of O.nilticus, subjected to sodium dodecyl sulfate at quantities found in the field, exhibits a range of histological alterations in comparison to the control group. The severity of these changes is depending on both the duration of exposure and the dosage administered. Figure 3B illustrates the presence of slight expansion in the cords of hepatocytes and the occurrence of dark patches on the second day of exposure to the surfactant at a concentration of 2.50mg/l SDS. The severity of the dilation was observed in conjunction with partial distigeration of the hepatocytes, as the concentration of the toxicant increased to 5.0mg/l during the same period of exposure (Figure 3C). Furthermore, at concentrations of 7.50mg/l, the liver of the fish exhibited a pronounced dilation of the sinusoid (Figure 3D). The liver exhibited significant impairment at a concentration of 10.0mg/l over the exposure periods, as evidenced by the occurrence of hepatocyte rupture (Figure 3E).
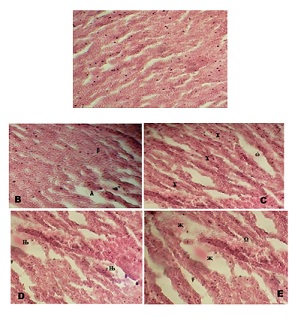 Figure 3: Liver of O.niloticus exposed to sublethal doses of sodium dodecyl sulfate on day 2; A: control fish showing architecture of a hepatic lobule. The nuclei hepatocytes (arrowhead) and the hepatic sinusoid (arrow with tail); No visible lesion was observed; B: Liver of the fish exposed to 2.50mg/l of SDA; mild dilation of the hepatic sinusoid (λ), and dark patches (β); C: 5.0mg/l SDA exposure revealed partial disintegration of the hepatocytes (Δ), and dilation of the sinusoid (ω); D: 7.50mg/l treatment: the fish’s liver shows severe dilation of the sinusoid (?); E: 10.0mg/l SDA exposure: severe dilation of the sinusoid (χ) and rupture of the hepatocytes (Ω); (H & E stain x300)
Figure 3: Liver of O.niloticus exposed to sublethal doses of sodium dodecyl sulfate on day 2; A: control fish showing architecture of a hepatic lobule. The nuclei hepatocytes (arrowhead) and the hepatic sinusoid (arrow with tail); No visible lesion was observed; B: Liver of the fish exposed to 2.50mg/l of SDA; mild dilation of the hepatic sinusoid (λ), and dark patches (β); C: 5.0mg/l SDA exposure revealed partial disintegration of the hepatocytes (Δ), and dilation of the sinusoid (ω); D: 7.50mg/l treatment: the fish’s liver shows severe dilation of the sinusoid (?); E: 10.0mg/l SDA exposure: severe dilation of the sinusoid (χ) and rupture of the hepatocytes (Ω); (H & E stain x300)
On the thirtieth day of the experiment, the liver of the treated fish exhibited distinct responses that were more severe, while maintaining the same concentrations as observed on the second day. At a concentration of 2.50mg/l, pronounced dilatation of the hepatic sinusoid and slight disintegration of the hepatocytes were seen (Figure 4). When the concentration was increased to 5.0 mg/L, the liver of the fish exhibited enlarged hepatocytes that contained micro vesicular lipid vacuoles in the cytoplasm, as well as severe dilatation of the sinusoid. At a concentration of 7.50mg/l of Sodium Dodecyl Sulfate, the liver of the fish exhibits significant shedding of hepatocytes. Furthermore, when exposed to a concentration of 10.0mg/l of SDS, the fish's liver displays severe dilatation, degeneration of the sinusoid, necrosis, rupture, and extensive shedding of hepatocytes.
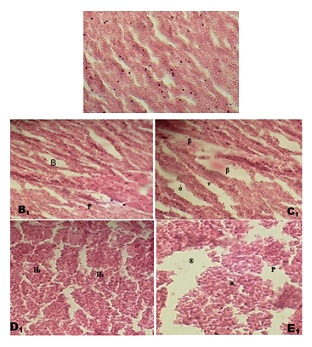 Figure 4: Liver of C. gariepinus subjected to sublethal sodium dodecyl sulphate dosages on day 10; Figure A1: fish’s liver exhibiting the hepatic lobule's structure in the control treatment. There was no evidence of a visible lesion lesion; Figure B1: 2.50mg/l of SDA exposure; severe dilation of the hepatic sinuosoid (B), and mild disintegration of the hepatocytes (P); Figure C1: 5.0mg/l SDA treated fish’s liver; enlarged hepatocytes containing micro vesicular lipid vacuoles in the cytoplasm (β), and severe dilation of the sinusoid (α);Figure D1: 7.50mg/l treatment: the fish’s liver shows desquamation of the hepatocytes (); Figure E1: 10.0mg/l SDA exposure: severe dilation and degenration of the sinusoid (®), necrosis (P) rupture and severe desquamation of the hepatocytes(χ). (H & E stain x300)
Figure 4: Liver of C. gariepinus subjected to sublethal sodium dodecyl sulphate dosages on day 10; Figure A1: fish’s liver exhibiting the hepatic lobule's structure in the control treatment. There was no evidence of a visible lesion lesion; Figure B1: 2.50mg/l of SDA exposure; severe dilation of the hepatic sinuosoid (B), and mild disintegration of the hepatocytes (P); Figure C1: 5.0mg/l SDA treated fish’s liver; enlarged hepatocytes containing micro vesicular lipid vacuoles in the cytoplasm (β), and severe dilation of the sinusoid (α);Figure D1: 7.50mg/l treatment: the fish’s liver shows desquamation of the hepatocytes (); Figure E1: 10.0mg/l SDA exposure: severe dilation and degenration of the sinusoid (®), necrosis (P) rupture and severe desquamation of the hepatocytes(χ). (H & E stain x300)
Discussion
Glutathione is a well-known ROS scavenger and was used as a biomarker of oxidative stress in many studies [12], and its synthesis depends on the cellular concentration of glutamate and glutamine [13]. This result demonstrated that the GST activity of erythrocytes in fish exposed to surfactants increased significantly in comparison to the control group, regardless of exposure duration or concentration. It is possible that the observed elevation in GST activity in stressed fish is attributable to the enzyme's status as a critical antioxidant, which serves as a fundamental defense against the lethal effects of reactive oxygen species and aids in coping with organic substances.
On day 2nd, the glucose content observed in fishes exposed to sodium dodecyl sulfate increases with the increase in the concentrations of the toxicant. However, on day 30th, the glucose level decreases, and abruptly. The initial increased can be attributed to the fact that glucose is the primary source of energy and its increased plasma level is a secondary indicator of stress response in animals. In our results, the elevated level of glucose in sodium dodecyl sulfate exposed fish indicates that the surfactant induced stress in the test fish. Increased blood glucose levels have also been reported in C. carpio and in Oncorhynchus mykiss after long-term exposure to sub-lethal concentrations of diazinon [14]. However, with long exposure as observed on day 30th, the glucose level declined with exposure duration. Prior research has suggested that prolonged chemical exposure may induce a biphasic response in blood glucose levels, consisting of an initial rise, followed by a decline, culminating in depletion. The observed occurrence could potentially be linked to the exhaustion of energy reserves caused by the strain caused by the substantial accumulation of the surfactant. Low glucose content in chronically exposed fishes could also be due to improper gluconeogenesis as caused by the surfactant.
Among the teleost fishes, the liver is the primary organ for biotransformation of organic xenobiotics. Therefore, the liver can be studied in environmental monitoring due to its high sensitivity to contaminants and alterations in its structure can be significant in the evaluation of the health of fish. The Major degenerative changes observed in this study included; dilation of the cords of hepatocytes, dark patches, partial distigeration of the hepatocytes, dilation of the sinusoid, rupture of the hepatocytes, desquamation of the hepatocytes, necrosis, and rupture of the hepatocytes. Similar observations were reported by Pacheco and Santos [15] in Anguilla Anguilla exposed to various environmental contaminants. Also, Marty [16] reported a similar histological lesion in demersal rockfish in Prince William Sound, Alaska, after the Exxon Valdez oil spill. The dilation of the sinusoid in the connective tissue in O.niloticus liver exposed to SDS in the present study was similar to the observation of Esam [17] in the livers of siganus canaliculatus following exposure to crude oil and dispersed oil. Desquamation of the hepatocytes, necrosis, and rupture of the hepatocytes are considered as markers of serious environmental change [18]. Other inflammations observed in this investigation can be seen as a response to leukocyte infiltration activation and clustering [19].
In this study prevalence and relative risks for most lesions were significantly higher in treated fish compared with the control groups, and the histopathological changes in fish were proportionate with the duration of exposure and the concentrations of sodium dodecyl sulphate. The fish exposed to low doses of the surfactant at the early stage of the test showed less significant histopathological changes for most observed lesions, and each liver alteration possesses distinctive histological features and affects specific areas of tissue related to function and might affect fish health. However, the degenerative alterations are given the highest importance factor because they are considered a direct effect of toxicants, they are generally irreversible, and their persistence or progression may lead to a partial or total loss of organ function. Changes in the liver, like those observed in this study, could cause serious physiological issues and provide accurate information on the stress caused by variety of environmental contaminants.
Conclusion
This study demonstrated that fish biochemical enzymes can change significantly when exposed to sodium dodecyl sulphate. These enzymes are useful biomarkers that can be used to detect surfactant pollution in aquatic systems. The findings also revealed that aquatic animals' health may be harmed by the presence of surfactants in water bodies. Furthermore, additional research is required to explain the correlation between GST and glucose induction and the magnitude of histopathological alterations in the liver of O.niloticus, as well as the physiological implications of alterations observed in the fish on other aquatic life.
Significance Statement
Surfactants are new environmental contaminants whose toxicity threatens ecological, evolutionary, nutritional, and environmental balances. Surfactants such as sodium dodecyl sulphate, linear alkyl benzene sulfonates, and alpha -olefin sulfonate may be harmful to aquatic life in freshwater reservoirs. Their physiological changes have the potential to reduce the survival rate of aquatic life in nature. As a result, measures should be taken to mitigate the potential contamination of the aquatic ecosystem by many contaminants that are carelessly dumped into water bodies, and further research should be conducted to strengthen the current findings. Furthermore, more research on their potential residual effects is needed to fully understand their hazardous impacts on aquatic ecosystems, as well as the use of environmentally friendly surfactants.
Ethical Statement
- Funding: PhD thesis; Personally funded
- Conflict of Interest: None
- Ethical approval: Not applicable
- Informed consent: not applicable
- Author contribution: Ikpesu, T.O.; Initiated, design, write and run the analysis
- Data Availability Statement: Data is available on request
References
- Nkpondion NN, Ugwumba OA, Esenowo IK (2016) The Toxicity Effect of Detergent on Enzymatic and Protein Activities of African Mud Catfish (Clarias gariepinus). J Environ Anal Toxicol 6: 361.
- Muthusamy K, Gopalakrishnan S, Ravi TK, Sivachidambaram P (2008) Biosurfactants: properties, commercial production and application. Current Science 94: 736-747.
- Rejeki S, Desrina D, Mulyana AR (2008) Chronic effects of detergent surfactant (Linear Alkylbenzene Sulfonate / LAS) on the growth and survival rate of sea bass (Lates calcalifer Bloch), larvae; Journal of Coastal Development 213.
- Schleheck D, Lechner M, Svhonenberger R, Marc JFS, Cook AM (2003) Desulfonation of the disulfodiphenyl ether carboxylates from linear alkyl diphenyletherdisulfonate surfactants. Appl Environ Microbiol 69: 938-944.
- Saparuddin A, Arbain V (2019) Biological Test of the Laundry Industry Toxicity of Detergents and Concentration of Hemoglobin in Tilapia (oreochromis niloticus). Earth Environ Sci. 382: 012-038.
- Rodríguez A, Gisbert E, Rodríguez G, Castelló OF (2005) Histopathological observations in European glass eels (Anguilla anguilla) reared under different diets and salinities. Aquaculture 244: 203-214.
- Abdel-Moneim AM, AL-Kahtani MA, Elmenshawy OM (2012) Histopathological biomarkers in gills and liver of Oreochromis niloticus from polluted wetland environments, Saudi Arabia. Chemosphere 88: 1028-1035.
- Orbea A, Ortiz ZM, Sole M, Porte C, Cajaraville MP (2002) Antioxidant enzymes and peroxisome proliferation in relation to contaminant body burdens of PAHs and PCBs in bivalve molluscs, crabs and fish form the Urdaibai and Plentzia estuaries (Bay of Biscay). Aquatic Toxicology 58: 75-98.
- Baker LA (1994) In Environmental Chemistry of Lakes and Reservoirs Advances in Chemistry Series 237. Journal of Environmental Quality 25: 1452-1452.
- Congleton JL, LaVoie WJ (2001) Comparison of blood chemistry values for samples collected from juvenile Chinook salmon by three methods. Journal of Aquatic Animal Health 13: 168-172.
- Gewaily MS, Abumandour MMA (2020) Gross morphological, histological and scanning electron specifications of the oropharyngeal cavity of the hooded crow (corvus cornix pallescens). Anat. Histol. Embryol 50: 72-83.
- Regoli F, Giuliani ME, Benedetti M, Arukwe A (2011) Molecular and biochemical biomarkers in environmental monitoring: A comparison of biotransformation and antioxidant defense systems in multiple tissues. Aquat. Toxicol 105: 56-66.
- Bharti S, Rasool F (2021) Analysis of the biochemical and histopathological impact of a mild dose of commercial malathion on Channa punctatus (Bloch) fish. Toxicol Reports 8: 443-455.
- Banaee M, Sureda A, Mirvaghefi AR, Ahmadi K (2011) Effects of diazinon on biochemical parameters of blood in rainbow trout (Oncorhynchus mykiss). Pesticide Biochemistry and Physiology 99: 1-6.
- Pacheco M, Santos MA (2002) Biotransformation, genotoxic, and histopathological effects of environmental contaminants in European eel (Anguilla Anguilla L.). Ecotoxicology and Environmental Safety 53: 331-347.
- Marty GD, Hoffman A, Okihiro MS, Hanes D (2003) Retrospective analysis: Bile hydrocarbons and histopathology of demersal rockfish in Prince William Sound, Alaska, after the Exxon Valdez oil spill. Mar Environ Res 56: 569-584.
- Esam A (2012) Histopathological Changes in the Livers of Rabbit Fish (Siganus canaliculatus) Following Exposure to Crude Oil and Dispersed Oil, Toxicologic Pathology 40: 1128-1140.
- Sayed AH, Younes HAM (2017) Melano-macrophage centers in Clarias gariepinus as an immunological biomarker for toxicity of silver nanoparticles. The Journal of Microscopy and Ultrastructure 5: 97-104.
- Brito IA, Freire CA, Yamamoto FY, Assis HCS, Souza BLR, et al. (2012) Monitoring water quality in reservoirs for human supply through multi-biomarker evaluation in tropical fish. Journal of Environmental Monitoring 14: 615-625.
Citation: Ikpesu TO (2023) Oxidative Stress and Histopathological Alteration of the Liver of Oreochromis niloticus Induced by Sodium Dodecyl Sulphate. J Environ SciCurr Res 6: 045
Copyright: © 2023 Ikpesu TO, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.

