
Panax Ginseng Preparations Enhance Long Term Potentiation in Rat Hippocampal Slices by Glutamatergic NMDA and Kainate Receptor Mediated Transmission
*Corresponding Author(s):
Wilfried DimpfelJustus-Liebig-University Giessen, Giessen, NeuroCode AG, Sportparkstr 9, D-35578 Wetzlar , Germany
Email:w.dimpfel@neurocode-ag.com
Alexander G Panossian
Phytomed AB, Bofinkvagen 1, Vaxtorp 31275, Sweden
Tel:+46 73330626,
Email:ap@phytomed.se
Abstract
Background: Root of the Korean red ginseng (Panax ginseng CA Meyer) is used in traditional medicinal systems to enhance cognitive function. In this study we compared the effects of HRG80 with a Standard Ginseng Preparation (SGP) on the excitability of pyramidal cells in the hippocampus of rats by using hippocampal long-term potentiation.
The aim of the study: The aim of this study was to compare the effects of HRG80 with SGP on the excitability of pyramidal cells in the hippocampus of rats, and to elucidate a possible mechanism of their action by using hippocampal long-term potentiation, a memory model based on modulation of glutamatergic neurotransmission.
Methods: Red Ginseng preparations were orally administered at daily doses of 10 mg/kg, 25 mg/kg, and 50 mg/kg to rats for 1 week before ex vivo analysis of the excitability of hippocampus slices was performed the following day. Hippocampal slices were stimulated in vitro with Single Stimuli (SS) or Theta Burst Stimuli (TBS) in order to activate the Schaffer Collaterals targeting pyramidal cells in the presence or absence of six various glutamatergic receptor antagonists.
Results: Both P.ginseng preparations induced a dose dependent increase in the population spike in the presence of SS as well as in the presence of TBS leading to Long-Term Potentiation (LTP) compared to the placebo (glucose 1% 1 ml/kg). Comparison of the efficacy of both P.ginseng preparations revealed asuperior action of HRG80 Ginseng, reached considerably and statistically significantlyhigher population spike peak amplitudes than SGPin the presence of both stimulation modi. Onlyglutamatergic NMDA and Kainate receptor antagonists selectively reversed the actions ofHRG80 and SGP.
Conclusion: Ginseng HRG80 preparation [65,66] from hydroponically cultivated roots is more active than SGP. Ginseng induced higher excitability of pyramidal cells by modulation of ionotropic glutamate NMDA and Kainate receptor mediated transmission.
Keywords
Hippocampus Slices; HRG80; Hydroponic Cultivation; Kainate Receptor Agonist; Long-Term Potentiation; Nmda Receptor Agonis; Panax Ginseng
ABBREVIATIONS
HRG80 – Hydroponically cultivated red ginseng preparation HRG80
SGP - Standard Ginseng Preparation
NMDA - Ionotropic glutamate agonist N-Methyl-D-aspartic Acid
AMPA - Ionotropic glutamate agonistamino-3-hydroxy-5-Methylisoxazoleproprionic Acid
CGS 19755 - Ionotropic glutamateNMDA receptor antagonist cis-4-[Phosphomethyl]-piperidine-2- Carboxylic acid
NBQX - Ionotropic glutamateAMPA receptor antagonist 2,3-Dioxo-6-nitro-1,2,3,4-tetrahydrobenzo[f] quinoxaline-7- Sulfonamide
UBP301 - Ionotropic glutamate Kainate receptor antagonist
YM298198 - Metabotropic glutamate Group I receptor antagonist
(RS)-APICA - Metabotropic glutamate Group II receptor antagonist
MSOP - Metabotropic glutamate Group III receptor antagonist
INTRODUCTION
Preparations of Panax ginseng CA Meyer, belonging to the Araliaceae plant family, have been used for thousands of years within traditional Chinese medicine [1]. Research on Panax ginseng has been conducted in 64 countries, mainly in Asia (South Korea and China). Publications are increasing exponentially. About 39% of them deal with preclinical and clinical pharmacology [2], including the effects on cognitive functions [3-14]. The main active constituents are believed to be tetracyclic triterpenoid glycosides - ginsenosides [15,16]. Red Ginseng preparation HRG80, obtained by hydroponic cultivation contains an 8-fold higher content of presumably more active rare ginsenosides than standard white Ginseng preparation (SGP), which is not steam cooked and therefore has minor amounts of rare ginsenosides. We hypothesized that HRG80 acts with higher efficacy than SGP, due to higher levels of rare ginsenosides [17-19].
The aim of this study was to compare the effects of HRG80 with SGP on the excitability of pyramidal cells in the hippocampus of rats, and to elucidate the mechanism of their action with respect to modulation of glutamatergic transmission. We used electric induction of hippocampal Long-Term Potentiation (LTP) in vitro, as a model of time and spatial dependent memory [20].
Hippocampal slice preparation is a validated model for direct analysis of the interaction of substances with living neuronal tissues, through theta burst stimulation [21,22]. Due to the preservation of the three-dimensional structure of the hippocampal tissue, substance effects on the excitability of pyramidal cells can be studied in a unique manner. The stimulation of Schaffer Collaterals leads to release of glutamate, resulting in excitation of the postsynaptic pyramidal cells. The result of electrical stimulation was recorded as a so-called population spike (pop-spike) (Figure 1).
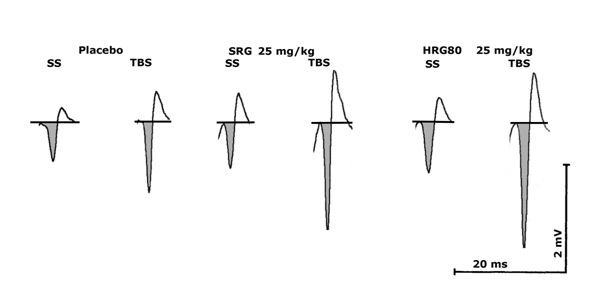
Figure 1: Documentation of an original signal (from one slice) showing the effects of using Single Stimuli (SS) or Theta Burst Stimulation (TBS) in the presence of Artificial Cerebro-Spinal Fluid (ASCF), glucose 1% - 1ml/kg, HRG80 25 mg/kg, SGP 25 mg/kg (for 1 week) in slices from 2 month old rats. The amplitude is calculated from baseline to the down reflection of the signal (shaded). Scales: Time is given in milliseconds (ms), amplitude in millivolts (mV).
The amplitude of the resulting population spike represents the number of recruited pyramidal cells. The advantages of the model are the possibility of recording in vitro for 8 hours, and also the ability to modify the excitability of the system. Analysis of hippocampal pyramidal cell activity by examining changes in amplitude of the population spike, in response to therapeutic interventions, is useful for assessment of their potential efficacy to investigate brain function under strictly controlled laboratory conditions.
In the present study, we used this model under ex vivo conditions. Ginseng preparations, HRG80 or SGP, were administered daily for a week, and the hippocampus was removed the following day for in vitro characterization of the sensitivity of the intra-hippocampal pathway to electric stimulation. When the repetitive administration succeeded in changing the communication structure within the hippocampus, either higher or lower population spike amplitudes were recorded. Within a second set of experiments of our study, the mechanism of action of the preparation under investigation was analyzed by testing several modulators of neurotransmission, with respect to their ability to change neuronal transmission in an agonistic or antagonistic manner, depending on the effect of the preparation.
MATERIALS AND METHODS
Test sample and reference standard
Powdered Red Ginseng HRG80 preparation (6.2% rare and 7.6% total ginsenosides) was obtained from hydroponically cultivated Korean ginseng (P.ginseng Meyer) root in controlled conditions to contain 12–15% total ginsenosides and 10-12% bioavailable (or rare) ginsenosides at Botalys S. A. (Batch No. PGC-190301-001; Ath, Belgium). Harvested roots were air-dried and steamed to red ginseng. The red ginseng was then powdered and sifted at 300 µm and standardized for the content of rare ginsenosides and the ginsenoside profile. HRG80preparation was standardized for the content of the rare ginsenosides Rh1, Rg2, Rg6, Rh4, Rg3, PPT, Rk1, C(K), Rh2, Rh3 and PPD (calculated as ginsenoside Rb1) and for the total ginsenosides Rg1, Re, Rf, Rh1, Rg2, Rb1, Rc, Rb2, Rd, Rg6, Rh4, Rg3, PPT, Rk1, C(K), Rh2, Rh3 and PPD.
The reference standard, P. ginseng Meyer powdered root (Batch No. 38837487; Arkopharma Laboratories, Carros, France), contained 0.8% rare and 2.6% total ginsenosides. The reference standard was analyzed and certified by Botalys S.A.
In vitro assay on hippocampal slices
Hippocampal slices were obtained from 22 adult male CDrats at the age of 2 months (Charles River Wiga, Sulzbach, Germany). Rats were kept under a reversed day/night cycle for 2 weeks prior to the start of the experiments to allow recording of in vitro activity from slices during the active phase of their circadian rhythm [23]. Animals were exsanguinated under ether anesthesia; the total brain was removed and the hippocampal formation was isolated under a micro stereoscopic vision system. The midsection of the hippocampus was fixed to the table of a vibrating microtome (Rhema Labortechnik, Hofheim, Germany) using a cyanoacrylate adhesive, submerged in chilled bicarbonate-buffered saline (artificial cerebrospinal fluid [ACSF]: NaCl: 124 mM, KCl: 5 mM, CaCl2: 2 mM, MgSO4: 2 mM, NaHCO3: 26 mM, glucose: 10 mM), and cut into 400 µm thick slices. All slices were pre-incubated for at least 1 h in Carbogen saturated ACSF (pH 7.4) in a pre-chamber before use [24].
During the experiment, the slices were held and treated in a special super-fusion chamber (List Electronics, Darmstadt, Germany) [25] at 35? [26]. The preparation was super-fused with ACSF at 180-230 mL/h. Electric stimulation (200 µA constant current pulses of 200 µs pulse width) of the Schaffer Collaterals within the CA2 area and recording of extracellular field potentials from the pyramidal cell layer of CA1 [24] was performed according to conventional electrophysiological methods, using the Labteam Computer system NeuroTool software package (MediSyst GmbH, Linden, Germany). Measurements were performed at 10 min intervals to avoid potentiation mechanisms. The results of four stimulations, each 20 s apart, were averaged for each time point. After obtaining three stable responses to SS, LTP was induced by applying a Theta Burst Stimulus (TBS). The mean amplitudes of three signals were averaged to give mean absolute voltage values (Microvolt) ± Standard Error (SE) of the mean for four slices for one of the experimental conditions. Four or five slices from one rat were used per day.
Slices were used one day after the daily oral administration of HRG80 Panax Ginseng, Arkopharma Panax Ginseng (SGP), or glucose 1%, for one week by use of an intra-gastric gavage. Ten slices from 2 animals were averaged to give one final value for each dosage. HRG80 Panax Ginseng was administered daily at a dosage of 10 mg/kg, 25 mg/kg, and 50 mg/kg. Arkopharma Panax Ginseng was administered daily at a dose of 10 mg/kg, 25 mg/kg, and 50 mg/kg.
Statistical analysis
The results are reported as mean ± SD (standard deviation) or ± SE for the indicated number of experiments. All statistical tests were two-sided tests and p-values <0.05 wereregarded as significant. The Wilcoxon and Mann Whitney Utests were also used throughout all experimental analyses for comparison of results obtained by vehicle administration andtiming with respect to electrophysiological data. Two-way ANOVA was used to examine interaction effectsand dose dependent responses between the two treatments, HRG80 and SGP.
RESULTS
Dose dependence of ginseng induced excitatory neurotransmission in rat hippocampal slice preparations ex vivo
The study comprised of two sets of experiments. The first one aimed to compare effectiveness of HRG80 with effectiveness of SGP by analysis of dose-response relationships.Both preparations were repeatedly administered to rats in three doses for one week. The hippocampal slices were prepared on the day following the last day of treatment, and the excitability of the pyramidal cells was tested in vitro by stimulation of the Schaffer collaterals by means of single electric stimuli, as well as theta burst stimulation leading to long-term potentiation.
Examples for responses (amplitudes of population spikes) to single stimuli (SS) or theta burst stimulation (TBS) are shown for a single slice from a rat under the placebo condition (glucose 1%, 1 ml/kg a day for 1 week), an HRG80treated animal (25 mg/kg a day for 1 week) and anSGP treated animal (25 mg/kg a day for one week) in Figure 1. The HRG80 treated animalsclearly showed higher amplitudes (given in mV) in the presence of Single Stimuli (SS), as well as after Theta Burst Stimulation (TBS).
The intra-gastric administration of placebo resulted in an average amplitude of 1151 µV in the presence of single electric stimuli (Figure 2). Theta Burst Stimulation (TBS) led to average amplitude of 2723 µV.
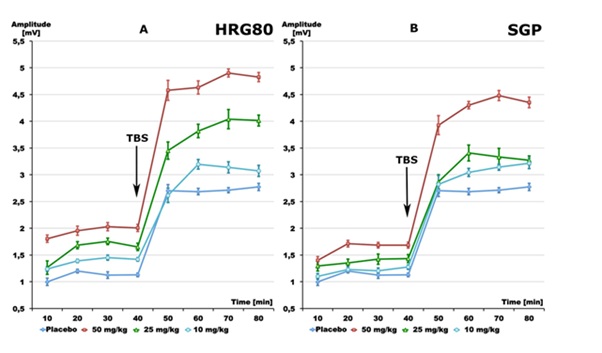
Figure 2: Effects of: A - HRG80 (10, 25 and 50 mg/kg), B - SGP (10, 25 and 50 mg/kg) and Placebo (glucose 1% 1 ml/kg) on pyramidal cell activity in terms of changes in population spike amplitudes (as voltage on the ordinate). Results as obtained after single stimuli (mean of 10-40 min) or after theta burst stimuli (mean of 50-80 min). Data are given as mean ± S.E.M. of n=10 slices from 2 animals/group. SS=single stimuli, TBS=theta burst stimuli.
Both preparations enhanced the responses to single and theta burst stimulation. However, higher doses of SGP were needed to achieve effects compatible to the effects of HRG80 (Figure 2). Repeated administration of HRG80 at the doses of 10 mg/kg, 25 mg/kg, and 50 mg/kg for 1 week led to statistically significant increases in the population spike amplitude in comparison to the placebo during single Shock Stimulation (SS) as well as during TBS. Peak values of 1996 µV after single stimuli and 4787 µV after TBS were measured after administration of 50 mg/kg (Figure 2A).Repeated administration of SGPat the doseof 10 mg/kg did not show increases in the amplitude of the population spike in comparison to controls after SS (Figure 2B). Only higher doses of 25 mg/kg or 50 mg/kg SGPsignificantly increased the population spike amplitude in comparison to the placebo during single Shock Stimulation (SS), as well as during TBS, reaching amplitudes of 1693 µV and 4377 µV, respectively (p<0.01), Figure 2B.
Direct comparison ofthe dose-response curves of HRG80 and SGPreveals a clear quantitative difference in the increasein amplitude of the population spikes in the presence of SS (Figure 3A, p <0.0001),as well as in the presence of TBS, (Figure 3B, p = 0.0001). The effect of HRG80 on the amplitude of the population spike is significantly stronger than the effect of SGP at the lowest dose of 10 mg/kg (Figure 3A).
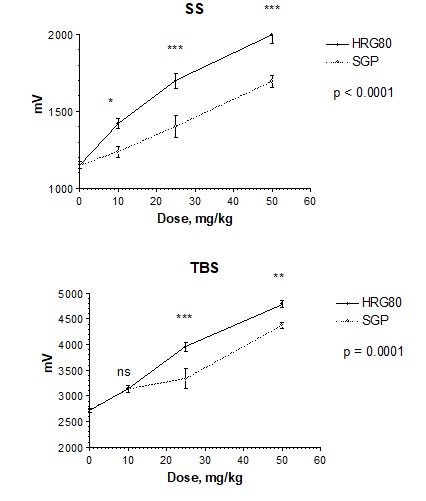
Figure 3: Effects of HRG80 and SGP supplementation for 1week on hippocampal pyramidal cell population spike amplitudes (µV, mean ± SEM) after A - single stimuli (SS, from 20-40 min), and B -theta burst stimuli (TBS, from 60-80 min)ex vivo. Statistically significant interaction effects between dose and response (mV) of two treatments (p = 0.0004) were observed in SS (p<0.0001, two-way ANOVA) and TBS (p=0.0001, two-way ANOVA), indicating that the HRG80 treatment was more beneficial than the ginseng control treatment SGP. *- p<0.05, ** - p <0.01, ***-p<0.001, ns – not significant in Bonferroni post-tests.
Effects of selective glutamatergic receptor antagonists on ginseng induced excitatory neurotransmission in the rat hippocampal slicepreparation ex vivo
Ginseng preparationsHRG80 and SGP were orally administered by gavage in equipotent doses of 25 mg/kg for HRG80 and 50 mg/kg SGP correspondingly for1 week.
In order to test a possible interaction of ginseng preparations with various glutamate receptors, HRG80 and SGP were orally administered by gavage in equipotent doses of 25 mg/kg for HRG80 and 50 mg/kg SGP correspondingly for 1 week and glutamatergic neurotransmission of isolated hippocampal slices was analyzed in vitro by addition of their selective antagonists: CGS 19755 - ionotropic glutamate NMDA receptor antagonist, NBQX - ionotropic glutamate AMPA receptor antagonist, UBP301 - ionotropic glutamate Kainate receptor antagonist, YM298198 - metabotropic glutamate Group I receptor antagonist, (RS)-APICA - metabotropic glutamate Group II receptor antagonist, and MSOP - metabotropic glutamate Group III receptor antagonist.
Tables 1 and 2 show that only NMDA and Kainate receptor antagonists prevent HRG80 (Table 1) and SGP (Table 2) induced enhancement of the population spike amplitude, in the presence of SS and TBS (Figures 4 and 5).
|
|
SS |
Significance of difference |
TBS |
Significance of difference |
|
|
[µV] |
HRG80 vs. antagonist p |
[µV] |
HRG80 vs. antagonist |
|
Mean (SEM) |
|
Mean (SEM) |
p |
|
|
|
60-80 min |
|
100-120 min |
|
|
HRG80 |
-1697.17 (45.92) |
|
-3957.57 (88.46) |
|
|
+CGS19755 0.25µM |
-1242.65 (46.98) |
<0.01 |
-2915.54 (87.26) |
<0.01 |
|
+NBQX 0.05 µM |
-1715.92 (51.32) |
n.s. |
-4061.13 (195.48) |
n.s. |
|
+UBP301 0.025 µM |
-1219.44 (34.97) |
<0.01 |
-2875.83 (59.10) |
<0.01 |
|
+YM298198 0.05 µM |
-1782.96 (29.19) |
n.s. |
-3797.79 (54.54) |
n.s. |
|
+RS-APICA 0.10 µM |
-1817.25 (37.85) |
n.s. |
-3778.46 (77.87) |
n.s. |
|
+MSOP 0.05 µM |
-1736.33 (55.23) |
n.s. |
-3776.17 (58.24) |
n.s. |
Table 1: Effects of six selective glutamate receptor antagonists on HRG80- (25 mg/kg daily for 1week, ex vivo) induced excitability of pyramidal cells in the hippocampus of rats. Hippocampal pyramidal cell population spike amplitudes (µV, mean ± SEM) after Single Stimuli (SS) and Theta Burst Stimuli (TBS). Summary of results from 8-10 slices with each antagonist.
|
|
SS |
Significance of difference |
TBS |
Significance of difference |
|
|
[µV] |
SGP vs. antagonist p |
[µV] |
SGP vs. antagonist p |
|
|
Mean (SEM) |
|
Mean (SEM) |
|
|
|
60-80 min |
|
100-120 min |
|
|
SGP |
-1693.67 (37.36) |
|
-4377.97 (54.25) |
|
|
+CGS19755 0.25µM |
-1154.16 (30.62) |
<0.01 |
-4178.96 (123.12) |
<0.01 |
|
+NBQX 0.05 µM |
-1850.46 (21.02) |
<0.05 |
-4164.75 (88.46) |
n.s. |
|
+UBP301 0.025 µM |
-1142.66 (18.22) |
<0.01 |
-2924.50 (157.62) |
<0.01 |
|
+YM298198 0.05 µM |
-1888.29 (31.29) |
<0.01 |
-4232.46 (113.46) |
n.s. |
|
+RS-APICA 0.10 µM |
-1827.79 (42.85) |
<0.05 |
-4110.25 (61.78) |
<0.01 |
|
+MSOP 0.05 µM |
-1882.83 (37.36) |
n.s. |
-4209.08 (54.25) |
n.s. |
Table 2: Effects of six selective glutamate receptors antagonists on SGP- (50 mg/kg daily for 1week, ex vivo) induced excitability of pyramidal cells in the hippocampus of rats. Hippocampal pyramidal cell population spike amplitudes (µV, mean ± SEM) after Single Stimuli (SS) and Theta Burst Stimuli (TBS). Summary of results from 8-10 slices (GRS) with each antagonist.
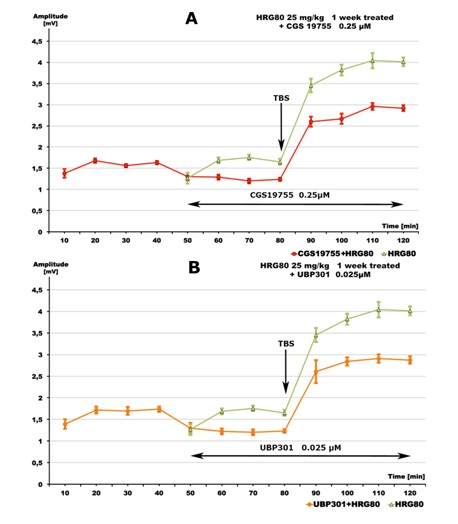 Figure 4: Inhibitory effects of: A - ionotropic glutamate NMDA receptor antagonistCGS 19755 (0.25 μM, red line), and B - ionotropic glutamate Kainate receptor antagonist UBP301 (0.025 μM, yellow line) on HRG80- (25 mg/kg daily for 1 week) induced increase of excitability of rat hippocampal pyramidal cells ex vivo (green lines). Data are given as mean ± S.E.M. of n=10 (green line) or n=8 (red line) slices from 2 animals/group. SS=single stimuli, TBS=theta burst stimuli.
Figure 4: Inhibitory effects of: A - ionotropic glutamate NMDA receptor antagonistCGS 19755 (0.25 μM, red line), and B - ionotropic glutamate Kainate receptor antagonist UBP301 (0.025 μM, yellow line) on HRG80- (25 mg/kg daily for 1 week) induced increase of excitability of rat hippocampal pyramidal cells ex vivo (green lines). Data are given as mean ± S.E.M. of n=10 (green line) or n=8 (red line) slices from 2 animals/group. SS=single stimuli, TBS=theta burst stimuli.
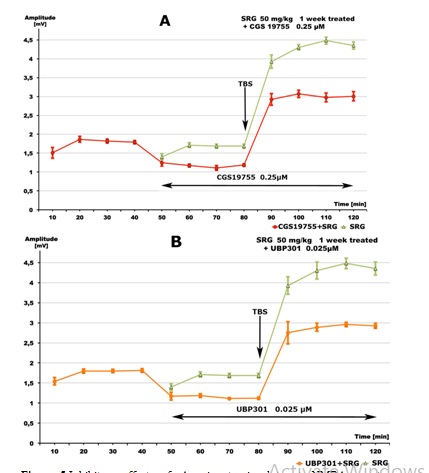
Figure 5: Inhibitory effects of: A - ionotropic glutamate NMDA receptor antagonist CGS 19755 (0.25 μM, red line), and B - ionotropic glutamate Kainate receptor antagonist UBP301 (0.025 μM, yellow line) on SGP- (50 mg/kg daily for 1 week) induced increase of excitability of rat hippocampal pyramidal cells ex vivo (green lines). Data are given as mean ± S.E.M. of n=10(green line) or n=8 (red line) slices from 2 animals/group. SS=single stimuli, TBS=theta burst stimuli.
DISCUSSION
Herbal preparations of the underground parts of P. ginseng have been used in the traditional medicine systems of Eastern Asia for centuriesfor many conditions, including aging-related neurodegeneration and memory decline [1, 27]. In the European Union, herbal preparations of P. ginseng have been used for at least for 30 years as a “tonic in case of tiredness, weakness, and decreased mental and physical capacity, as well as to improve concentration and to improve the general condition during convalescence” [28]. An increasing body of scientific evidences suggests that ginseng is an effective and safe treatment for impaired cognitive functions [5,6,8,9,12-14,29-31].
Ginsenosides, the main active ingredients of Panax ginseng, exhibit neuroprotective effects in different animal models of neurodegenerative diseases [32-34]. However, biological activity of various ginsenosides in various test models is quite different due to their structure dependent bioavailability and selectivity to various receptors [33,35].
In our study, we compared biological activity of hydroponically cultivated red ginseng preparation HRG80, containing 7-fold higher amounts of rare ginsenosides and 3-fold more total ginsenosides than SGP with the aim of obtaining evidence supporting claims that rare ginsenoside enriched ginseng preparations have superior activity.
The results of ourstudy revealed that ginseng preparations HRG80 and SGPsignificantly increase population spikes in the presence of SS in all tested doses, except of the dose of 10 mg/kg of SGP (Figure 2B).
This observation is in line with a previous publication by Zhu et al. 2015 [34], where chronic intraperitoneal injection of ginsenoside Rg1 at the doses of 1 - 10 mg/kg for 30 days facilitated weak TBS-induced Long-Term Potentiation (LTP) in acute hippocampal slices from middle-aged animals and promoted memory and hippocampal long-term potentiation.
The results of our study are also in line with another publication [36], where red ginseng had excitatory effects on substantia gelatinosa neurons via the activation of glutamate receptors.
The amplitudes of the population spikes after administration of 10 mg/kg of SGP were not significantly different from those after placebo administration, whereas HRG80 induced a highly statistically significant increase in the population spike. A medium dosage of 25 mg/kg of HRG80 reached the maximum value of 50 mg/kg of SGP (Figure 2A). Overall, HRG80 was more active thanSGP in increasing of population spikes both in the presence of SS (p <0.0001, Figure 3A) and in the presence of TBS, (p = 0.0001, Figure 3B). Consequently, we conclude that the superior activity of HRG80 is presumably due to the inherent high amount of rare ginsenosides, assuming no other substantial differences exist in the chemical composition of HRG80 and SGP.
As a matter of fact, besides ginsenosides, a lysophosphatidic acid-proteins complex, gintonin [37] has been reported to increase LTP [3] via interaction with glutamate NMDA receptors in rats [38].
Memory improvement effects of ginsenosides are associated both with their effects on neurogenesis [39] and synaptic plasticity-related protein expression [40-42]. These mechanisms include a plethora of intermediate steps and molecular targets, including glutamate mediated synaptic neurotransmission, which plays an important role in impairment of synaptic plasticity (learning) and long-term potentiation of memory.
Hypoactivity of glutamate mediated neurotransmission is associated with impaired neuronal plasticity, while hyperactivity of the glutamatergic system leads to neurodegeneration and neuronal dysfunction in the central nervous system [43]. Many drugs and natural compounds have biphasic dose dependent effects on glutamate mediated neurotransmission, which are mostly characterized as adaptive stress response (hormesis) [44-51]. The dose range of the reversal effect from hypo- to hyperactivity of neurotransmission depends on the neurotoxicity of the ligands.
As an example, the neurotoxin Kainic Acid (KA) activates a glutamate ionotropic receptor, resulting in impairment of hippocampus-dependent learning and spatial memory due to degeneration of pyramidal neurons and astrocyte damage, amongst others [32]. Kainate receptors play a crucial role in the control of synaptic integration and spike transmission efficacy at hippocampal mossy fiber synapses and are likely important for spatial information processing [52].
In contrast, ginsenosides, which are not toxic over a wide range of doses, protect glia, neurons, and cognitive function in a rat model of neurodegeneration.Pretreatment with ginsenosides at the doses of 30 or 40 mg/kg in rats significantly protected their pyramidal neurons against KA-induced acute excitotoxicity and delayed their injury. Ginsenosides also prevented memory impairments and protected astrocytes from KA induced acute excitotoxicity [32]. Their action leads to maintaining glutamate homeostasis which is very important for improving memory and cognitive functions.
Overall, maintenance of synaptic homeostasis that, is common for adaptogens per se [53], is key for effective cognitive functioning. Ginseng is an adaptogen [53-56], which in relatively small doses activates adaptive stress resonance and therefore protects against further stressful exposures, e.g. KA excitotoxicity.
In the second set of experiments of our study we aimed to elucidate the possible mechanism of action related to glutamatergic transmission within the hippocampus. Six different glutamate receptor antagonists were tested to reverse ginseng induced modulation of excitability of the pyramidal cells.
The neurotransmitter glutamate is known to activate several classes of metabotropic receptors and three major types of ionotropic receptors: Alpha-Amino-3-hydroxy-5-methyl-4-isoxazolepropionic Acid (AMPA), Kainate and N-Methyl-d-Aspartate (NMDA) [57]. Since the synapses between Schaffer collaterals and the hippocampal pyramidal cells use glutamate as a neurotransmitter, several glutamatergic receptors contribute to the enhancement of transmission. Glutamate not only interacts with GTP-binding protein coupled receptors (i.e., metabotropic glutamate receptor subtypes) but also binds to various ionotropic ligand-gated glutamate receptors, which include AMPA, Kainate and NMDA receptors [58,59].
Tables 1 and 2 show that HRG80- as well as SGP-induced excitatory neurotransmission in the hippocampal slices is selectively mediated by only two ionotropic glutamate receptors - NMDA and Kainate receptors. It is very unlikely that the minor effects of other glutamate receptors antagonist NBQX, YM298198 and (RS) APICA (Tables 1 and 2) have therapeutic significance.
Overall, both HRG80 and SGP seem to act by interfering only with glutamatergic NMDA receptor- and Kainate receptor-mediated neurotransmission in the rat hippocampus.
The results of our study are in line with the results of other studies where panaxadiol saponins (termed as the Rb fraction) protected the pyramidal neurons and maintained microglial homeostasis against Kanic acid-induced excitotoxicity [32].
Involvement of NMDA receptors in the neuroprotective activity of ginsenosides hasbeen demonstrated in several studies [35,60-62], however none of them suggest that ginseng activates LTP via activation of glutamatergic NMDA receptors. In contrast, Kimand colleagues (2002) “suggest that the inhibition of NMDA receptors by ginseng, in particular by ginsenoside Rg3, could be one of the mechanisms for ginseng-mediated neuroprotective actions [35]”.
It is noteworthy that inhibition of NDMA receptors was observed in vitro in comparatively high concentrations - 100 mg/l, while in our study activation was observed at doses of 25-50 mg/kg in rats, which corresponds to a concentration in blood of 10 mg/l (assuming that the oral bioavailability of ginsenosides is about 1-5%). That is in line with the hormesis concept related to dose-dependent reversal effects of drugs [44-51].
In a preceding investigation into Gingko extract marketed for improvement of mental functions, an increase in pyramidal cell response was shown on SS. However, there was also a difference in the effect of Ginseng, since the extent of the increase of LTP was significantly lower and hardly reached statistical significance in the presence of Ginkgo extract [63,64]. Thus, Ginseng is similar in its action in comparison to Gingko regarding improvement of communication at Schaffer collaterals after single shock challenge but differs quantitatively with respect to TBS for induction of long-term potentiation. The present results indicate a profound difference in the mechanism of action between Ginseng and Ginkgo based on the use of a total of different glutamate receptor modulators.
The effect of Ginseng could be traced to depend only on NMDA- and Kainate-receptor mediated signaling. The effect of ginkgo extract was much less specific since it could be attenuated by the presence of an NMDA receptor antagonist, one competitive and two non-competitive AMPA antagonists, as well as antagonists of the metabotropic mGluI and mGluII glutamate receptors[Dimpfel, unpublished]. Thus, one can conclude from the experiments in which glutamate receptor antagonistswere explored that Ginseng mediated signaling is specific - via specific interaction only with NMDA receptors and Kainate receptors.
CONCLUSION
Ginseng HRG80 preparation from hydroponically cultivated roots is more active than SGP in an electrophysiological hippocampal long-term potentiation model. HRG80- and SGP-induced activation of pyramidal cells in the hippocampus is selectively mediated by two ionotropic glutamate receptors, NMDA and Kainate, suggesting their potential beneficialeffects on aging related decline of cognitive functions, and specifically on spatial memory.
DECLARATIONS
Ethics approval
The principles of laboratory animal care were followed in all trials and the local authority (“Regierungspräsidium” Giessen), responsible for animal care, was informed according to German Health Guidelines. Details of the acclimatization, housing conditions, and surgery have been reported (Dimpfel, 1991). German Animal Protection Law (Tierschutzgesetz) states that animals are allowed to be sacrificedfor the removal of organs for scientific purposes. Permissionto keep animals are renewed by governmental authorities every 3 years.
Availability of data and material
The datasets used and/or analyzed during the current study are available from the corresponding author upon reasonable request.
Conflict of Interest
A.P. is self-employed at Research and Development Company Phytomed AB (Sweden) and has an independent contractor agreement with Europharma, USA. He is not a member of any pharmaceutical industry-sponsored advisory board and has no shares or financial interest in any pharmaceutical company. Authors W.D. and L.S. have no conflicts of interests and significant financial benefits in any pharmaceutical company. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript, or in the decision to publish the results.
Authors' Contributions
WD and AP planned the experiments, carried out data analysis, and wrote the manuscript; LS performed the experiments;all authors critically revised and approved the final version of the manuscript.
FUNDING
This work was supported in part by EuroPharma USA, Green Bay, WI, USA and Botalys SA Ath, Belgium. Sponsors of the research were Mr. Terrence Lemerond, EuroPharma Inc., USA; Pierre-Antoine Mariage, Botalys SA, Belgium; and Paul-Evence Coppee, Botalys SA, Belgium (grant number 2019-01).
ACKNOWLEDGEMENT
The authors acknowledge the support of Botalys SA for the supply of investigational agents and their characterization. Quality control was performed by Ingrid K. Keplinger-Dimpfel, NeuroCode AG, Wetzlar, Germany.
REFERENCES
- Lee SM, Bae BS, Park HW, Ahn NG, Cho BG, et al. (2015) Characterization of Korean Red Ginseng (Panax ginseng Meyer): History, preparation method, and chemical composition. J Ginseng Res 39: 384-391.
- Xu W, Choi HK, Huang L (2017) State of Panax ginseng Research: A Global Analysis. Molecules 22: 1518.
- Kim S, Kim M-S, Park K, Kim H-J, Jung S-W, et al. (2016) Hippocampus dependent cognitive enhancement induced by systemic gintonin administration. J Ginseng Research 40: 55-61.
- Choi J, Kim TH, Choi TY, Lee MS (2013) Ginseng for health care: a systematic review of randomized controlled trials in Korean literature. PLoS One. 8: 59978.
- Park K, Jin H, Rhee, HY, Kim S, Lee SE, et al. (2013) A randomized, double-blind, placebo-controlled clinical trial of Korean ginseng as a functional food in mild cognitive impairment. Alzheimers Dement 9: 804.
- Yeo HB, Yoon HK, Lee HJ, Kang SG, Jung KY, et al. (2012) Effects of Korean Red Ginseng on cognitive and motor function: A Double-blind, Randomized, Placebo-controlled Trial. J Ginseng Res 36: 190-197.
- Fu LM, Li JT (2011) A systematic review of single Chinese herbs for Alzheimer's disease treatment. Evid Based Complement Alternat Med 2011: 640284.
- Heo JH, Lee ST, Oh MJ, Park HJ, Shim JY, et al. (2011) Improvement of cognitive deficit in Alzheimer's disease patients by long term treatment with korean red ginseng. J Ginseng Res 35: 457-461.
- Heo JH, Lee ST, Chu K, Oh MJ, Park HJ, et al. (2008) An Open-Label Trial of Korean Red Ginseng as an Adjuvant Treatment for Cognitive Impairment in Patients With Alzheimer's Disease. Eur J Neurol. 15: 865-868.
- Geng J, Dong J, Ni H, Lee MS, Wu T, et al. (2010) Ginseng for cognition. Cochrane Database Syst Rev 12: CD007769.
- Lee MS, Yang EJ, Kim JI, Ernst E (2009) Ginseng for cognitive function in Alzheimer's disease: a systematic review. J Alzheimers Dis 18: 339-344.
- Lee ST, Chu K, Sim JY, Heo JH, Kim M (2008) Panax ginseng enhances cognitive performance in Alzheimer disease. Alzheimer Dis Assoc Disord 22: 222-226.
- Reay JL, Kennedy DO, Scholey AB (2006) Effects of Panax Ginseng, Consumed With and Without Glucose, on Blood Glucose Levels and Cognitive Performance During Sustained 'Mentally Demanding' Tasks. J Psychopharmacol. 20: 771-781.
- Reay JL, Kennedy DO, Scholey AB (2005) Single doses of Panax ginseng (G115) reduce blood glucose levels and improve cognitive performance during sustained mental activity. J Psychopharmacol 19: 357-365.
- Kiefer D, Pantuso T (2003) Panax ginseng. Am Fam Physician 68: 1539-1542.
- Smith I, Williamson EM, Putnam S, Farrimond J, Whalley BJ (2014) Effects and Mechanisms of Ginseng and Ginsenosides on Cognition. Nutr Rev 72: 31.
- Yu S, Zhou X, Li F, Xu C, Zheng F, et al. (2017) Microbial transformation of ginsenoside Rb1, Re and Rg1 and its contribution to the improved anti-inflammatory activity of ginseng. Sci Rep 7: 138.
- Jakaria M, Haque ME, Kim J, Cho DY, Kim IS, et al. (2018) Active ginseng components in cognitive impairment: Therapeutic potential and prospects for delivery and clinical study. Oncotarget 9: 33601-33620.
- Zheng MM, Xu FX, Li YJ, Xi XZ, Cui XW, et al. (2017) Study on Transformation of Ginsenosides in Different Methods. Biomed Res Int 2017: 8601027.
- Bliss TVP, Lomo T (1973) Long-lasting potentiation of synaptic transmission in the dentate area of the anaesthetized rabbit following stimulation of the perforant path. J Physiology 232: 331- 356.
- Dingledine R (1984) Brain Slices. Plenum Press, New York, USA.
- Lynch G, Schubert P (1980) The Use of in Vitro Brain Slices for Multidisciplinary Studies of Synaptic Function. Ann Rev Neurosci 3: 1-22.
- Dimpfel W, Dalhoff B, Hofmann W, Schlüter G (1994) Electrically evoked potentials in the rat hippocampus slice in the presence of aminophylline alone and in combination with quinolones. European Neuropsychopharmacology 4: 151-156.
- Dimpfel W, Spüler M, Dalhoff A, Hoffmann W, Schlüter G (1991) Hippocampal Activity in the Presence of Quinolones and Fenbufen in Vitro. Antimicrobial Agents and Chemotherapy 6: 1142-1146.
- Haas HL, Schaerer B, Vosmansky M (1979) A simple perfusion chamber for the study of nervous tissue slices in vitro. J Neurosci Methods 1: 323-325.
- Schiff SJ, Somjen GG (1985) The Effects of Temperature on Synaptic Transmission in Hippocampal Tissue Slices. Brain Research 345: 279-284.
- Radad K, Gille G, Liu L, Rausch WD(2006) Use of ginseng in medicine with emphasis on neurodegenerative disorders. J Pharmacol Sci 100: 175-186.
- European Medicines Agency (2013) Assessment report on Panax ginsengA. Meyer, radix Based on Article 16d(1), Article 16f and Article 16h of Directive 2001/83/EC as amended (traditional use). European Medicines Agency, Amsterdam, Netherlands.
- Kennedy DO, Scholey AB, Wesnes KA (2001) Dose dependent changes in cognitive performance and mood following acute administration of Ginseng to healthy young volunteers. Nutr. Neurosci 4: 295-310.
- Kennedy DO, Reay JL, Scholey AB (2007) Effects of 8 weeks administration of Korean panax ginseng extract on the mood and cognitive performance of healthy individuals. J Ginseng Res. 31: 34-43.
- Kennedy DO, Scholey AB (2003) Ginseng: potential for the enhancement of cognitive performance and mood. PharmacolBiochemBehav 75: 687-700.
- Xu K, Zhang Y, Wang Y, Ling P, Xie X, et al. (2014) Ginseng Rb fraction protects glia, neurons and cognitive function in a rat model of neurodegeneration. PLoS One 9: 101077.
- Zhang BB, Hu XL, Wang YY, Li JY, Pham TA, et al. (2019) Neuroprotective Effects of Dammarane-Type Saponins from Panax notoginseng on Glutamate-Induced Cell Damage in PC12 Cells. Planta Med 85: 692-700.
- Zhu G, Wang Y, Li J, Wang J (2015) Chronic treatment with ginsenoside Rg1 promotes memory and hippocampal long-term potentiation in middle-aged mice. Neuroscience 4: 81-89.
- Kim S, Ahn K, Oh TH, Nah SY, Rhim H (2002) Inhibitory effect of ginsenosides on NMDA receptor-mediated signals in rat hippocampal neurons. Biochem Biophys Res Commun 296: 247-254.
- Yin H, Park SA, Park SJ, Han SK (2011) Korean Red Ginseng Extract Activates Non-NMDA Glutamate and GABAA Receptors on the Substantia Gelatinosa Neurons of the Trigeminal Subnucleus Caudalis in Mice. J Ginseng Res 35: 219-225.
- Hwang SH, Shin EJ, Shin TJ, Lee BH, Choi SH, et al. (2012) Gintonin, a ginseng-derived lysophosphatidic acid receptor ligand, attenuates Alzheimer's disease-related neuropathies: involvement of non-amyloidogenic processing. J Alzheimers Dis 31: 207-223.
- Shin T-J, Kim H-J, Kwon B-J, Choi SH, Kim HB, et al. (2012) Gintonin, a Ginseng-Derived Novel Ingredient, Evokes Long-Term Potentiation Through N-methyl-D-aspartic Acid Receptor Activation: Involvement of LPA Receptors. Mol Cells 34: 563-572.
- Hou J, Xue J, Lee M, Liu L, Zhang D, et al. (2013) Ginsenoside Rh2 improves learning and memory in mice. J Med Food 16: 772-776.
- Zhao H, Li Q, Pei X, Zhang Z, Yang R, et al.(2009) Long-term Ginsenoside Administration Prevents Memory Impairment in Aged C57BL/6J Mice by Up-Regulating the Synaptic Plasticity-Related Proteins in Hippocampus. Behav Brain Res 201: 311-317.
- Yang L, Zhang J, Zheng K, Shen H, Chen X (2014) Long-term ginsenoside Rg1 supplementation improves age-related cognitive decline by promoting synaptic plasticity associated protein expression in C57BL/6J mice. J GerontolA Biol Sci Med Sci 69: 282-294.
- Shi YQ, Huang TW, Chen LM, Pan XD, Zhang J, et al. (2010) Ginsenoside Rg1 attenuates amyloid-beta content, regulates PKA/CREB activity, and improves cognitive performance in SAMP8 mice. J Alzheimers Dis 19: 977-989.
- Parsons CG, Stöffler A, Danysz W (2007) Memantine: A NMDA Receptor Antagonist That Improves Memory by Restoration of Homeostasis in the Glutamatergic System--Too Little Activation Is Bad, Too Much Is Even Worse. Neuropharmacology 53: 699-723.
- Calabrese EJ, Mattson MP (2017) How does hormesis impact biology, toxicology, and medicine? NPJ Aging Mech Dis. 3:13.
- Calabrese V, Cornelius C, Dinkova-Kostova AT, Calabrese EJ, Mattson MP (2010) Cellular Stress Responses, The Hormesis Paradigm, and Vitagenes: Novel Targets for Therapeutic Intervention in Neurodegenerative Disorders. Antioxid Redox Signal 13: 1763-1811.
- Mattson MP (2008) Hormesis and disease resistance: activation of cellular stress response pathways. Hum Exp Toxicol 27: 155-162.
- Mattson MP (2008) Dietary factors, hormesis and health. Ageing Res Rev 7: 43-48.
- Mattson MP (2008) Hormesis defined. Ageing Res Rev 7: 1-7.
- Mattson MP, Cheng A (2006) Neurohormetic phytochemicals: Low-dose toxins that induce adaptive neuronal stress responses. Trends Neurosci 29: 632-639.
- Son TG, Camandola S, Mattson MP (2008) Hormetic dietary phytochemicals. Neuromolecular Med 10: 236-246.
- Stranahan AM, Mattson MP (2012) Recruiting adaptive cellular stress responses for successful brain ageing. Nat. Rev Neurosci. 13: 209-216.
- Sachidhanandam S, Blanchet C, Jeantet Y, Cho YH, Mulle C. (2009) Kainate Receptors Act as Conditional Amplifiers of Spike Transmission at Hippocampal Mossy Fiber Synapses. The J of Neuroscience 29: 5000.
- Panossian A (2017) Understanding adaptogenic activity: specificity of the pharmacological action of adaptogens and other phytochemicals. Ann NY Acad Sci 1401: 49-64.
- Liao LY, He YF, Li L, Meng H, Dong YM, et al. (2018). A preliminary review of studies on adaptogens: comparison of their bioactivity in TCM with that of ginseng-like herbs used worldwide. Chin Med 13: 57.
- Nocerino E, Amato M, Izzo AA (2000) The aphrodisiac and adaptogenic properties of ginseng. Fitoterapia 1: 1-5.
- Patel S, Rauf A (2017). Adaptogenic Herb Ginseng (Panax) as Medical Food: Status Quo and Future Prospects. Biomed Pharmacother 85: 120-127.
- Bennett DA, Lehmann J, Bernard PS, Liebman JM, Williams M, Wood, et al. (1990) CGS 19755: A Novel Competitive N-methyl-D-aspartate (NMDA) Receptor Antagonist With Anticonvulsant, Anxiolytic and Anti-Ischemic Properties. Prog Clin Bio Res 361: 519-524.
- Hollmann M, Heinemann S (1994) Cloned glutamate receptors. Annu. Rev Neurosci 17: 31-108.
- Moriyoshi, K, Masu M, Ishii T, Shigemoto R, Mizuno N, et al. (1991) Molecular cloning and characterization of the rat NMDA receptor. Nature 354: 31-37.
- Shen Y, Bhattarai JP, Park SJ, Lee GS, Ryu PD, et al. (2018) Korean red ginseng excitation of paraventricular nucleus neurons via non-N-methyl-D-aspartate glutamate receptor activation in mice. J Vet Sci 19: 172-178.
- Kim JH, Cho SY, Lee JH, Jeong SM, Yoon IS, et al. (2007) Neuroprotective effects of ginsenoside Rg3 against homocysteine-induced excitotoxicity in rat hippocampus. Brain Res 1136: 190-199.
- Zhang YF, Fan XJ, Li X, Peng LL, Wang GH, et al. (2008) Ginsenoside Rg1 Protects Neurons From Hypoxic-Ischemic Injury Possibly by Inhibiting Ca2+ Influx Through NMDA Receptors and L-type Voltage-Dependent Ca2+ Channels. Eur J Pharmacol 586: 90-99.
- Dimpfel W (1995) Effects of Memantine on Synaptic Transmission in the Hippocampus in vitro. Arzneimittelforschung 45: 1- 5.
- Dimpfel W, Kler A, Kriesl E, Lehnfeld R, Keplinger-Dimpfel IK (2006) Neurophysiological characterization of a functionally active drink containing extracts of ginkgo and ginseng by source density analysis of the human EEG. Nutr Neurosci 9: 213-324.
- Mariage PA, Hovhannisyan A, Panossian AG (2020) Efficacy of Panax ginseng Meyer Herbal Preparation HRG80 in Preventing and Mitigating Stress-Induced Failure of Cognitive Functions in Healthy Subjects: A Pilot, Randomized, Double-Blind, Placebo-Controlled Crossover Trial. Pharmaceuticals (Basel) 13: 57.
- Lemerond T and Panossian AG (2020) Panax ginseng Meyer Herbal Preparation HRG80 for Preventing and Mitigating Stress-Induced Failure of Cognitive Functions in Healthy Subjects. J Altern Complement Integr Med 6: 100.
Citation: Dimpfel W, Schombert L, Panossian AG (2020) Panax Ginseng Preparations Enhance Long Term Potentiation in Rat Hippocampal Slices by Glutamatergic NMDA and Kainate Receptor Mediated Transmission. J Altern Complement Integr Med 6: 106.
Copyright: © 2020 Wilfried Dimpfel, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.

