Polycyclic Aromatics Hydrocarbons and Organochlorine Pesticides in the Environment: Sources, Routes, Effects, and Fate
*Corresponding Author(s):
Adelodun AADepartment Of Marine Science And Technology, The Federal University Of Technology, PMB 704, Akure, Nigeria
Tel:+234 9055487919,
Abstract
Persistence Organic Pollutants (POPs) contamination of sediment, soil, and groundwater is one of the major threats to environmental and human health due to their toxic effects. Thus, this short review is aimed at evaluating the sources, exposure routes, effects, and the fate of polycyclic aromatic hydrocarbons (PAHs) and organochlorine pesticides (OCPs) in the environment. As useful as these chemicals may be, their adverse effects on human health and the total environment are of grievous concerns. With the adverse effects, have serious health concerns. “The findings from this short communication provides succinct albeit detailed information on the current environmental status and health risks posed by the residues of PAHs and OCPs, especially in our soils and waters. To this effect, the future environmental effect and eventual fate of pesticides could be progressively monitored and mitigated." research thus, provide information on the current and health risk residue levels of organochlorine pesticides and PAHs in soil from this region with which future environmental performance on the use of pesticides on the environment could be progressively monitored.
Keywords
Carcinogenicity; Genotoxicity; Organochlorine pesticides; Persistent organic pollutants; Polycyclic aromatic hydrocarbons; Teratogenicity; Toxicity
INTRODUCTION
Since the advent of industrialization, human exposure to pesticides has become a primary and huge occupational and environmental hazard [1]. Several health issues are associated with the continuous use of pesticides to ensure adequate agricultural yield [2]. As a result, agriculturalists are most susceptible to pesticide-related maladies. Whereas, some non-occupational hazards could result from exposure to polluted ecosystems. Based on available statistics, over a million of acute and chronic illnesses, even death have been attributed to pesticide poisoning globally [3]. The after-effect of abuse or overuse of pesticides on environmental health cannot be overemphasized. The wide range of living organisms prone to one pesticide or the other include microbes, invertebrates, plants, Pisces, and vertebrates [4,5].
Some pesticides are environmentally refractory, i.e., they tend to be stable in the ecosystem, giving them a long life span. These pesticides are popularly referred to as Persistent Organic Pollutants (POPs). Although these pesticides are effective for their intended use, they are, however, environmentally recalcitrant and toxic [6]. In this mini-review, we examined the nature, route of exposure, and the fate of Polycyclic Aromatic Hydrocarbons (PAHs) and Organochlorine Pesticides (OCPs), the two most prominent groups of POPs. But first, an overview of POPs.
AN OVERVIEW OF POPS
The nature and classes of Environmental POPs
Characteristically, POPs are organics which, albeit toxic, are resistant to degradation. They bioaccumulate within living tissues and could be dispersed by air and water, as well as by migratory species across international boundaries [6]. Being volatile organic compounds, POPs evaporate quickly in warm regions of the world, thereby are transported through the atmosphere and condense in the mountainous areas where the temperatures are usually at the sub-zero [7]. Therefore, it has been observed that the Polar Regions are characteristic of high levels of POPs [8]. Figure 1 provides a generalized route of environmental POPs.
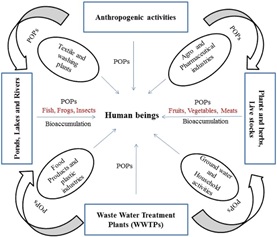 Figure 1: Possible routes of environmental POPs to humans [4].
Figure 1: Possible routes of environmental POPs to humans [4].
By classification, POPs include Polychlorinated Biphenyls (PCBs), Perfluorinated Compounds (PFCs), Brominated Compounds (BFR), Organochlorinated Pesticides (OCPs), and Polycyclic Aromatic Hydrocarbons (PAHs). Most of these classes are anthropogenic in origin. However, the sources of dioxins and furans could be natural (i.e., volcanic or vegetation fire origins) [9,10]. Generally, POPs are anthropogenic products, pre-designed for control of agricultural pest invasions, as well as for industrial use. As for the latter, it could be premeditated (e.g., polychlorinated biphenyls, hexachlorobenzene, brominated compounds, perfluorinated compounds) or unintended by-products of chemical processes or inceration (dioxins and furans) [11]. Because of their long-term, trans-boundary phenomena, the adverse effects of POP pollution on humans and the ecosystem, in general, are accumulative [12].
Sources of Environmental POPs
Several natural and anthropogenic release POPs into the environment. Aside from agricultural and zootechnic sources, waste dumps and landfills emissions contribute to air-borne POPs. Similarly, unclean burning of domestic plastic waste also plays some part [13]. Elsewhere, fluid wastes generated from the clean-up of pesticide facilities are often POPs-laden [13]. Also, wastes from electrical power [10] and e-waste recycling facilities [14,12] have been identified as viable sources of POPs [14] Prominent among other sources, are chemical and petrochemical industries, roads and railroads construction and maintenance, and wastewater treatment plants [15].
POLYCYCLIC AROMATIC HYDROCARBONS (PAHS)
Nature of PAHs
By their nomenclature, PAHs are a class of organics made up of two or more fused unsubstituted aromatic rings. They are plain aromatics, without any heteroatom [16]. The United States Environmental Protection Agency (USEPA) has identified sixteen PAHs in the environment (Figure 2). This group of (POPs) is generally resistant to all forms of degradation. Their refractiveness ensures their long life in the environment [17].
Similar to the general sources of POPs, PAHs are both human-made and naturally formed [16]. They are products of incomplete combustion of petroleum products [18]. PAHs are usually found as complex mixtures, with each component having a different degree of toxicity. Some of these PAHs are carcinogens and mutagens [19].
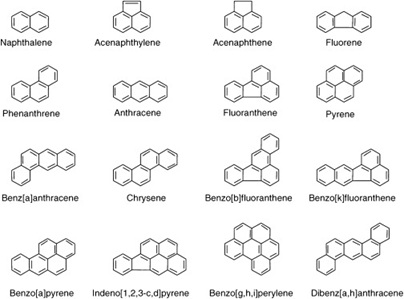 Figure 2: Chemical structures of identified polycyclic hydrocarbons.
Figure 2: Chemical structures of identified polycyclic hydrocarbons.
The environmental fate of PAHs is primarily driven by their ease of dispersion and solubility in the atmosphere and hydrosphere, respectively [20]. Some PAHs are easily dispersed, encouraging transboundary movement on a global scale, while exhibiting environmental persistence [21]. Generally, PAHs are poorly hydrophilic, i.e., they are highly lipophilic. However, when found in water, they may undergo photodecomposition upon contact with the UV region of insolation [22]. During photochemical reactions in the troposphere, PAHs may react with other air pollutants (such as O3, NOx, and SOx), yielding more deleterious secondary pollutants (such nitro-PAHs. dinitro-PAHs, and sulphonic acids, respectively). There is a general knowledge that the denser the PAH (based on the number of benzene rings), the more toxic it is. Therefore, PAHs of four and more condensed aromatic rings more environmentally unfriendly than those of three or two rings 923].
Further, PAHs are mainly of anthropogenic origin and have no significant natural sources [16]. Although there are thousands of PAHs in the environment, in practice, only 16 PAHs are regarded as priority pollutants.
Sources of PAHs
Because they are lipophilic, PAHs are usually found in soil, sediment, and lipophilic tissues, rather than in the atmosphere or water bodies. For instance, natural oil deposits (such as crude oil and coal) contain wide varieties and large quantities of PAHs [24].
Besides, some household and human activities possess or generate PAHs. Common among them are cigarette smoke, smoldering fireplaces, gas-burner, and charred food materials. Other potential sources of PAHs in the environment include disposal from public sewage treatment, irrigation with coke oven effluent, leachate from bituminous coal storage sites, and use of soil compost and fertilizers [17]. Primary among the outdoor sources of PAHs are gas stations, machine shops, rail, chemical manufacturing plants, incinerators, chemical waste storage facilities, oil refineries, landfills, automobile engines, etc. [25].
Some natural sources of PAHs include volcanic eruptions and forest fires. Most PAHs found in the environment are products of incomplete combustion or pyrolysis [26].
Environmental Fate of PAHs
When dispersed into the atmosphere from the thermal oxidation of fuels, PAHs may adsorb onto fine or coarse dust particles. From there, they may be carried under the air current and get deposited into another environmental sphere. Or, they may undergo photo-oxidation, which breaks down the PAH within a week [20]. Due to their high hydrophobicity, PAHs have an inherent preference of adsorbing onto soils or sediments at the bottom of water bodies. When the condition is suitable, they may solubilize in an oily fluid in the contaminated water. Therefore, the toxicity of PAHs is primarily driven by metabolism and photo-oxidation, which, in turn, determines the occurrence, number, and magnitude of secondary pollutants generated [22].
Noteworthily, some mixed microbial populations have been identified to be capable of remediating PAH pollution of sediments and soils. Some processes have been effectively viable over a short period of a few weeks to months. PAHs in soils are unlikely to exert toxic effects on terrestrial invertebrates, except when the soil is contaminated [27].
Health Effects of PAH Exposure
The negative health impacts of PAH exposure are usually evinced in the reproductive, developmental, and immune aspects of humans [28]. Generally, the toxicokinetic studies of PAH on mammals have identified olfactory, oral, and dermal routes of poisoning [20]. Based on gender, Figure 3 summarizes the various health effects humans are susceptible to upon exposure to PAHs.
As regards plants, PAHs are commonly adsorbed via translocation. The rate of such uptake is driven by the level of the PAH in the soil or underlay water, the maturity of the plants, the hydrophilicity of the PAH, and other physicochemical properties of the water/soil system.
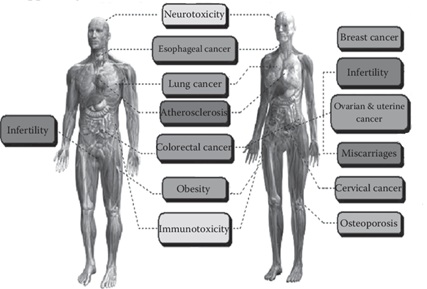 Figure 3: Human health impacts of PAH exposures [29].
Figure 3: Human health impacts of PAH exposures [29].
Acute effects
The health impact of PAHs is mostly based on the toxicity of the PAH, length of exposure, dosage, and the route of exposure. Other minor factors include the health status-quo and age of the affected organism, in this case, man. Up till today, the propensity of most PAHs to induce acute health defects is unclear. Exposure via inhalation and ingestion has been traced to soil tilling, an occupational hazard of agricultural practitioners who have been exposed to excessive levels of PAHs. Symptoms often experienced include eye irritation, nausea, vomiting, and diarrhea [30].
Chronic effects
On the other hand, the health defects attributed to chronic exposure to PAHs include deteriorating immune system, cataract, damage to internal organs, such as the kidney, lungs, and liver, resulting in jaundice. Sometimes, some difficulty with breathing (i.e., asthma-like symptoms), redness and skin inflammation, etc. may ensue. Specifically, naphthalene, the lightest PAH, has been indicted for causing hemolysis, the breakdown of red blood cells [31].
ORGANOCHLORINE PESTICIDES (OCPS)
Nature of OCPs
As the name implies, OCPs are chlorinated organic compounds popularly and effectively used for the control of agricultural pests. They are a class of POPs characterized by high environmental refractiveness [2]. Due to that fact, and that they are non-polar compounds, bearing only carbon, hydrogen, and chlorine, OCPs are ubiquitously found in the environment [32]. Representative compounds in this group include DDT, methoxychlor, dieldrin, chlordane, toxaphene, mirex, kepone, lindane, and benzene hexachloride.
When classified broadly, OCPs could be grouped into five viz: dichloro-diphenyl-drichloro ethane (DDT) and analogues, Kelevan, and Murex; and the toxaphenes [33]. Because chlorine has a relative atomic weight, of most OCPs are much denser than water, thereby they could easily sink and settle underwater, attaching themselves onto solid surfaces of soils and sediments.
In terms of peculiar applications, OCPs have been earlier successfully used in the control of malaria and typhus, before their use was banned due to their toxic residual effect [2]. Data have shown that approximately 40% of all pesticides are OCPs [34]. The chemical structure of some OCPs is depicted in Figure 4. DDT is the most popularly used OCP. Similar to PAHs, OCPs are chemically and biologically refractory. Also, they are highly lipophilic, making them exhibit high biomagnification and bioaccumulation potencies [35].
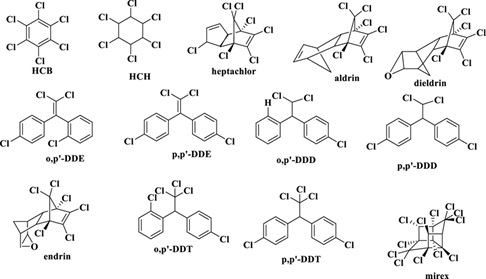 Figure 4: Examples of organochlorine pesticide.
Figure 4: Examples of organochlorine pesticide.
Sources of OCPs
Similar to most pesticides, OCPs are primarily applied to agricultural farmlands to control pests. Usually, OCPs enter into the hydrosphere via run-offs from industrial discharges, municipal wastewaters, and accidental discharge from factories [36]. PCBs are used as dielectric and coolant fluids in electrical converters, carbonless copy paper [37]. Also, they are found in heat transfer fluids, plasticizers, plastic, and rubber products [38]. OCP residues linger in the environment over many years, and their persistence encourages long-distance transport after volatilization from the primary source of emission, be it from production or application [39].
Environmental Fate of OCPs
Generally, OCPs resist any form of physical, chemical, or biological degradation [40]. The use of agricultural pesticides for agricultural production has led not only to the increase in yield but also to increase environmental pollution. Due to their high environmental reactiveness and persistence, OCPs dispersed in water are transferred along in the food chain, bioaccumulating (bioconcentrating) in aquatic animals up to it gets to man [36].
As for DDT, upon exposure to some microbes, especially Enterobacter aerogenes in the soil, it undergoes degradation into DDE or DDD, as depicted in figure 5.
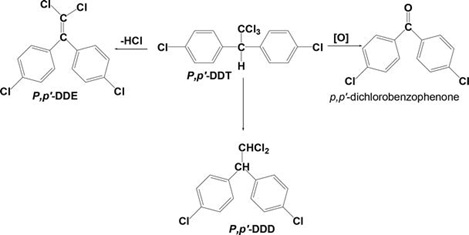 Figure 5: Degradation routes of DDT into DDE, DDE, and dichlorobenzophenone.
Figure 5: Degradation routes of DDT into DDE, DDE, and dichlorobenzophenone.
Health Effects of OCP Exposure
Expectedly, OCPs have a high tendency to store up in the fatty tissues of all forms of animals, as well as in the tissues of plants. Most of them they have been reportedly found concentrated in the fillets of fishes [41]. When in the human body, these chemicals can easily find their ways into the urinary and reproductive systems [42]. Apart from their bioaccumulation in fatty tissues, OCPs can be absorbed orally, transdermally, by inhalation, and through the gastrointestinal tract [43].
Toxicokinetic studies have shown that OCPs usually access humans via the dermal and olfactory routes. Those that are commonly exposed to are cyclodienes, hexachlorocyclohexane, and endosulfan. Humans could get exposed to OCPs through the ingestion of fatty food, such as meat, fish, poultry, and dairy products, etc. [44]. Many of the organochlorine molecules are carcinogens and neurotoxic [45]. On many occasions, different types of OCPs have been implicated in cases of hypertension, cardiovascular disorders, among other life-threatening diseases. Also, OCPs are endocrine-disrupting chemicals (EDCs), altering the molecular circuitry and function of the endocrine system [43].
Traces of OCP residues have been found in the blood samples of farmworkers and their families where OCPs have been previously applied. Worse is the effect of such poisoning on the central nervous system during acute and highly concentrated exposure. In recent years, traces of dioxins have been found in human ovarian follicular fluid, causing endometriosis. Other autoimmune illnesses associated with such exposure include multiple sclerosis and eczema [46]. Especially in children, dioxins cause learning disability (LD) [47].
Consequently, because of the measures put in place by various countries to ban the use of PAHs and OCPs for pest control, as well as strict embargos on all chemical and thermochemical processes that form these chemicals, there are expectations of a steady reduction in their environmental presence. Similarly, the mitigative measures and enforcement of laws implemented by relevant governing institutions (such as WHO, USEPA) are already yielding positive results as regards the future outlook of PAHs and OCPs in the environment [48]. Although, these efforts are being sabotaged by recalcitrant farmers who have prioritized their immediate commercial gain over the long-term health of their family, neighbors, customers, and the nations. While efforts are incessantly made toward the control of these organic pollutants, scientists are encouraged to research for environmentally benign alternatives to PAHs and OCPs, to ensure that agricultural products are sufficiently available to cater for the ever-growing human population. Thence, to achieve a successful reduction of the environmental, health, and economic impacts of PAH and OCP pollutions, all efforts from all levels of global citizenship must be harmonized.
CONCLUSION
We have provided a mini-review of the nature, sources, environmental fate and health impacts of Polycyclic Aromatic Hydrocarbons (PAHs) and Organochlorine Pesticides (OCPs). Although several detailed reviews are available, this short review provides an updated information, as well as a condensed easy-to-read appraisal of these serious environmental problem that seems recalcitrant to mitigate. With the information provide, man can easily plan towards avoiding or controlling the pollution of these unwanted organics in our environment.
ACKNOWLEDGEMENT
We are grateful to Dr. Olayinka A. Ibigbami of the Department of Chemistry, Ekiti State University, Ado-Ekiti, Nigeria, for his insight and material support during the preparation of this manuscript.
REFERENCES
- Jacob J, Cherian J (2013) Review of environmental and human exposure to persistent or-ganic pollutants, Asian Society of Science 9: 107-120.
- Aktar, MW, Sengupta D, Chowdhury A (2009) Impact of pesticides use in agriculture: Their benefits nd hazards. Interdisciplinary Toxicology 2: 1-12.
- Caldas ED (2016) Pesticide poisoning in Brazil. Reference module in Earth systems and environmental sciences 419-427
- Isman R, Kumar S, Karmoker J, Kamruzzaman Md, Biswas N, et al. (2018) Bioaccumulation and adverse effects of persistent organic pollutants (POPs) on ecosystems and human exposure: A review study on Bangladesh perspectives. Environmental Technology and Innovation 12: 115-131.
- Usta C, (2012) Microorganisms in biological pest control- A review (Bacterial toxin application and effect of environmental factors). Current Progress in Biological Research (DOI: 10.5772/55786).
- Jaruga AU, Lewinska K, Mammadov E, Karczewska A, Smreczak, et al. (2020) Residues of persistent organic pollutants (POPs) in agricultural soils adjacent to historical sources of their storage and distribution- The Case study of Azerbaijan. Molecules 25: 1815-1820.
- Yang R, Wang Y, Li A, Zhang Q, Jing C, et al. (2010) Organochlorine pesticides and PCBs in fish from lakes of the Tibetan Plateau and the implications. Environmental Pollution. 158: 2310-2316.
- Gouin T, Wilkinson D, Hummel S, Meyer B, Culley A, (2010) Polycyclic aromatic hydrocarbons in air and snow from Fairbanks, Alaska. Atmospheric Pollution research 1: 9-15.
- Jacob J (2013) A Review of the Accumulation and Distribution of Persistent Organic Pollutants in the Environment. International Journal of Bioscience, Biochemistry and Bioinformatics 3: 657-661.
- El-Shahawi MS, Hamza A, Bashammakh AS, Al-Saggaf WT (2010) An overview on the accumulation, distribution, transformations, toxicity and analytical methods for the monitoring of persistent organic pollutants. Talanta 80: 1587-1597.
- Guo W, Pan B, Sakkiah S, Yavas G, Ge W, et al. (2019) Persistent organic pollutants in food: Contamination sources, health effects and detection methods. International Journal of Environmental Research and Public Health 16: 4361.
- Lana NB, Berton P, Covaci A, Ciocco NF, Barrera-Oro E, et al. (2014) Fingerprint of persistent organic pollutants in tissues of Antarctic notothenioid fish. Science of the Total Environment 499: 89-98.
- Webster L, Russell M, Walsham P, Hussy I, Lacaze JP, et al. (2014) Halogenated persistent organic pollutants in relation to trophic level in deep sea fish. Marine pollution bulletin 88: 14-27.
- Tang B, Zeng YH, Luo XJ, Zheng XB, Mai BX (2015) Bioaccumulative characteristics of tetrabromobisphenol A and hexabromocyclododecanes in multi-tissues of prey and predator fish from an e-waste site, South China. Environmental Science and Pollution Research 22: 1-7.
- Schmid P, Kohler M, Gujer E, Zennegg M, Lanfranchi M (2007) Persistent organic pollutants, brominated flame retardants and synthetic musks in fish from remote alpine lakes in Switzerland. Chemosphere 67: 16-21.
- Fetzer JC (2000) The Chemistry and Analysis of the large polycyclic aromatic hydrocarbon. Polycyclic aromatic compound (New York: Wiley) 27: 143-162.
- AgarwalT, Khillare P, Shridhar V, Ray S (2009) Pattern, sources and toxic potentials of PAHs in the agricultural soils of Delhi, India. Journal of Hazardous Material 163: 1033-1039.
- Srogi K (2008) Levels and congener distributions of PCDDs, PCDFs and dioxin-like PCBs in environmental and human samples: a review. Environmental Chemistry Letters 6: 1-28.
- Alharbi OML, Basheer AA, Khattab RA, Ali I (2018) Health and environmental effects of persistent organic pollutants. Journal of Molecular Liquids 263: 442-453.
- Manzetti S (2013) polycyclic aromatic hydrocarbons in the environment: environmental fate and transportation. Polycyclic Aromatic Compounds 33: 311-330.
- Kim KH, Jahan SA, Kabir E, Brown RJ (2013) A review of airborne polycyclic aromatic hydrocarbons (PAHs) and their human health effects. Environmental International 60: 71-80.
- Srogi K (2007) Monitoring of environmental exposure to polycyclic aromatic hydrocarbons: a review. Encironmental Chemistry Letters 5: 169-195.
- Choi H, Harrison R, Komulainen H, Komulainen H, Saborit JMD (2010 )Polycyclic aromatic hydrocarbons. In: WHO Guidelines for Indoor Air Quality: Selected Pollutants, World Health Organization, Geneva, USA.
- Abdel-Shafy HI, Mansour MSM (2016) A review on polycyclic aromatic hydrocarbons: Source, environmental impact, effect on human health and remediation. Egyptian Journal of Petroleum 25: 107-123.
- Meeker JD, Sathyanarayana S, Swan SH (2009) Phthalates and other additives in plastic: human exposure and associated health outcomes. Philosophical Transactions of the Royal Society B 364: 2097-2113.
- Meador JP (2008) polycyclic aromatic hydrocarbons. Reference Module in Earth Systems and Environmental Sciences. Encyclopedia of Ecology 2008: 2881-2891.
- Peter HA (2003) Petroleum and individual Polycyclic aromatic hydrocarbon. In: Hoffman DJ, Rattner BA, Buston GA, Cairns J (eds.). Lewis Publisher, Handbook of Ecotoxicology, London, New york Washington. Pg. No: 342-359.
- Peterson ME, Talcot PA (2006) Small Animal Toxicology (2nd ed). Elsevier St. Louis, Missouri, USA. No: 941-949.
- Ramesh A, Hood DB, Guo Z, (2012) In book: Global Contamination Trends of Persistent Organic Chemicals. (1st ed). CRC, Taylor & Francis Group, Pg. No: 97-128.
- Kadi MW, Ali N, Albar HMSA (2018) Phthalates and polycyclic aromatic hydrocarbons (PAHs) in the indoor settled carpet dust of mosques, health risk assessment foe public. Science of Total Environment 627: 134-140.
- Volney G, Tatusov M, Yen AC, Karamyan N (2018) Napthalene toxicity: methemoglobinemia and acute intravascular hemolysis. Cureus 10: 3147.
- Adeyemi D, Anyakora C, Ukpo G, Adedayo A, Darko G (2011) Evaluation of the levels of organochlorine pesticide residues in water samples of Lagos lagoon using solid phase extraction method. Environmental Chemistry and Ecotoxicology 3: 160-166.
- Pope JV, Thomas MS, Rosen CL (1994) Toxicity, organochlorine pesticides: Clinical. Medscape, New York. Pg no: 259-278.
- Gupta PK (2004) Pesticide exposure--Indian scene. Toxicology 198: 83-90.
- Aiyesanmi AF, Idowu GAA (2012) Organochlorine pesticides residues in soil of cocoa farms in Ondo State central district, Nigeria. Environment and Natural Resources Research 2: 65-72.
- Sarkar SK, Bhattacharya BD, Chatterjee M, Alam A (2008) Occurrence, distribution and possible sources of organochlorine residues in tropical coastal environment of India: An overview. Environmental International 34: 1062-1071.
- Babut M, Arts GH, Caracciolo AB, Carluer N, Domange N, et al. (2013) Pesticide risk assessment and management in a globally changing world–Report from a European interdisciplinary workshop. Environmental Science and Pollution Research 20: 8298-8312.
- Kordybach BM, Smreczak B, Pawlas AK (2014) Evalation of the status of contamination of arable soils in Poland with DDT and HCH residues; National and Regional Scales. Polish Journal of Environmental Studies 23: 139-148.
- Lammel G, Klanova J, Eric L, Ilic P, Kohoutek J, et al. (2011) Sources of organochlorine pesticides in air in an urban Mediterranean environment: volatilization from soil. Journal of Environmental Monitoring 13: 3358.
- Darko G, Acquaah SO (2007) Levels of organochlorine pesticides residues in meat. International Journal of Environmental Science and Technology 4: 1735-1742.
- Bentzen TW, Follman EH, Amstrup SC, York GS, Woller MJ, et al. (2008) Dietary biomagnification of Organochlorine contaminants in Alaskan polar bears. Canadian Journal of Zoology 86: 177-191.
- Burns JS, Williams PL, Sergeyev O, Korrick SA, Lee MM, et al. (2012) Serum concentrations of organochlorine pesticides and growth among Russian boys. Environment and Health Perspective 120: 303-308.
- Idowu GA, Aiyesanmi AF, Owolabi BJ (2013) Organochlorine pesticides residue levels in river water and sediment from cocoa-producing areas of Ondo State central senatorial district, Nigeria. Journal of Environmental Chemistry and Ecotoxicology 5: 242-249.
- Rusiecki JK, Baccarelli A, Bollati V, Tarantini L, Mooore LE, et al. (2008) Global DNA hypomethylation is associated with high serum-persistent organic pollutants in Greenlandic inuit. Environmental Health Perspectives 116: 1547-1552.
- Kaiser J (2000) Endocrine disrupters. Panel cautiously confirms low-dose effects. Science 290: 695-697.
- Jayaraj R, Megha P, Sreedev P (2016) Organochlorine pesticides, their toxic effects on living organisms and their fate in the environment. Interdisciplinary toxicology 9: 90-100.
- Lee D, Jacobs DR, Porta M (2007) Association of serum concentrations of persistent organic pollutants with the prevalence of learning disability and attention deficit disorder. Journal of Epidemiology and Community Health 61: 591-596.
- PES (2020) Taking Action (Control Measures). Pesticide Environmental Stewardship. North Carolina, United States.
Citation: Adelodun AA (2020) Polycyclic Aromatics Hydrocarbons and Organochlorine Pesticides in the Environment: Sources, Routes, Effects and Fate. J Protein Res Bioinform 2: 011
Copyright: © 2020 Adelodun AA, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.