
Procalcitonin in Older Patients; Promoting Antibiotic Stewardship in Complex Patients
*Corresponding Author(s):
Kordo SaeedDepartment Of Microbiology, Royal Hampshire County Hospital, Hampshire Hospitals NHS Foundation Trust, Winchester, United Kingdom
Tel:+44 1962825927,
Email:kordosaeed@nhs.net
Abstract
Procalcitonin (PCT) is a biomarker that increases in bacterial infection and sepsis. Recent meta-analysis has supported its use in older patients. We evaluated the real life use of PCT in 55 older patients (defined as over 65) in a district general hospital in the UK, with retrospective case notes review over a 3 month period. PCT was negative (<0.25 µg/L) in 39/55 (70.9%). In this group, 64.1% were not started on antibiotics and 20.5% had antibiotic treatment stopped. This paper highlights the use of PCT can assist antimicrobial stewardship in a complex population who are vulnerable to the development of Clostridium difficile and other resistant infections.
Keywords
INTRODUCTION
Diagnosis of infection in older patients is an ongoing challenge, with atypical presentation coupled with comorbidity and poor functional status. It is important to recognize infection and treat quickly, however there is potential to over prescribe antibiotics in older patients. With the rise in antimicrobial resistance and Clostridium difficile it is essential we support better antibiotic stewardship and prevent unnecessary prescriptions [1].
Procalcitonin (PCT) is a biomarker that increases in bacterial infection and sepsis. It can be used in initial diagnosis of bacterial sepsis and to determine the duration of treatment with antibiotics [2]. Its use has been shown to be superior to C-Reactive Protein (CRP); a commonly used biomarker in the diagnosis of bacterial infection [3]. While PCT has been available for many years its use had not become widespread in the UK.
In older patients initial research suggested that PCT was not reliable in distinguishing those with infection from those without, however recent meta analysis found that it can be used as a rule-in diagnostic test or moreover a rule-out test, particularly in those over 75 [4-6].
In our organization, PCT has been available with microbiology approval for over 5 years. We evaluated how this biomarker had been used in our older population (defined as over 65 by the WHO) to provide a picture of how this test can be used in to aid diagnosis and treatment in a district hospital setting.
METHODS
Retrospective case notes review was carried out on all patients over the age 65 who had a PCT taken over a 3 month period between 1st April 2014 and 7th July 2014. PCT was measured in the microbiology laboratory during routine working hours using the Brahms Vidas EIA method (bio-Mérieux, Basingstoke, UK). The Royal Hampshire County Hospital a district general in the south of England with approximately 300 beds; Inclusion criteria was any patient on a medical or surgical ward over the age of 65. This age criteria were set in line with the WHO definition and ensure complex patients were captured in the data collection. Patients in Intensive care were excluded. Intensive care patients by definition have multiple organ failure and are a more distinct group. Work has been carried out specifically looking at PCT in the ITU setting [1] where patients had serial PCTs only the initial one was included in this analysis. Repeat PCT was not specifically looked at unless a patient had deteriorated and required by the clinician who was directly looking after the patient. PCT has been used as a rule-in rule-out test for infection and we felt that the paper should focus more on its “real life” application and use within our hospital. To focus in on the application of the PCT on clinical practice data on White Cell Count (WCC), CRP, antibiotic use, cultures (taken 48 hours prior to PCT and 24 hours post PCT) and infective foci was also collected to gain an impression of the type of patients PCT was used for the their presentation.
At any time during or after admission, discussion with microbiology was required before a PCT test could be run to ensure appropriate patients were being tested. Patients were eligible for a PCT if they had a potential infective source, or symptom and did not fit the criteria for SIRS (defined as two of the following -a temperature >38.3 or <36°C, a respiratory rate >20 min-1 /PaCO2 <32 mmHg (4.3 kpa), heart rate >90 bpm /WCC <4 x 109/l or >12 x 109/l). In patients that had died with a negative PCT, death certificates and further notes were obtained.
RESULTS
PCT was measured in 55 patients aged 66-98. The most common potential septic source was chest (19 patients), following skin and soft tissue (9 patients) and genitourinary (6 patients). Other indications included potential discitis, septic arthritis, CNS infection, endocarditis, pyrexia of unknown origin and gastrointestinal infection.
PCT was negative (<0.25 µg/L) i.e., not supporting bacterial infection in 39/55 (70.9%). Figure 1 shows the correlation of WCC and CRP in the group with a negative PCT. In this group, 64.1% were not started on antibiotics and 20.5% had antibiotic treatment stopped. One patient had antibiotic treatment started and 12.8% continued on antibiotics despite a low PCT value.
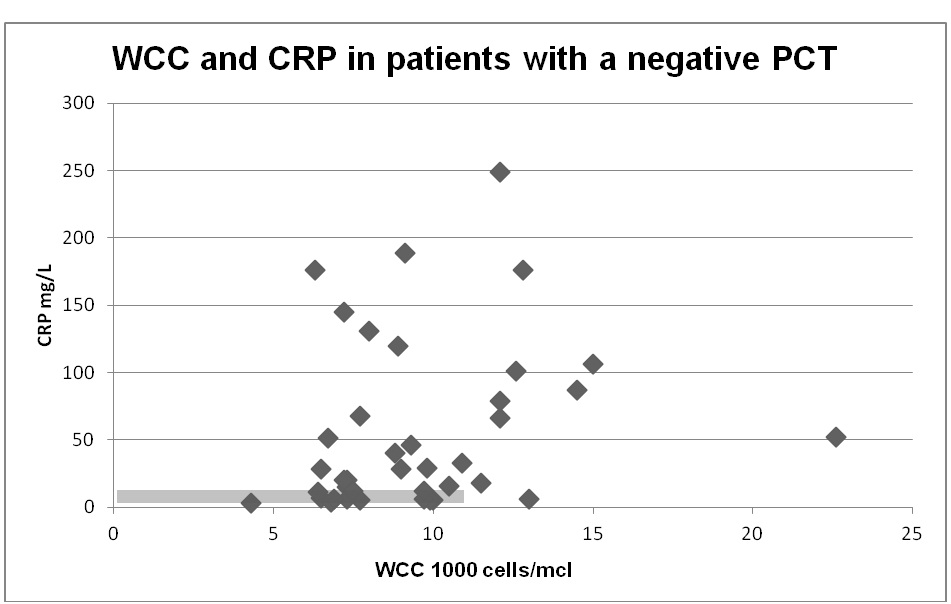
Figure 1: WCC and CRP in patients with a negative PCT (<0.25 µg/L). The grey box indicates that would have constituted a negative result for infection defined as WCC < 11 000 cells/mcl and CRP <10 mg/L.
Eight of the 39 patients with a negative PCT had a positive microbiological culture, all of these were from a non-sterile site in the preceding 48 hours and 24 hours after the PCT was taken. Six of these were urine cultures, and two were wound/skin swabs. Of the six urine cultures four were deemed to be colonized (scanty growth, mixed growth, Pseudomonas and Candida in catheter samples). The remaining two were a Proteus and Escherichia coli from MSU in two female patients where urine was not thought to be the source of infection. The two positive skin swabs represented a mixed anaerobe which is most likely a contaminant and a Staphylococcus aureus. The Staphylococcus aureus was in a patient with a wound did not look infected clinically. There were no positive cultures from normally sterile sites.
In the group where antibiotics had not been started prior to the test results, WCC was greater than 11 in 4 patients and CRP ranged from 5-249 mg/L (median 33 mg/L). In patients with a negative PCT where antibiotics were stopped, WCC was greater than 11 in 3 and CRP was 4-106 mg/L (median 21 mg/L).
Ten patients (18.2%) had died by the time the data was collected. Six patients had a positive PCT, and had been treated with antibiotics appropriately according to local guidelines and/or clinical grounds. PCT had been negative in 4 patients that had subsequently died 14-26 days after the PCT measurement.
In three patients infection was listed on the death certificate. Pneumonia was listed in two patients, and chest infection in another. The first patient died 26 days after the initial PCT, a repeat positive PCT was done the day before they died and they had received further antibiotic treatment. The second patient died 16 days after the initial PCT, they had declined further treatment and had been transferred to a nursing home for end of life care. The final patient died 14 days after the initial PCT. A repeat PCT had not been carried out on clinical grounds; several other comorbidities were listed as the cause of death in addition to pneumonia. These included heart failure, ischemic heart disease and COPD and cerebrovascular accident. In conclusion, we felt that PCT results had been appropriate at the time they were taken, and that no patient had, to our knowledge received inappropriate treatment as a result of a low PCT result.
DISCUSSION
PCT is predominately being used to rule-out infection in older patients with low suspicion of infection in our trust. Clinicians are using this tool in patients with a low suspicion of bacterial infection, as evidenced by the fact that 64.1% had not started antibiotics pending results. Interestingly, other biomarkers such as WCC and CRP were elevated in these patients.
CRP while well evaluated as a marker of infection and is routinely used to identify infection, it can be raised in a number of clinical scenarios including trauma, malignancy and even cognitive disorders such as delirium [7]. Delerium in the older patient is a poor prognostic indicator and although multifactorial is often attributed to infection. Concern has been raised about the number of patients treated for presumed urinary tract infections due to asymptomatic bacteremia and delirium [8]. The presence of asymptomatic bacteriuria on ongoing challenge in the elderly; In keeping with other research, eight patients in this study with no urinary symptoms had a positive urinary culture [9]. Interestingly all patients with urine as the potential source had a negative PCT, and only one had a positive culture which was a Candida from a catheter sample which was regarded as colonization and not infection. Delerium was not looked at specifically at this in study, however the high proportion of negative PCTs in the context of urinary source emphasizes the issues around diagnosis and treatment in these patients.
While PCT is well evaluated in sepsis and respiratory infection, its use in more local infections such as cellulitis is less well understood [2,10]. Cellulitis can be challenging to diagnose clinically as other conditions such as a deep vein thrombosis can mimic its presentation. The local nature of infection has raised questions over the sensitivity of serum PCT in this setting [10-12]. One patient had a Staphylococcal aureus positive culture with a negative PCT. While this was likely to represent colonization, in the context taken it, PCT should be used in caution in soft tissue infection.
Low PCT values should also always be taken in the clinical context. A low PCT don’t always exclude the diagnosis of infection and in 8 patients in this cohort antibiotics were continued despite a negative PCT. In this situation serial PCT can be helpful along with clinical examination history and other biomarkers. This reinforces that PCT cannot be used in place of history, examination and clinical experience, but instead as an adjunct to try and improve diagnosis and antimicrobial stewardship.
The number of patients in our sample that had subsequently died indicates the frailty of the patient demographic where PCT was requested. Three patients who had low initial PCT passed away and infection been part of the cause of death. However these deaths happened 14-26 days after the PCT measurement, i.e., the decision to withhold antibiotics at the time of PCT measurement was correct and subsequently these patients developed potential new infections. Additionally all these had multiple and complex comorbidities. Many patients had a prolonged admission with often several courses of antibiotic treatments during their stay. In these patients antibiotic stewardship is particularly important, as these patients are vulnerable to the development of resistant infections, and Clostridium difficile.
In conclusion our results support the use of PCT in the setting of older medicine in conjunction with clinical findings, offering insight into the application of this biomarker in a district general hospital.
REFERENCES
- Saeed K, Dryden M, Bourne S, Paget C, Proud A (2011) Reduction in antibiotic use through procalcitonin testing in patients in the medical admission unit or intensive care unit with suspicion of infection. J Hosp Infect 78: 289-292.
- Meisner M (2014) Update on procalcitonin measurements. Ann Lab Med 34: 263-273.
- Simon L, Gauvin F, Amre DK, Saint-Louis P, Lacroix J (2004) Serum procalcitonin and C-reactive protein levels as markers of bacterial infection: a systematic review and meta-analysis. Clin Infect Dis 39: 206-217.
- Stucker F, Herrmann F, Graf JD, Michel JP, Krause KH, et al. (2005) Procalcitonin and infection in elderly patients. J Am Geriatr Soc 53: 1392-1395.
- Lee SH, Chan RC, Wu JY, Chen HW, Chang SS, et al. (2013) Diagnostic value of procalcitonin for bacterial infection in elderly patients - a systemic review and meta-analysis. Int J Clin Pract 67: 1350-1357.
- Lai CC, Chen SY, Wang CY, Wang JY, Su CP, et al. (2010) Diagnostic value of procalcitonin for bacterial infection in elderly patients in the emergency department. J Am Geriatr Soc 58: 518-522.
- Ritchie CW, Newman TH, Leurent B, Sampson EL (2014) The association between C-reactive protein and delirium in 710 acute elderly hospital admissions. Int Psychogeriatr 26: 717-724.
- Balogun SA, Philbrick JT (2013) Delirium, a Symptom of UTI in the Elderly: Fact or Fable? A Systematic Review. Can Geriatr J 17: 22-26.
- Woodford HJ, George J (2011) Diagnosis and management of urinary infections in older people. Clin Med 11: 80-83.
- Viallon A, Zeni F, Lambert C, Pozzetto B, Tardy B, et al. (1999) High sensitivity and specificity of serum procalcitonin levels in adults with bacterial meningitis. Clin Infect Dis 28: 1313-1316.
- Arakaki RY, Strazzula L, Woo E, Kroshinsky D (2014) The impact of dermatology consultation on diagnostic accuracy and antibiotic use among patients with suspected cellulitis seen at outpatient internal medicine offices: A randomized clinical trial. JAMA Dermatol 150: 1056-1061.
- Saeed K, Ahmad N, Dryden M (2014) The value of procalcitonin measurement in localized skin and skin structure infection, diabetic foot infections, septic arthritis and osteomyelitis. Expert Rev Mol Diagn 14: 47-54.
Citation: Eddy F, Joyce A, Dryden M, Saeed K (2015) Procalcitonin in Older Patients; Promoting Antibiotic Stewardship in Complex Patients. J Infect Non Infect Dis 1: 002.
Copyright: © 2015 Kordo Saeed, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.

