
Quercetin Prophylaxis: A Novel Approach to Prevent Hypoxia-Mediated Increase in Oxidative Stress, Pde-5 Activity and Transvascular Leakage in the Lungs of Rats
*Corresponding Author(s):
Sagi SSKDepartment Of Nutrition, Defence Institute Of Physiology And Allied Sciences, New Delhi, India
Tel:+91 01123883214,
Email:saradasks@gmail.com / s9510003@gmail.com
Abstract
Background and aims
An increase in the vascular permeability of alveolar epithelium has found to be the major manifestation of hypoxia exposure. This study examined the efficacy of quercetin in preventing the hypoxia-mediated increase in Phosphodiesterase-5 (PDE-5) levels in the lung, leading to the diminution of alveolar smooth muscles vasoconstriction and curtailed transvascular leakage.
Methods and results
Male SD rats (n=6 per group) were preconditioned with quercetin (50mg/Kg BW) and tadalafil (10mg/Kg BW) 1h prior to hypobaric hypoxia (7,620m for 6h). The prophylactic efficacies of the drugs were compared by carrying out biochemical estimations (ROS, MDA, GSH and transvascular leakage), western blotting analysis, mRNA expression studies, ELISA and by determining peNOS levels in the lungs of rats under hypoxia. Further, the compatibility of the drugs (analytes) toward PDE-5 (ligand) was ascertained by SPR spectroscopy and molecular docking. The results revealed that the animals fed with quercetin prior to hypoxia have significantly (P<0.001) down-regulated the oxidative stress causing reduction in PDE-5 activity followed by the decrease in 5’GMP and ET-1 levels, resulting into significant increase in NO synthesis due to the accelerated eNOS phosphorylation compared to tadalafil. Apart from this, SPR and docking results have also confirmed the better association of quercetin with PDE-5 compared to tadalafil.
Conclusion
Quercetin is noted to be a direct antagonist of PDE-5 in the lungs of rats under hypoxia, which by abrogating oxidative stress, prevents fluid accumulation in the lungs as a consequence of HAPE.
Keywords
HAPE; Oxidative stress; PDE-5; Quercetin; Vascular leakage
APPENDICES
cGMP: Cyclic Guanosine Monophosphate
eNOS: Endothelial Nitric Oxide Synthase
ET-1: Endothelin-1
GAPDH: Glyceraldehyde 3-Phosphate Dehydrogenase
GSH: Reduced Glutathione
5’GMP: Guanosine 5’-Monophosphate
HAPE: High Altitude Pulmonary Edema
KD: Dissociation Constant
MDA: Malondialdehyde
NO: Nitric Oxide
PDE: Phosphodiestrase
peNOS: Phosphorylated eNOS
PKG: Protein Kinase G
ROS: Reactive Oxygen Species
SD: Sprague Dawley
INTRODUCTION
High Altitude Pulmonary Edema (HAPE) is a form of non-cardiogenic pulmonary edema, which is amongst one of the formidable and multifactorial pulmonary disorders with limited treatments [1]. It generally affects healthy, non-acclimatized individuals ascending to an altitude of 2,500 m or above [2]. Initially, the onset of HAPE is characterized by the dyspnoea, coughs and reduced workout performance which progresses to exaggerated Pulmonary Capillary Pressure (Pcp), Pulmonary Artery Pressure (Pap) and inflated vasoconstriction in the later stages [3]. However, literature has also elaborated on the putative roles of hypoxia-induced oxidative stress in causing vasoconstriction which finally ends-up to the fluid build-up in the lungs [4].
Vasoconstriction primarily results from endothelial dysfunctioning due to imbalanced bioavailability of dilators especially- Nitric Oxide (NO) and Prostacyclin Analogs (PGI2) vs elevation in constrictors such as- thromboxane, Angiotensin II (Ang II), Endothelin-1 (ET-1) [5]. Research had also confirmed that exacerbation in ROS production due to hypoxia is amongst major factors for the occurrence of vasoconstriction in the smooth muscles of lung endothelium [5,6]. In physiological systems, active ROS levels, which can be produced in healthy smooth muscles, endothelial and adventitious cells in the systemic and pulmonary vasculatures are responsible for routine regulation of cellular and physiologic responses [7]. ROS refers collectively to stable oxidants such as- Hydrogen Peroxide (H2O2) and unstable oxidants and/or free radicals namely- Nitric Oxide (NO), Peroxynitrite (ONOO-) and Hydroxyl Moiety (OH-), etc. [7]. These free radicals have very short half-life as they are highly reactive and scavenged by numerous anti-oxidants such as- GSH, GPx, SOD, catalase, etc. [8]. Oxidant stress is reported to occur because of the recurring external insults evoking elevated ROS generation which overburdens anti-oxidant systems [8]. Recent studies on hypoxia-induced oxidant injuries have reported that apart from heart failure, atherosclerosis, respiratory distress and ventricular atrophy, oxidative stress also contributes in damaging endothelial-derived prostacyclin synthases especially- Prostaglandin-I Synthases (PGIs) and Endothelial Nitric Oxide Synthases (eNOS) resulting in impaired vasodilation by diminishing the capacity of blood vessels [9-13]. Apart from the increased emergence of free radicals, excess production of ET-1 from vascular endothelium as a manifestation of hypoxia is also reported to be a leading cause of the reduction in eNOS synthesis from L-arginine in the lung endothelium [14].
The endothelins are a member of 21 amino acid peptides family existing mainly in three distinct forms namely- ET-1, ET-2 and ET-3, of which ET-1 differs from ET-2 and ET-3 by two and six amino acids respectively. ET-1 is reported to be the most profound and best-characterized isoforms of endothelins [15]. As per the literature, the lung comprises of highest ET-1 levels secreted by smooth muscles, endothelium and alveoli. Not only in the lungs, but ET-1 is also reported to circulate in the plasma [15]. Whereas, ET-2 with almost similar functions as that of ET-1 is found commonly in kidneys, myocardium and placental tissues. On the contrary, ET-3 which is best studied in the lungs, kidneys, Gastrointestinal Tract (GIT) and Central Nervous System (CNS) is also reported to circulate in the plasma as well [15]. In recent years, the role of ET-1 in regulating the eNOS phosphorylation/dephosphorylation via cGMP signaling, which is in turn regulated by Phosphodiesterases (PDEs) is found to be an area of interest for the researchers [16]. Cyclic Guanosine Monophosphate (cGMP) is one of the important intracellular secondary messengers, playing a crucial role in regulating various cellular responses such as- cell survival, proliferation and apoptosis via signal transduction cascades [17]. cGMP pool present in the cells is controlled by highly selective PDEs [18]. PDEs, represent a superfamily of hydrolases expressing ubiquitously in the cell [19]. Till the date, 11 different PDE gene families (PDE-1 to PDE-11), consisting of 21 genes producing more than 100 proteins by alternative splicing of mRNA have been indexed in the literature [20]. These 11 PDE families are grouped into 3 distinct categories based on their substrate specificities: 1) PDE-4, 7 and 10 specifically hydrolyzes cAMP; 2) PDE-5, 6 and 9 selectively hydrolyze cGMP; 3) PDE-1, 2, 3, 10 and 11 have dual selectivities of acting on both cAMP and cGMP respectively [21]. Among the 11 PDEs reported, PDE-5 is found to be mainly associated with bronchial and alveolar smooth muscle relaxation and contraction via eNOS phosphorylation/dephosphorylation [22]. The abundance of PDE-5 in alveolar smooth muscles and its role in regulating their contraction and relaxation via eNOS phosphorylation has made PDE-5 an important drug target for minimizing the HAPE manifestations in the sojourns or high landers [23]. Researchers have attributed special notoriety to PDE-5 superfamily after the development of selective and potent PDE-5 inhibitors namely- sildenafil, tadalafil and vardenafil, etc. [24]. However, the direct exposure to these drugs have been associated with some considerable side-effects alongside the suppression of PDE-5 activity such as- sildenafil and tadalafil supplementation has been reported to cause cutaneous flushing and headache [24,25]. Vardenafil, a potent PDE-5 inhibitor is associated with moderate-severe visual disturbances [25].
Based on the above-mentioned facts, the present study has been programmed to minimize the increase in ET-1 levels, oxidative stress and PDE-5 activity by prophylactic administration of quercetin, a phyto-flavonoid. Quercetin is a naturally occurring, a dietary flavonoid found ubiquitously in all plant parts and products [26]. The reported health benefits of quercetin intake on mammalian systems include its- anti-oxidant, anti-carcinogenic, anti-inflammatory, anti-apoptotic, anti-aging, anti-coagulating, anti-tumoral and neuroprotective behaviour respectively [27,28]. The carcinogenicity and toxicity study of quercetin carried out on approximately 200 male and female rats administered with different doses of quercetin ranging from 0, 1000, 10,000 or 40,000 ppm along with the estimated dose of 40-1900 mg/Kg BW/day delivered to rats, demonstrated normal animal physiology [29]. As mentioned in the literature, the oral supplementation of quercetin has been reported to be the safest route of administration [30]. The LD50 value of quercetin is found to be 161 mg/Kg BW when fed orally to the rats [31]. Thus, the present study was undertaken to investigate the prophylactic potential of quercetin against tadalafil (a known PDE-5 inhibitor) in minimizing hypoxia-induced transvascular leakage by attenuating ROS production, inhibiting PDE-5 activity, curtailing ET-1 expressions and elevating eNOS phosphorylation in the lungs of rat under hypoxia.
MATERIALS AND METHODS
Chemicals and reagents
Quercetin (3’3’5’7’-pentahydroxyflavone) was procured from Sigma Aldrich (St. Louis MO, USA), Tadalafil from Cipla (10mg), Rolipram from Sigma Aldrich, Dimethyl sulphoxide (DMSO) from Sisco Research Laboratory (SRL, Maharashtra), Flourscein sodium salt from Sigma Aldrich and 2,7-Dichlorofluorescein Diacetate (DCFH-DA) assay from Sigma Aldrich. All the other chemicals and reagents were of analytical grade, PDE-5 receptor from Biovision (USA).
Drug preparation
Drugs quercetin and tadalafil were prepared freshly by dissolving into a vehicle (0.5% of DMSO) and supplemented orally to the animals 1h prior to the hypoxia exposure.
Experimental animals
Male Sprague Dawley (SD) rats weighing180-200 gm were obtained from the central animal facility, DIPAS-DRDO, Delhi, India. Animals were maintained in polypropylene cages with dimensions of 32 in. 24in. 16in. covered with paddy husk as bedding material. The animals were kept in the animal house facility of the institute with free retrieval to standard water and chow for 12 h day and night cycles with maintained standard temperature (25±1°C) and relative humidity (55±2%). All protocols involving animal studies were reviewed and approved by the Institutional Animal Ethics Committee (IAEC), DIPAS-DRDO, Delhi, India, accredited to Committee for Control and Supervision of Experiments on Animals (CPCSEA), Government of India. We have followed the standards outlined in the guide for the Care and Use of Laboratory Animals (National Academy of Sciences, Washington, DC).
Experimental set-up
The animals were assigned into six different groups and each group comprised of 6 rats (n=6): Group 1 served as control or Normoxia (N) that received the only vehicle, group 2 (H) received the only vehicle and was exposed to hypoxia for 6 h, group 3- (NQ) supplemented with quercetin (50 mg/Kg BW) without exposure, group 4 (HQ) received quercetin (50 mg/Kg BW) and exposed to hypoxia for 6 h, group 5 (NT) supplemented with tadalafil (10 mg/Kg BW) without exposure and group 6- (HT) received tadalafil (10 mg/Kg BW) and exposed to hypoxia for 6 h to carry out biochemical estimations. The Reason for opting 50mg/Kg BW dose of quercetin was based on the dose standardization studies carried out in our laboratory, as this dose (50mg/Kg BW) showed a significant reduction in transvascular leakage in the lung of rats exposed to hypoxia compared to other doses tested [32].
Exposure to hypobaric hypoxia
Animals were exposed to a simulated hypobaric hypoxia chamber (Matrix Sarrod, India) at an altitude of 7,620 m (280 mm Hg) for the duration of 6h at 25±2ºC. Reason for choosing 6h exposure is based on previous findings of our lab revealing significantly enhanced vascular leakage at 6h of hypoxia exposure [33]. Fresh air was flushed at the rate of 4 L per hours with the relative humidity of 55±2% inside the chamber. The partial pressure of arterial oxygen (PaO2) in normoxic animals was found to be 95±2 mm Hg, while in hypoxic rat PaO2 was 36±2 mm Hg, stipulating the reduced barometric pressure at 7,620 m. The animals were fed with food and water ad libitum during hypoxic exposure.
Surface Plasmon Resonance (SPR) spectroscopy
Surface Plasmon Resonance (SPR) spectroscopy was performed to assess the binding affinities of analytes (quercetin, tadalafil and rolipram) towards the ligand (PDE-5), immobilized over gold chip of the dynamic SPR (Autolab Esprit) apparatus, which works on the principle-based on Taylor Dispersion Analysis (TDA) of biomolecular interaction quantification [34,35]. According to TDA, analyte concentration changes gradually and uniformly, whereas the association of analytes with the respective ligand is represented in a sigmoidal gradient pattern. Similarly, the variable concentration of analytes dissolved in 0.5% Dimethyl Sulphoxide (DMSO) ranging from 20µM to 200µM was run against the ligand (PDE-5), which was immobilized in sodium acetate (pH ∼ 5.0, 10mM) and coated over a gold chip. Only in cases of SPR spectroscopic & molecular modelling studies, both tadalafil and rolipram have served as positive controls against quercetin for analyzing & comparing the binding potentials of analytes with the ligand, however, in rest of the study only tadalafil has been used as a positive control against quercetin.
Biochemical parameters
Method of sacrifice: Animals were sacrificed by using the cocktail of Ketamine hydrochloride (80 mg/Kg BW) and Xylazine (20 mg/Kg BW) as an anesthesia post hypobaric hypoxia exposure (6h).
Sample preparation: Lungs of both hypoxia exposed and unexposed rats were perfused using cold PBS (1X), excised out, washed with saline (0.9% NaCl) and then homogenized (10%) using 0.154 M KCL containing DTT, PMSF and PIC for performing biochemical estimations.
Quantification of oxidative stress: The ROS production in the lung of rats exposed to hypoxia (6h) was estimated by carrying out 2,7-Dichlorofluorescein Diacetate (DCFH-DA) assay. Wherein, an assay mixture was prepared by using tissue homogenate, DCFH-DA and potassium dihydrogen phosphate buffer. It was incubated for 15 min. at Room Temperature (RT) and the fluorescence emitted upon oxidation of DCFHDA to DCF was quantified at an excitation of 485 nm and emission of 530 nm using a spectrofluorimeter (Synergy H1, Biotek, Germany) [36].
Malondialdehyde Estimation (MDA): To assess the lipid peroxidation in the lung of rats exposed to hypoxia, Thiobarbituric Acid Reactive Substances (TBARS) assay was performed. The method involved heating-up of the assay mixture consisting of lung tissue homogenates from different animals, TCA, TBA and HCL in a boiling water bath for 1 h at 80°C. After an hour, the assay mixture was allowed to cool down and centrifuged at 2000 rpm for 10 min. at 4°C. Later the absorbance of the supernatant obtained was measured spectrophotometrically (Synergy H1, Biotek, Germany) at 532 nm [37].
Estimation of reduced glutathione (GSH) levels: Measurement of GSH in the lungs of rats was carried out by using 5,5’-dithiobis (2-nitrobenzoic acid) (DTNB) assay reported by Tietze (1969) [38], which later on was modified by Adams et.al., in 1983 [39]. In this method, the lung homogenates and precipitating reagent were mixed thoroughly and incubated at room temperature for 5 min. Further, this reaction mixture was centrifuged (1200g, 20 min. and 4ºC) and the supernatant obtained was mixed with DTNB reagent and phosphate buffer. The resultant color developed was quantified spectrophotometrically (Synergy H1, Biotek, Germany) at 412 nm (OD).
Protein expression studies: The protein content in the lung homogenate of rats from different groups was estimated using Lowry’s method [40]. Further, the western blotting analysis was conducted to ascertain the prophylactic potential of quercetin on enhanced expressions of PDE-5 and diminished expressions of peNOS under hypoxia. The protein contents present in the samples were segregated using 10% SDS-PAGE (PDE-5, peNOS and β-actin). Later, the separated proteins were electro-blotted onto the nitrocellulose membrane having 0.45μm thickness and the membrane was further blocked by using 5% Bovine Serum Albumin (BSA) prepared in PBS (1X) for overnight. Further, the membrane was washed and probed with primary antibodies (Santa Cruz biotechnology, 1:5000 dilutions) and allowed to incubate at RT for 2 h. After the primary antibody incubation, followed by 4-5 washings with PBST, the membrane was probed with enzyme-linked, HRP-conjugated secondary antibodies (Santa Cruz 1:15000 dilutions) and incubated for 2-3 h at RT. After thorough washing (5-6 times) with PBST, the membranes were developed by using chemiluminescent peroxidase substrate (Luminata forte, Millipore, USA) and the resulting bands were visualized in Gel doc (UVP, Cambridge, UK). Further, the optical density of the bands was measured using lab works software (UVP-Bioimaging systems, C.A., USA).
mRNA expression studies: The right lobe of both hypoxia exposed and unexposed animal lungs were homogenized using 1ml TRIzol reagent (Molecular Research Company, Inc., U.S.A.). Later, the RNA was isolated by using chloroform, precipitated with 75% ethanol and isopropanol and reconstituted in DNase/RNase-free water. The concentration of RNA was measured by absorbance at 260 nm. Reverse transcription was then performed using the invitrogen cDNA synthesis kit (CA, USA) as per manufacturer’s instructions and specific oligonucleotide sequences of PDE-5, eNOS and GAPDH were designed using Primer 3plus tool and later customized (Table 1). The resulting PCR products obtained were then separated by electrophoresis loading onto a 1.2% agarose gel. After staining with ethidium bromide all DNA bands were captured using the Gel Doc system (UVITEC, Cambridge, USA).
|
Proteins |
Sequences |
Product Length (bp) |
Tm (°C) |
|
|
Direction |
Reverse (5' - 3') |
|||
|
PDE-5 |
Sense |
AGCCCTTTGCTTCACTTTCA |
224 |
60 |
|
eNOS |
Sense |
GGCTGAGTACCCAAGCTGAG |
231 |
60 |
|
GAPDH |
Sense |
CCGTTGTCCCAATCTGTTCT |
204 |
59.6 |
Table 1: mRNA of PDE-5, eNOS and GAPDH protein’s primer sequences.
Cyclic GMP (cGMP) content estimation: The cGMP content in the lung homogenate of rats was assessed by using a commercially available cGMP diagnostic kit (Biovision, C.A., USA), following the procedure elaborated in the manufacturer’s guidelines.
Estimation of 5’GMP contents: The content of 5’GMP in the lung homogenate of rats was ascertained with the help of 5’GMP standard ELISA kit (Randox, U.K.), following the procedure elaborated in the manufacturer’s guidelines.
Protein Kinase G (PKG) expression: The PKG expressions in the lung homogenates of both hypoxia exposed and unexposed rats were ascertained by using rat PKG ELISA kit (Sunlong biotek co.ltd., Zhejiang, China), following the procedures detailed in manufacturer’s guidelines.
Measurement of Endothelin-1 (ET-1) activity in the lung of rats: The assessment of enzymatic activity of ET-1 in the lung homogenate of rats was carried out kinetically by using a laboratory-based, commercially feasible ET-1 diagnostic kit (Biovision, C.A., USA).
Assessment of nitric oxide synthases (NOS) in the lungs of rats: To determine the expression levels of NOS in lung homogenate of both hypoxia exposed and unexposed rats, we have used commercially available NOS detection ELISA kit (Biovision, C.A., USA).
Determination of Vascular permeability: The vascular leakage assessment in the lung of animals was carried out with few modifications in the method reported by Schoch et.al. (2002), using sodium fluorescein dye as a fluorescent tracker [41]. Hypoxia (6h) exposed animals were drawn out of the simulating hypobaric hypoxia chamber half-an-hour before the completion of exposure. Animals were intravenously administered with sodium fluorescein dye (1.5 mg/Kg BW) dissolved in saline and were re-exposed to the hypoxia chamber for the remaining half-an-hour. After the completion of 6h of hypoxia exposure, rats were anesthetized and their lungs were perfused using cold PBS (1X) to eliminate the excess dye from the vascular bed of the lungs. The lungs were then isolated and placed in formamide (3%) for 18 h at room temperature. After 18 h, the tissues were centrifuged (3000 rpm, 10 min. and 4°C) and the resulting supernatant obtained was used for ascertaining the fluorescence spectrophotometrically (Synergy H1, Biotek, Germany) at an excitation of 485 nm and emission of 530 nm.
Comparing the binding potentials of quercetin, tadalafil and rolipram using molecular docking approach
Binding mode of tadalafil, quercetin and rolipram in the binding pocket of Phosphodiesterase (PDE)-5 was studied using a computational modeling approach. Crystal co-ordinates of PDE-5 complexed with tadalafil (PDB: 1XOZ) [42] were taken; tadalafil and solvent molecules were removed from the complex. Hydrogen atoms and OPLS force field parameters [43] were added to native protein structure and ligands (tadalafil, quercetin and rolipram) for performing all the modeling studies. The active site region of the PDE-5 was used as a binding site for docking studies of ligands (tadalafil, quercetin and rolipram). 3D coordinates of tadalafil, quercetin and rolipram for docking studies were obtained from their respective co-crystal complex (PDB: 1XOZ), (PDB: 4DFU) [44] and (PDB: 1TBB) [45]. Each ligand was prepared for docking studies by adding hydrogen atoms and OPLS force field parameters followed by energy minimization. The prepared ligands were docked in the defined binding site using molecular docking program Glide [46] which uses a systematic conformational search algorithm for a conformational sampling of the ligand in the binding site and score them using an empirical approach. Docking of tadalafil was used as validation to test the ability of Glide to generate the correct docked pose. The docked conformation having highest score (maximum negative) was taken as the most probable binding mode of the ligand. The crystal complex of PDE-5 and tadalafil and selected docked complex of quercetin and rolipram were energy minimized to remove any steric clashes followed by refinement using Molecular Dynamics (MD) simulation in the presence of solvents to relax the protein structure (as it was treated rigid during docking process) and it also optimizes the binding of the ligand in presence of solvents. MD simulation was performed for 20 ns using DESMOND [47] with the time step of 2 fs in the presence of SHAKE constraints under NPT condition using OPLS force field parameters [48-50]. The refined docked complex was analyzed to understand the potential binding mode and binding interactions of tadalafil, quercetin and rolipram with PDE-5.
RESULTS
Affinity of analytes (Quercetin, Tadalafil and Rolipram) towards ligand (PDE-5)
The association kinetics of the drugs (quercetin, tadalafil and rolipram) using a concentration gradient ranging from 20µM to 200µM against PDE-5 exhibited a sigmoidal pattern of association curve by using surface plasma resonance (SPR). SPR was performed as represented in figure 1. Therefore, to obtain the binding affinity of quercetin in comparison to the known agonist tadalafil as a positive control against PDE-5 expressed in terms of Dissociation Constant (KD) value, which is considered to be inverse of affinity constants (binding affinity α 1/KD). Based on the Michaelis-Menten’s equation (KD is inversely proportional to affinity), quercetin and tadalafil with lesser KD values (57 µM and 83 µM) respectively exhibited better binding with PDE-5 over rolipram with higher KD (200 µM) (Figure 1).
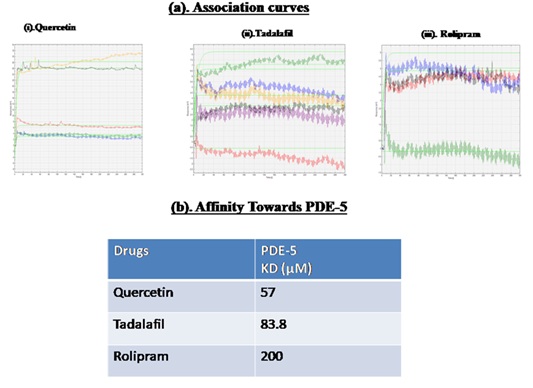 Figure 1: Binding potential of quercetin compared to tadalafil with respect to PDE-5 represented in terms of (a). Association constant curves and (b). Dissociation constant (Kd) values. Affinity Between differential concentrations of analytes (quercetin, tadalafil and, rolipram) ranging from 20µM, 40 µM, 60 µM, 80 µM, 100 µM and 200 µM) towards PDE-5 as ligand optimized confirmed 200 µM to be optimal dose concentration of analytes to be run against respective ligand and represented sigmoidally. In addition to this, the calculated Kd values confirmed the potent binding efficiency of quercetin and tadalafil than rolipram towards PDE-5.
Figure 1: Binding potential of quercetin compared to tadalafil with respect to PDE-5 represented in terms of (a). Association constant curves and (b). Dissociation constant (Kd) values. Affinity Between differential concentrations of analytes (quercetin, tadalafil and, rolipram) ranging from 20µM, 40 µM, 60 µM, 80 µM, 100 µM and 200 µM) towards PDE-5 as ligand optimized confirmed 200 µM to be optimal dose concentration of analytes to be run against respective ligand and represented sigmoidally. In addition to this, the calculated Kd values confirmed the potent binding efficiency of quercetin and tadalafil than rolipram towards PDE-5.
Reactive oxygen species (ROS) production
The levels of ROS generation in the lung of animals exposed to hypoxia (6h) were found to be significantly up-regulated (nearly 2-foldη) (p<0.001) compared to control (0h). Whereas, quercetin prophylaxis has significantly (p<0.001) restored the levels of ROS production compared to hypoxia control (6h) and tadalafil pretreated animals under hypoxia. However, quercetin and tadalafil pre-conditioning to the rats under normoxia showed unmodified levels of ROS compare to normoxia control (0h) (Figure 2).
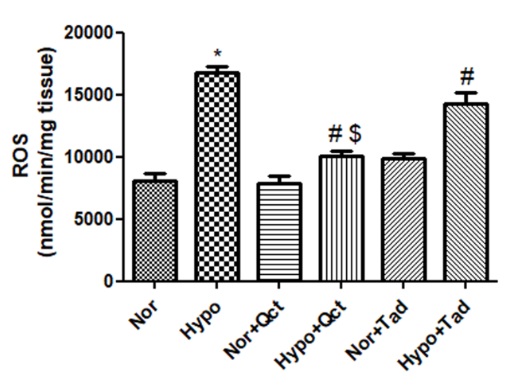 Figure 2: Reactive Oxygen Species (ROS) production in the lungs of the rats exposed to hypoxia at 7,620 m, for 6 h. Values are mean±SD (n=6). *P<0.001 normoxia vs hypoxia group, #P<0.05 hypoxia vs hypoxia+quercetin and $P<0.05 hypoxia+quercetin vs hypoxia+tadalafil. Nor- normoxia, Hypo- hypoxia, Nor+Qct- normoxia+quercetin Hypo+Qct- hypoxia+ quercetin, Nor+Tad- normoxia+tadalafil and Hypo+Tad- hypoxia+tadalafil.
Figure 2: Reactive Oxygen Species (ROS) production in the lungs of the rats exposed to hypoxia at 7,620 m, for 6 h. Values are mean±SD (n=6). *P<0.001 normoxia vs hypoxia group, #P<0.05 hypoxia vs hypoxia+quercetin and $P<0.05 hypoxia+quercetin vs hypoxia+tadalafil. Nor- normoxia, Hypo- hypoxia, Nor+Qct- normoxia+quercetin Hypo+Qct- hypoxia+ quercetin, Nor+Tad- normoxia+tadalafil and Hypo+Tad- hypoxia+tadalafil.
Modulations in lung lipid peroxidation (MDA) levels
Lungs of hypoxia exposed (6h) animals demonstrated a significant increase (2.5-folds↑) in lipid peroxidation compared to normoxia control (0h). Whereas, oral administration of quercetin prior to hypoxia exposure has appreciably curtailed (p<0.001) (2-folds ↓) MDA levels compared to hypoxia control (6h) and hypoxia+tadalafil group (1- fold↓). However, the normoxic animals fed with quercetin and tadalafil exhibited no modifications in MDA levels compared to normoxia (Figure 3).
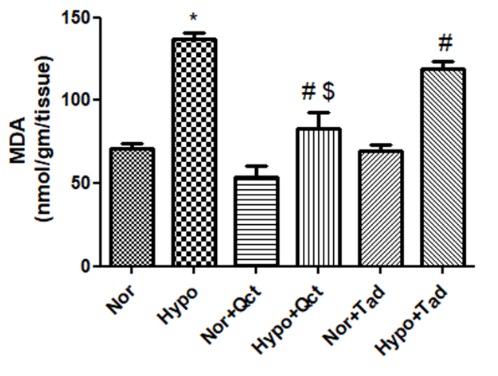 Figure 3: Effect of quercetin pre-conditioning on MDA levels in the lung of rats exposed to hypoxia at 7,620 m for 6 h. Values are mean±SD (n=6). *P<0.001 normoxia vs hypoxia, #P<0.05 hypoxia vs hypoxia+quercetin and $P<0.05 hypoxia+quercetin vs hypoxia+tadalafil. Nor- normoxia, Hypo- hypoxia, Nor+Qct- normoxia+quercetin Hypo+Qct- hypoxia+ quercetin, Nor+Tad- normoxia+tadalafil and Hypo+Tad- hypoxia+tadalafil.
Figure 3: Effect of quercetin pre-conditioning on MDA levels in the lung of rats exposed to hypoxia at 7,620 m for 6 h. Values are mean±SD (n=6). *P<0.001 normoxia vs hypoxia, #P<0.05 hypoxia vs hypoxia+quercetin and $P<0.05 hypoxia+quercetin vs hypoxia+tadalafil. Nor- normoxia, Hypo- hypoxia, Nor+Qct- normoxia+quercetin Hypo+Qct- hypoxia+ quercetin, Nor+Tad- normoxia+tadalafil and Hypo+Tad- hypoxia+tadalafil.
Effect of quercetin on the levels of reduced glutathione (GSH) in lungs of rat under hypoxia
There was a significant reduction (p<0.001) in the levels of GSH observed in the lung of rats under hypoxia (nearly 2-foldη) compared to normoxia control (0h). Whereas, quercetin supplementation 1h prior to hypoxia (6h) exposure resulted in a significant increase (1.8- foldsι) in the production of GSH compared to hypoxia control (6h) and a group of animals pre-treated with tadalafil during hypoxia. However, the supplementation of quercetin and tadalafil to the animals under normoxia showed unmodified levels of GSH compare to normoxia (Figure 4).
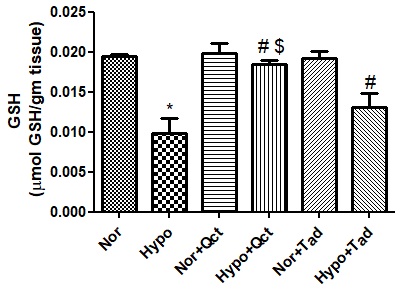 Figure 4: GSH levels in the lung of rats exposed to hypoxia at 7,620 m for 6 h. Values are mean±SD (n=6). *P<0.001 normoxia vs hypoxia, #P<0.05 hypoxia vs hypoxia+quercetin and $P<0.05 hypoxia+quercetin vs hypoxia+tadalafil. Nor- normoxia, Hypo- hypoxia, Nor+Qct- normoxia+quercetin Hypo+Qct- hypoxia+ quercetin, Nor+Tad- normoxia+tadalafil and Hypo+Tad- hypoxia+tadalafil.
Figure 4: GSH levels in the lung of rats exposed to hypoxia at 7,620 m for 6 h. Values are mean±SD (n=6). *P<0.001 normoxia vs hypoxia, #P<0.05 hypoxia vs hypoxia+quercetin and $P<0.05 hypoxia+quercetin vs hypoxia+tadalafil. Nor- normoxia, Hypo- hypoxia, Nor+Qct- normoxia+quercetin Hypo+Qct- hypoxia+ quercetin, Nor+Tad- normoxia+tadalafil and Hypo+Tad- hypoxia+tadalafil.
Effect of quercetin in modulating the hypoxia-induced regulation in PDE-5 and pe NOS protein and mRNA expressions
Protein expression profiling revealed a significant increase in the expression of PDE-5 (nearly 2-foldη) leading to the significant alleviation in phosphorylated eNOS levels (nearly 2-foldι) during 6 h of hypoxia exposure as compared to control. Whereas, quercetin supplementation prior to hypoxia exposure (6 h) showed, a significant (p<0.001) reduction in PDE-5 (nearly 1.5-foldι) expressions compared to hypoxia control (6h) and hypoxia+tadalafil group. On contrary, a significant up-regulation in the phosphorylated eNOS levels (p<0.001) was observed in the group of rats pretreated with quercetin (nearly 2-foldη) and tadalafil (1.5-foldι) compared to hypoxia (6 h). The animals receiving quercetin prior to hypoxia exhibited higher eNOS expressions compared to the group fed with tadalafil. However, animals under normoxia fed with the drugs (quercetin and tadalafil) demonstrated unmodified expressions of PDE-5 and peNOS compared to control (0 h) (Figure 5). Apart from this, we have also evaluated mRNA expressions of PDE-5 and eNOS using qualitative PCR. mRNA expressions of PDE-5 in the lungs of rats under hypoxia were observed to be up-regulated whereas, phosphorylated eNOS expressions were found to be reduced compared to control (0h). Nonetheless, the quercetin supplementation 1h prior to hypoxia exposure has down-regulated the levels of PDE-5 along with a concomitant elevation in eNOS levels compared to hypoxia (6 h) and hypoxia+tadalafil group (Figure 5).
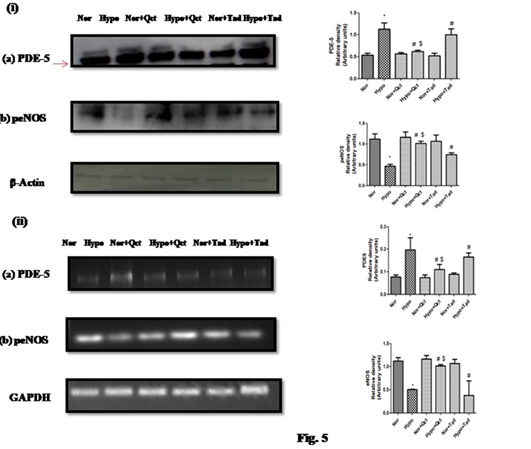 Figure 5: Effect of quercetin supplementation on the (i) Protein expression of (a) PDE-5 and (b) eNos and (ii) mRNA expressions of (a) PDE-5 and (b) eNOS in the lung of rats exposed hypoxia at 7,620 m for 6 h. Values are mean±SD (n=6). *P<0.001 normoxia vs hypoxia, #P<0.05 hypoxia vs hypoxia+quercetin and $P<0.05 hypoxia+quercetin vs hypoxia+tadalafil. Nor- normoxia, Hypo- hypoxia, Nor+Qct- normoxia+quercetin Hypo+Qct- hypoxia+ quercetin, Nor+Tad- normoxia+tadalafil and Hypo+Tad- hypoxia+tadalafil.
Figure 5: Effect of quercetin supplementation on the (i) Protein expression of (a) PDE-5 and (b) eNos and (ii) mRNA expressions of (a) PDE-5 and (b) eNOS in the lung of rats exposed hypoxia at 7,620 m for 6 h. Values are mean±SD (n=6). *P<0.001 normoxia vs hypoxia, #P<0.05 hypoxia vs hypoxia+quercetin and $P<0.05 hypoxia+quercetin vs hypoxia+tadalafil. Nor- normoxia, Hypo- hypoxia, Nor+Qct- normoxia+quercetin Hypo+Qct- hypoxia+ quercetin, Nor+Tad- normoxia+tadalafil and Hypo+Tad- hypoxia+tadalafil.
Effect of quercetin prophylaxis on cGMP contents in the lungs of rats under hypoxia
Animals exposed to hypoxia demonstrated significantly (p<0.001) reduced cGMP contents (nearly 3.5-foldι) compared to normoxia. Whereas the animals supplemented with quercetin 1 h prior to hypoxia (6 h) exposure presented a significant increase in cGMP contents (nearly 3.2-foldη) compared to hypoxia (6 h) and animals fed with tadalafil prior to hypoxia exposure (1.8-foldη). However, the normoxic groups fed with quercetin and tadalafil exhibited unmodified c GMP contents compared to normoxia (Figure 6).
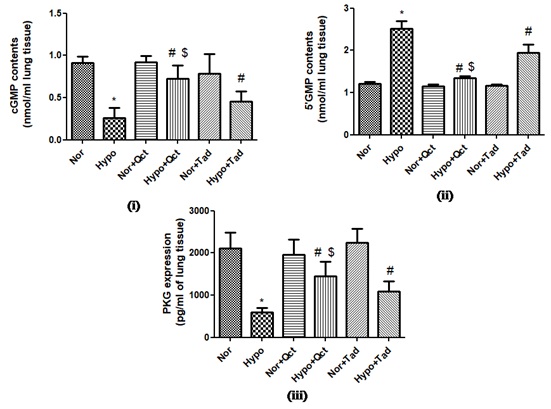 Figure 6: Effect of quercetin supplementation on (i) Contents of cGMP, (ii) 5’GMP contents and (iii) PKG expressions in the lungs of rat exposed to hypoxia at 7,620 m for 6 h. Values are mean±SD (n=6). *P<0.001 normoxia vs hypoxia, #P<0.05 hypoxia vs hypoxia+quercetin and $P<0.05 hypoxia+quercetin vs hypoxia+tadalafil. Nor- normoxia, Hypo- hypoxia, Nor+Qct- normoxia+quercetin Hypo+Qct- hypoxia+ quercetin, Nor+Tad- normoxia+tadalafil and Hypo+Tad- hypoxia+tadalafil.
Figure 6: Effect of quercetin supplementation on (i) Contents of cGMP, (ii) 5’GMP contents and (iii) PKG expressions in the lungs of rat exposed to hypoxia at 7,620 m for 6 h. Values are mean±SD (n=6). *P<0.001 normoxia vs hypoxia, #P<0.05 hypoxia vs hypoxia+quercetin and $P<0.05 hypoxia+quercetin vs hypoxia+tadalafil. Nor- normoxia, Hypo- hypoxia, Nor+Qct- normoxia+quercetin Hypo+Qct- hypoxia+ quercetin, Nor+Tad- normoxia+tadalafil and Hypo+Tad- hypoxia+tadalafil.
Effect of quercetin supplementation on 5’GMP content
The contents of 5’GMP in the lung homogenate of rats under hypoxia was found to be elevated (nearly 2.5-foldη) significantly (p<0.001) compared to normoxia. Whereas, the animals under hypoxia pretreated with quercetin resulted in a significant (p<0.001) reduction in 5’GMP content (nearly 2.5-foldι) compared to hypoxia and hypoxia+tadalafil (nearly 1-foldι). However, animals under normoxia fed with the drugs (quercetin and tadalafil) expressed no change in the content of 5’GMP compared to normoxia (Figure 6(ii)).
The prophylactic potential of quercetin in regulating PKG expressions in the lung of animals exposed to hypoxia
Animals exposed to hypoxia elicited significant reduction (p<0.001) in PKG expressions (nearly 3.5- folds ι) compared to hypoxia (6 h). Whereas, animals preconditioned with quercetin and exposed to hypoxia, exhibited a significant elevation (p<0.001) in PKG expressions (3- folds η) compared to hypoxia (6 h) and the hypoxia+tadalafil group (1-fold η). However, the animals under normoxic group fed with quercetin and tadalafil showed no change in PKG expressions compared to normoxia (Figure 6).
The prophylactic potential of quercetin on ET-1 activity in the lungs of rat exposed to hypoxia
The catalytic activity of ET-1 expression in the lungs was found to be significantly up- regulated (nearly 2-foldη) by hypoxia treatment compared to normoxia. Whereas, administration of quercetin and tadalafil prior to hypoxia exposure has resulted in a significant reduction (p<0.001) in the activity of ET-1 (nearly 2-foldι and 1-foldι) compared to hypoxia (6 h). However, the decrease in the ET-1 activity was found to be more significant in the group of animals fed with quercetin during hypoxia compared to tadalafil pre-treated rats under hypoxia. The animals fed with quercetin and tadalafil under normoxia demonstrated unmodified ET-1 activity compared to normoxia control (0h) (Figure 7).
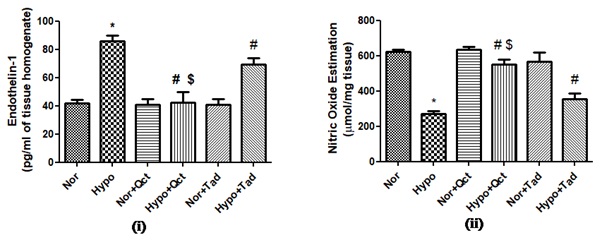 Figure 7: Prophylactic potential of quercetin on: (i) ET-1 and (ii) NO in the lungs of rat exposed to hypoxia at 7,620 m for 6 h. Values are mean±SD (n=6). *P<0.001 normoxia+hypoxia, #P<0.001 hypoxia vs hypoxia+quercetin and $P<0.001 hypoxia+quercetin vs hypoxia+tadalafil. Nor- normoxia, Hypo- hypoxia, Nor+Qct- normoxia+quercetin Hypo+Qct- hypoxia+ quercetin, Nor+Tad- normoxia+tadalafil and Hypo+Tad- hypoxia+tadalafil.
Figure 7: Prophylactic potential of quercetin on: (i) ET-1 and (ii) NO in the lungs of rat exposed to hypoxia at 7,620 m for 6 h. Values are mean±SD (n=6). *P<0.001 normoxia+hypoxia, #P<0.001 hypoxia vs hypoxia+quercetin and $P<0.001 hypoxia+quercetin vs hypoxia+tadalafil. Nor- normoxia, Hypo- hypoxia, Nor+Qct- normoxia+quercetin Hypo+Qct- hypoxia+ quercetin, Nor+Tad- normoxia+tadalafil and Hypo+Tad- hypoxia+tadalafil.
Effect of quercetin in augmenting nitric oxide (NO) levels in the lung of rats under hypoxia
Rats exposed to hypoxia (6 h) exhibited significant (p<0.001) reduction in the levels of NO (nearly 3-foldι) compared to normoxia. Whereas, the quercetin supplementation prior to hypoxia showed a significant increase (p<0.001) in NO levels (nearly 2.5-foldη) compared to hypoxia control (0 h) and hypoxia + tadalafil group (nearly 1.2-foldη). However, normoxic animals fed with quercetin and tadalafil elicited similar levels of NO as that of normoxia (Figure 7).
Vascular permeability
Hypoxia exposed animals exhibited a significant elevation (p<0.001) in transvascular leakage (3163.50±89.21 rfu/gm tissue) over normoxia control (0h) (1192.83±69.44 rfu/gm tissue). However, preconditioning with quercetin and tadalafil resulted in significant attenuation (p<0.001) in transvascular leakage (1089±67 rfu/gm tissue) (2884.16±67.4 rfu/gm tissue) compared to hypoxia control (6 h) group. Whereas, the normoxic rats fed with quercetin and tadalafil showed no change in relative fluorescence compared to normoxia respectively (Figure 8).
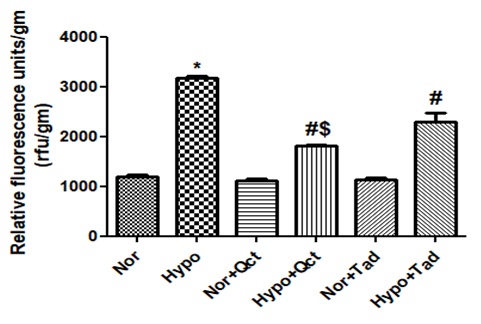 Figure 8: Prophylactic administration of quercetin on transvascular leakage in the lung of rat exposed hypoxia at 7,620 m for 6 h. Values are mean±SD (n=6). *P<0.001 normoxia vs hypoxia, #P<0.05 hypoxia vs hypoxia+quercetin and $P<0.05 hypoxia+quercetin vs hypoxia+tadalafil. Nor- normoxia, Hypo- hypoxia, Nor+Qct- normoxia+quercetin Hypo+Qct- hypoxia+ quercetin, Nor+Tad- normoxia+tadalafil and Hypo+Tad- hypoxia+tadalafil.
Figure 8: Prophylactic administration of quercetin on transvascular leakage in the lung of rat exposed hypoxia at 7,620 m for 6 h. Values are mean±SD (n=6). *P<0.001 normoxia vs hypoxia, #P<0.05 hypoxia vs hypoxia+quercetin and $P<0.05 hypoxia+quercetin vs hypoxia+tadalafil. Nor- normoxia, Hypo- hypoxia, Nor+Qct- normoxia+quercetin Hypo+Qct- hypoxia+ quercetin, Nor+Tad- normoxia+tadalafil and Hypo+Tad- hypoxia+tadalafil.
In Silico analysis
The modeled complex of quercetin and rolipram were compared with the binding mode of crystal complex of tadalafil with PDE-5 [PDB: 1XOZ]. Binding position of docked quercetin and rolipram was found to be almost identical to co-crystallized tadalafil (Figure 9). The core scaffold of all three ligands almost coincides. The central aromatic ring in all three occupies the same space. Substituted benzodioxol ring moiety on central pyridine group of tadalafil projects in neighboring sub-pocket while smaller ligands (quercetin and rolipram) superimposes very well with core scaffold of tadalafil. Binding mode analysis of tadalafil in crystal complex shows that it is mainly stabilized by hydrophobic interactions with side chains of Ile768, Val782, Leu804 and Met816. It has only one hydrogen-bonded interaction with the side chain of Gln817 (Figure 9). In contrast, quercetin was found to have four hydrogen-bonded polar interactions though both reside in the same binding pocket (Figure 9). However, quercetin loses the non-polar interactions with Ile768 and Met816. Indole nitrogen of tadalafil forms a hydrogen bond with amide oxygen of the Gln817 side chain while one of the hydroxyl moieties of quercetin forms a hydrogen bond with amide oxygen of Gln817 side chain. Additionally, its hydroxyl group also forms two hydrogen bonds with a side chain of Gln775. Rolipram has two hydrogen bonds with PDE-5 and vander Waal interactions with non-polar side chains of Leu725, Leu765, Leu804 and Met816. Cyclopentyl ring of rolipram projects into the sub-pocket where benzodioxal ring of tadalafil was observed in the crystal complex and cyclopentyl moiety has non-polar interaction with Met816 similar to tadalafil (Figure 9). Pyrrolidine ring of rolipram forms a hydrogen bond with the main chain of Leu765 and oxygen atom on pyrrolidine forms a hydrogen bond with amide nitrogen of Gln817.
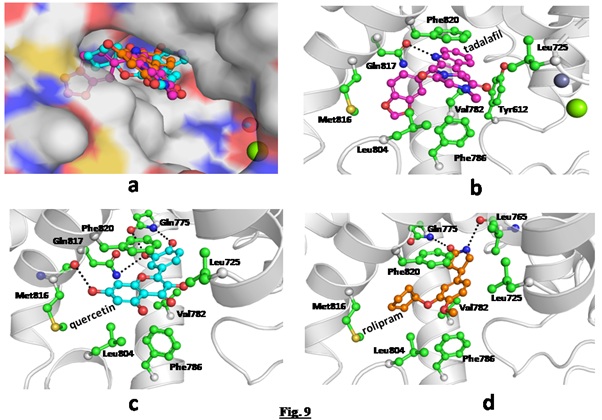 Figure 9: (a) Modelled complex of quercetin and rolipram superimposed on crystal complex of tadalafil with PDE-5; (b) Crystal complex of tadalafil (ball and stick, magenta) and (c) Modelled complex of quercetin (ball and stick, cyan) and (d) Rolipram (ball and stick, orange). Interacting side chains of PDE-5 with ligands are shown as ball and stick in green and hydrogen bonds are shown as black dotted lines.
Figure 9: (a) Modelled complex of quercetin and rolipram superimposed on crystal complex of tadalafil with PDE-5; (b) Crystal complex of tadalafil (ball and stick, magenta) and (c) Modelled complex of quercetin (ball and stick, cyan) and (d) Rolipram (ball and stick, orange). Interacting side chains of PDE-5 with ligands are shown as ball and stick in green and hydrogen bonds are shown as black dotted lines.
DISCUSSION
Every year thousands of people travel to high altitude areas for recreational purposes, trekking, skiing, pilgrimage, rescue operations, mining operations or army deployed at border areas and also several sports training centres and/or to participate in the sports events at high altitude areas. The effects of high altitude on humans are extensive due to the lack of enough oxygen (hypoxia). Hypoxia with low barometric pressure is the major putative problem that mainly causes 3 critical high altitude illnesses from mild AMS to severe HACE and HAPE. The hypoxemia depends on the height reached, the speed, the route, time to reach and also exertion at high altitude. High Altitude Pulmonary Edema (HAPE) is a life-threatening form of high altitude illness that involves abnormal accumulation of fluid in the lungs and in fact, is the most common fatal expression of severe high altitude illness occurs mainly due to inadequate acclimatization. It is well known that clinically HAPE diagnosis is characterized by fatigue, dyspnea and dry cough with exertion. If left untreated, it can progress to dyspnea at rest, rales, cyanosis, tachycardia and may further lead to mortality [51]. Therefore the present study has employed the application of quercetin, a phyto-flavonoid against already existing medication (tadalafil) in the prevention of high altitude pulmonary edema.
Amongst eleven different PDEs, PDE-5 is reported to be widely distributed in the lungs of the mammalian system and is responsible for regulating the contraction and relaxation of alveolar smooth muscles by catalyzing inactivation/hydrolysis of secondary messengers, Cyclic Adenosine Monophosphate (cAMP), cyclic guanosine monophosphate (cGMP), etc. [52]. Under stressed condition such as- hypoxic stress, the activity of PDE-5 has been reported to be exaggerated resulting into the impairment of cGMP levels leading to contraction of vascular smooth muscles of alveoli due to reduction in the synthesis of nitric oxide synthases in the lungs as an outcome of hypoxic exposure [53,54]. We have used one of the potent phosphodiesterase inhibitors tadalafil (recommended to prevent HAPE) to correlate our results with naturally occurring phytoflavonoid quercetin. Tadalafil is a potent Phosphodiesterase-5 (PDE-5) inhibitor approved for the treatment of Pulmonary Arterial Hypertension (PAH) by the World Health Organization (WHO) [54]. Tadalafil decreases the activity of PDE-5 so that more cyclic GMP is available to the blood vessels for their relaxation or widening within the lungs. Contraction & relaxation of blood vessels in the lungs decreases the pulmonary blood pressure to the heart and improve its function. Thus, the present study has been designed to combat the hypoxia-mediated increase in the PDE-5 activity followed by attenuating cGMP hydrolysis to yield 5’GMP, which finally ends-up with an increase in the production of nitric oxide in the lung parenchyma leading to relaxation of airway smooth muscles in the lungs of rats. To accomplish this objective, the study has employed the prophylaxis of quercetin (a phytoflavonoid), which is a well-known antioxidant and anti-inflammatory biomolecule with bioprotective and phytonutrient properties [51].
Hypoxia is reported to be a potent stimulator of reactive oxygen species production [55]. The emergence of ROS as a manifestation of hypobaric hypoxia is also reported to be a leading cause of lipid peroxidation [56]. Lipid peroxidation is a well-discussed mechanism of cellular injury as well as an indicator of oxidative stress, elicited by the levels of MDA (a physiological marker for lipid peroxidation) [56]. Our results have reported that quercetin significantly quenched the ROS production and also inhibited the MDA levels in the lungs of rats under hypoxia. Further the antioxidant molecule GSH was significantly enhanced under the quercetin regiment. Interestingly, the quercetin prophylaxis has also exhibited a significant reduction in hypoxia induced oxidative stress compared to tadalafil, indicating that quercetin is a more potent antioxidant molecule than tadalafil.
Among the most important for pulmonary vascular homeostasis are factors that utilize Cyclic Guanosine Monophosphate (cGMP) as an intracellular secondary messenger; the most important being nitric oxide [57]. The cGMP content in the cell is strictly monitored by PDEs, which during the stress conditions hydrolyzes 3’,5’-cyclic phosphate moiety of cGMP into 5’ nucleotide component of 5’GMP [58]. This 5’GMP is further reported to result in the development of vascular remodeling of smooth muscles or transvascular leakage in the lungs [57,58]. Our study demonstrated that quercetin prophylaxis under hypoxia has significantly curtailed the cGMP hydrolysis or 5’GMP synthesis leading to the subsided activity of PDE-5 compared to control (hypoxia, 6h). This crucial step is very much required to stabilize the cGMP which further activates the downstream molecule, protein kinase G. PKG is a cytosolic protein that plays a putative role in the regulation of cell cycle, apoptosis, gene expressions and GTP synthesis [58]. The increased PKG levels under quercetin prophylaxis indicate the inactivation of PDE-5 followed by enhanced cGMP. As per the literature tadalafil is a known potent inhibitor of PDE-5 responsible for inhibiting the PDE-5 activity. However, when both the drugs (tadalafil and quercetin) were compared, quercetin showed better results than tadalafil in subsiding the catalytic potential of PDE-5 in the lungs during hypoxia. This could be the main reason that vascular leakage in case of quercetin pre-treated rats was observed to be reduced as compared to tadalafil pre-treated and control (hypoxia h) rats.
Literature has also revealed that excess production of free radicals and an increase in MDA levels are also associated with the attenuation in NO production in the lung vasculature [59]. Under physiological conditions, NO, a well-studied vasoprotective molecule of pulmonary endothelium is reported to be produced continuously from eNOS [59,60]. Mechanistically, shortfall of tetrahydrobiopterin (BH4) (eNOS cofactor), L-arginine (eNOS substrate) and eNOS S-glutathionylation are the factors responsible for eNOS uncoupling [61]. Whereas, under pathological conditions, especially during oxidant injury, eNOS is impaired because of endothelial dysfunctioning primarily due to enhanced superoxide production resulting in fleeting NO inactivation [61]. Secondly, due to the uncoupling of O2 reduction from NO synthesis as a result of persisting oxidative stress [62]. Our protein analysis studies demonstrated the increased expression of PDE-5 and reduced eNOS levels under hypoxia, while quercetin pretreatment was able to lower the PDE-5 expression with increased eNOS levels. Our corresponding mRNA studies substantially supported these findings.
Apart from this, the emergence of free radicals generation, elevation in the catalytic activity of Endothelin-1 (ET-1) in the lung endothelium during hypoxia is a leading cause of vasoconstriction [63]. Hay et.al. 1993 had revealed that ET-1 released from vascular endothelial cells is an effective endothelium-derived vasoconstrictor protein with mitogenic activities, which is a key regulator for the synthesis of cytokines responsible for the alveolar inflammation [64]. Studies have also indicated that high altitude hypoxia is known to induce an inflammatory response and may contribute to the development of high altitude pulmonary edema (HAPE) by causing damage to the lung endothelial cells and thereby capillary leakage [32,65]. Endothelin-1 (ET-1) is a peptide that may act through activation of NF-κB and degradation of IκB-α [66]. Recent studies have also indicated the relation of increased ET-1 with hypoxic vasoconstriction [63,64]. The increased ET-1 levels (P<0.001) in the present study specified the changes in vascular endothelium as evidenced by reduced NO levels and an increased transvascular leakage in the lungs of rat under hypoxia. Both quercetin and tadalafil appreciably enhanced the NO levels and reduced transvascular leakage as compared to control. Quercetin prophylaxis might have improved the oxygen-derived vasodilation in the lungs during hypoxia that can be associated with the reduction in ET-1 net secretion from vascular endothelium. However, Maggiorni et al., (2006) have reported that both dexamethasone (a steroid recommended for HAPE prophylaxis) and tadalafil decrease systolic pulmonary artery pressure and may reduce the incidence of HAPE in adults with a history of HAPE [67]. However, the same group of authors in 2009 have revealed that corticosteroids, but not phosphodiesterase-5 inhibition with tadalafil had partially prevented the exercise capacity in subjects with intense hypoxic pulmonary vasoconstriction at high altitude [68]. This may be due to the fact that dexamethasone works as an anti-inflammatory and an anti-oxidant molecule in the prevention of hypoxia-induced oxidative stress and inflammation. But as reported earlier from our laboratory that quercetin prevented the hypoxia-induced oxidative stress and inflammation in the brain of rats better than dexamethasone [69] and in the lungs of rats as well [32]. It seems reasonable to explain that quercetin is a potent antioxidant and anti-inflammatory molecule that prevented vascular leakage by enhancing NO production via inhibiting PDE-5.
After these pre-clinical studies, we reasoned that, whether quercetin has strong binding properties to inhibit the PDE-5 similar to tadalafil (a known PDE-5 inhibitor)? Further, we extended our study to cross-compare these results with another potent PDE-4 inhibitor rolipram. It is to be noted that, our rolipram studies were restricted up to SPR binding and molecular docking studies only, hence not shown any rolipram results further. The SPR binding (affinity) studies showed KD (µM) of quercetin, tadalafil and rolipram as 57 µM, 83.8 µM and 200 respectively. It is well known that the lower the KD value, the higher is the binding activity; which was obtained with Quercetin compared to other dugs tested (tadalafil and rolipram).
To cross confirm and also to strengthen the SPR results we further carried out docking studies. Computational modeling analysis shows that quercetin and rolipram bind in an active site of PDE-5 similar to tadalafil. Though non-polar interactions are predominant between tadalafil and PDE-5 while polar hydrogen-bonded interactions are dominant between quercetin and PDE-5. Yet their binding mode is very similar. Quercetin and rolipram have the aromatic ring as the core scaffold is similar to tadalafil. Moreover, the aromatic core scaffold of all three ligands was found to have stacking interactions with the side chain of Phe820 of PDE-5. Tadalafil binding is stabilized mainly by non-polar interactions and has one hydrogen bonded interaction. While four hydrogen-bonded polar interactions are predominant force in the binding of quercetin which is a flavonol and having several hydroxyl groups. In the case of rolipram, cyclopentyl moiety of rolipram occupies the position similar to benzodioxal moiety of tadalafil and also has similar van der Waal interactions with side chains of Leu84 and Met816. Pyrrolidine moiety of rolipram forms a hydrogen bond with PDE-5 similar to the indole moiety of tadalafil as these two moieties occupy the same space in the binding pocket. The similarity in binding interactions of quercetin and rolipram with tadalafil sternly suggests that quercetin and rolipram bind in the active site of PDE-5 similar to tadalafil (PDB: 1XOZ). Docking score also indicates that quercetin has a higher binding affinity (ΔG= -10.74 kcal/mol) than tadalafil (ΔG= -9.65 kcal/mol) and rolipram (ΔG= -8.85 kcal/mol) with the lowest binding affinities. This observation is also substantiated by binding strength determined by SPR experiments.
Besides investigating the effects of preventive treatment on reducing oxidative stress, the present study sheds further light on the mechanism of the use of natural phenolic phyto- flavanoid to reduce vasoconstriction (reduced ET-1) followed by increased NO production under quercetin supplementation compared to tadalafil treatment. The reason for this could be due to the fact that quercetin is a known potent anti-oxidant and anti-inflammatory molecule. Interestingly, our study emphasized on the reduction of hypoxia-mediated increase in ROS production responsible for increase in PDE-5 activity, leading to the hydrolysis of cGMP to produce 5’GMP, which further results in the reduction in PKG expressions followed by increased in ET-1 production from vascular endothelium which will end up in the dephosphorylation of eNOS tending to exceed transvascular leakage in the lungs. Quercetin being a natural molecule does not imparts any major side-effects, whereas tadalafil is a synthetic drug and its direct exposure drug is reported to be associated with number of side- effects like headache, stomach upset, back pain, muscle pain, stuffy nose, flushing, or dizziness [25,70] which altogether may indicate that tadalafil cannot be used for longer durations against high altitude pulmonary edema.
Ideally the agent to be used as a countermeasure against the multifactorial illnesses like HAPE must be characterized by- least or no side-effects occurrence upon exposure, easy & quick administration to large numbers of individuals and efficacy i.e. when given as a rescue treatment after the exposure to hypobaric hypoxia conditions, should be beneficial without any side-effects. Our experiments on oral delivery of tadalafil and quercetin to rats under hypoxia showed that both were effective in reducing the occurrence of pulmonary edema. Thus, to reverse the effects of hypoxia on eNOS phosphorylation, quercetin was administered prophylactically to the animals before hypoxia exposure, which has resulted in the significant reduction in transvascular leakage by inhibiting the ROS generation and PDE-5 mediated hydrolysis of cGMP into 5’GMP responsible for regulating PKG expressions, leading to the reduction in ET-1 production. As a result, eNOS phosphorylation stimulated followed by the accelerated NO synthesis leading to a significant decrease in a fluid influx into the lungs of a rat under hypoxia. This cascade has been demonstrated schematically in figure 10.
Figure 10: Schematic representation of quercetin mediated increase in NO expressions in the lung of the rats exposed to hypoxia at 7,620 m for 6 h. Hypoxia exposure increased the production of Reactive Oxygen Species (ROS), MDA and curtailed the levels of GSH in the lung of rats, which in turn elevated the activities of ET-1, PDE-5 and down-regulated the expressions of peNOS and NO levels in the lungs of rat. These elevated ET-1 and PDE-5 activities under hypoxic conditions, further down-regulated the expression of PKG and cGMP contents along with the increase in levels 5’GMP resulted in fluid build-up in the lungs of rat under hypoxia. However, the entire process got reversed after the supplementation of quercetin prior to hypoxia. Quercetin primarily attenuated the production of intracellular ROS, MDA, ET-1 and inhibited the PDE-5 activity followed by augmented levels of GSH, PKG expressions and cGMP contents along with the up-regulation in peNOS and NO levels leading to significantly reduced levels of transvascular leakage in the lung of rats exposed to hypoxia. Where, ⊥ - inhibition, ↑- up-regulation and ↓- down-regulation.
The only limitation associated with the present study was that we could not perform invasive hemodynamic measurements that may give additional information (right heart catheterization and arterial blood gas analyses). In conclusion, quercetin prophylaxis lessens the oxidative stress leading to reduced phosphodiesterase-5 activity & expression which further contributes to attenuate transvascular leakage in the lung of rats under hypoxia.
CONCLUSION
The findings from the present study have unraveled that quercetin prophylaxis to male SD rats prior to acute hypoxia exposure was found to be effective in minimizing the severity of HAPE by inhibiting the hypoxia-mediated increase in PDE-5 activity responsible for controlling cGMP pool in the cell leading to reduction in fluid flux into the alveolar bed in the lungs of rats compared to tadalafil.
DECLARATION SECTION
Acknowledgment: We are, very thankful to the Director, DIPAS, DRDO, India, for providing all the support and facilities for conducting this experiment.
Funding: The study was conducted under the project entitled “Improving performance under different operational environments using suitable interventions” funded by the Defence Research and Development Organization, Government of India. Grant No.: DIP-265
Availability of data and materials: The data supporting the results in our manuscript have been clearly stated in the materials and method section. All the data presented in the manuscript is in a machine-readable format.
Author’s contributions: SKS has conceived and designed the experiments. AT has performed the experiments, prepared the manuscript, graphs and figures. MK and PK performed and analyzed in silico experiments. AT and SKS have analyzed the manuscript. BK has done the final investigation of the study.
Ethical approval for animal studies: All rat experiments in this study were carried out following the recommendations and approval of the institutional ethical committee (IEC). We followed the guidelines from the Universities of Federation for Animal Welfare (UFAW) for animal-based research work.
Consent for publication: Not applicable.
COMPETING INTEREST
The manuscript has not been published and is not under consideration for publication elsewhere. All authors have given their consent for its publication. The authors have declared that no competing interests exist.
REFERENCES
- Bartsch P, Mairbaurl H, Swenson ER, Maggiorini M (2003) High altitude pulmonary oedema. Swiss Med Wkly 133: 377-384.
- Schoene RB (2008) Illnesses at high altitude. Chest 134: 402-416.
- Demarco VG, Whaley-Connell AT, Sowers JR, Habibi J, Dellsperger KC (2010) Contribution of oxidative stress to pulmonary arterial hypertension. World J Cardiol 2: 316-324.
- DeMarco VG, Habibi J, Whaley-Connell AT, Schneider RI, Heller RL, et al. (2008) Oxidative stress contributes to pulmonary hypertension in the transgenic (mRen2)27 rat. American journal of physiology Heart and circulatory physiology 294: 2659-2668.
- McGoon MD, Kane GC (2009) Pulmonary hypertension: Diagnosis and management. Mayo Clinic proceedings 84: 191-207.
- Meyrick BO, Reid LM (1982) Crotalaria-induced pulmonary hypertension. Uptake of 3H-thymidine by the cells of the pulmonary circulation and alveolar walls. The American journal of pathology 106: 84-94.
- Archer SL, Nelson DP, Weir EK (1989) Detection of activated O2 species in vitro and in rat lungs by chemiluminescence. Journal of applied physiology 67: 1912-1221.
- Taniyama Y, Griendling KK (2003) Reactive oxygen species in the vasculature: Molecular and cellular mechanisms. Hypertension 42: 1075-1081.
- Redout EM, van der Toorn A, Zuidwijk MJ, van de Kolk CW, van Echteld CJ, et al. (2010) Antioxidant treatment attenuates pulmonary arterial hypertension-induced heart failure. American journal of physiology Heart and circulatory physiology 298: 1038-1047.
- Harrison D, Griendling KK, Landmesser U, Hornig B, Drexler H (2003) Role of oxidative stress in atherosclerosis. Am J Cardiol 91: 7-11.
- Weiland JE, Davis WB, Holter JF, Mohammed JR, Dorinsky PM, et al. (1986) Lung neutrophils in the adult respiratory distress syndrome. Clinical and pathophysiologic significance. Am Rev Respir Dis 133: 218-225.
- Redout EM, Wagner MJ, Zuidwijk MJ, Boer C, Musters RJ, et al. (2007) Right-ventricular failure is associated with increased mitochondrial complex II activity and production of reactive oxygen species. Cardiovasc Res 75: 770-781.
- Zou MH, Cohen R, Ullrich V (2004) Peroxynitrite and vascular endothelial dysfunction in diabetes mellitus. Endothelium 11: 89-97.
- Liu JQ, Zelko IN, Erbynn EM, Sham JS, Folz RJ (2006) Hypoxic pulmonary hypertension: role of superoxide and NADPH oxidase (gp91phox). Am J Physiol Lung cell Mol Physiol 290: 2-10.
- Arinami T, Ishikawa M, Inoue A, Yanagisawa M, Masaki T, et al. (1991) Chromosomal assignments of the human endothelin family genes: The endothelin-1 gene (EDN1) to 6p23-p24, the endothelin-2 gene (EDN2) to 1p34, and the endothelin-3 gene (EDN3) to 20q13.2-q13.3. Am J Hum Genet 48: 990-996.
- Alonso D, Radomski MW (2003) The nitric oxide-endothelin-1 connection. Heart Fail Rev 8: 107-115.
- Ashman DF, Lipton R, Melicow MM, Price TD (1963) Isolation of adenosine 3', 5'-monophosphate and guanosine 3', 5'-monophosphate from rat urine. Biochem Biophys Res Commun 11: 330-334.
- Bender AT, Beavo JA (2006) Cyclic nucleotide phosphodiesterases: molecular regulation to clinical use. Pharmacological reviews 58: 488-520.
- Keravis T, Lugnier C (2010) Cyclic nucleotide phosphodiesterases (PDE) and peptide motifs. Current pharmaceutical design 16: 1114-1125.
- Francis SH, Blount MA, Corbin JD (2011) Mammalian cyclic nucleotide phosphodiesterases: molecular mechanisms and physiological functions. Physiol Rev 91: 651-690.
- Conti M, Beavo J (2007) Biochemistry and physiology of cyclic nucleotide phosphodiesterases: essential components in cyclic nucleotide signaling. Annu Rev Biochem 76: 481-511.
- Sheridan BC, McIntyre RC, Meldrum DR, Fullerton DA (1997) Phosphodiesterase inhibition overcomes pulmonary vasomotor dysfunction in acute lung injury. Journal of surgical research 71: 145-149.
- Bates MG, Thompson AA, Baillie JK (2007) Phosphodiesterase type 5 inhibitors in the treatment and prevention of high altitude pulmonary edema. Curr Opin Investig Drugs 8: 226-231.
- Fiorito J, Saeed F, Zhang H, Staniszewski A, Feng Y, et al. (2013) Synthesis of quinoline derivatives: Discovery of a potent and selective phosphodiesterase 5 inhibitor for the treatment of Alzheimer's disease. European journal of medicinal chemistry 60: 285-294.
- Yu G, Mason H, Wu X, Wang J, Chong S, et al. (2003) Substituted pyrazolopyridopyridazines as orally bioavailable potent and selective PDE5 inhibitors: Potential agents for treatment of erectile dysfunction. Journal of medicinal chemistry 46: 457-460.
- Sak K (2014) Cytotoxicity of dietary flavonoids on different human cancer types. Pharmacognosy reviews 8: 122-146.
- Kelly GS (2011) Quercetin. Monograph. Alternative medicine review. A journal of clinical therapeutic 16: 172-194.
- Harwood M, Danielewska-Nikiel B, Borzelleca JF, Flamm GW, Williams GM, et al. (2007) A critical review of the data related to the safety of quercetin and lack of evidence of in vivo toxicity, including lack of genotoxic/carcinogenic properties. Food and chemical toxicology 45: 2179-2205.
- Dunnick JK, Hailey JR (1992) Toxicity and carcinogenicity studies of quercetin, a natural component of foods. Fundamental and applied toxicology 19: 423-431.
- Yang LL, Xiao N, Li XW, Fan Y, Alolga RN, et al. (2016) Pharmacokinetic comparison between quercetin and quercetin 3-O-β-glucuronide in rats by UHPLC-MS/MS. Scientific reports 6: 35460.
- Chen R, Lin J, Hong J, Han D, Zhang AD, et al. (2014) Potential toxicity of quercetin: The repression of mitochondrial copy number via decreased POLG expression and excessive TFAM expression in irradiated murine bone marrow. Toxicology reports 1: 450-458.
- Tripathi A, Kumar B, Sagi SSK (2019) Prophylactic efficacy of Quercetin in ameliorating the hypoxia induced vascular leakage in lungs of rats. PloS one14: 0219075.
- Sarada S, Himadri P, Mishra C, Geetali P, Ram MS, et al. (2008) Role of oxidative stress and NFkB in hypoxia-induced pulmonary edema. Experimental biology and medicine 233: 1088-1098.
- Quinn JG (2012) Modeling Taylor dispersion injections: Determination of kinetic/affinity interaction constants and diffusion coefficients in label-free biosensing. Analytical biochemistry 421: 391-400.
- Rich RL, Quinn JG, Morton T, Stepp JD, Myszka DG (2010) Biosensor-based fragment screening using FastStep injections. Analytical biochemistry 407: 270-277.
- Cathcart R, Schwiers E, Ames BN (1983) Detection of picomole levels of hydroperoxides using a fluorescent dichlorofluorescein assay. Analytical biochemistry 134: 111-116.
- Knight JA, Pieper RK, McClellan L (1988) Specificity of the thiobarbituric acid reaction: its use in studies of lipid peroxidation. Clinical chemistry 34: 2433-2438.
- Tietze F (1969) Enzymic method for quantitative determination of nanogram amounts of total and oxidized glutathione: Applications to mammalian blood and other tissues. Anal Biochem 27: 502-522.
- Adams JD, Lauterburg BH, Mitchell JR (1983) Plasma glutathione and glutathione disulfide in the rat: regulation and response to oxidative stress. J Pharmacol Exp Ther 227: 749-754.
- Lowry OH, Rosebrough NJ, Farr AL, Randall RJ (1951) Protein measurement with the Folin phenol reagent. J Biol Chem 193: 265-275.
- Schoch HJ, Fischer S, Marti HH (2002) Hypoxia-induced vascular endothelial growth factor expression causes vascular leakage in the brain. Brain. A Journal of Neurology 125: 2549-2557.
- Card GL, England BP, Suzuki Y, Fong D, Powell B, et al. (2004) Structural basis for the activity of drugs that inhibit phosphodiesterases. Structure 12: 2233-2247.
- Dahlgren MK, Schyman P, Tirado-Rives J, Jorgensen WL (2013) Characterization of biaryl torsional energetics and its treatment in OPLS all-atom force fields. J Chem Inf Model 53: 1191-1199.
- Shakya T, Stogios PJ, Waglechner N, Evdokimova E, Ejim L, et al. (2011) A small molecule discrimination map of the antibiotic resistance kinome. Chemistry & biology 18: 1591-601.
- Zhang KY, Card GL, Suzuki Y, Artis DR, Fong D, et al. (2004) A glutamine switch mechanism for nucleotide selectivity by phosphodiesterases. Molecular cell 15: 279-286.
- Friesner RA, Banks JL, Murphy RB, Halgren TA, Klicic JJ, et al. (2004) Glide: A new approach for rapid, accurate docking and scoring. 1. Method and assessment of docking accuracy. J Med Chem 47: 1739-1749.
- Hollingsworth SA, Dror RO (2018) Molecular Dynamics Simulation for All. Neuron 99: 1129-1143.
- Kumar M, Dahiya S, Sharma P, Sharma S, Singh TP, et al. (2015) Structure based in silico analysis of quinolone resistance in clinical isolates of Salmonella Typhi from India. PloS one 10: 0126560.
- Pandey G, Bakhshi S, Kumar M, Thakur B, Jain P, et al. (2019) Prognostic and therapeutic relevance of cathepsin B in pediatric acute myeloid leukemia. American journal of cancer research 9: 2634-2649.
- Tripathi A, Kumar M, Kaur P, Kumar B, Sagi SSK (2020) Efficacy of Quercetin as a potent sensitizer of β2-AR in combating the impairment of fluid clearance in lungs of rats under hypoxia. Respiratory physiology & neurobiology 273: 103334.
- Gonzalez-Gallego J, Sanchez-Campos S, Tunon MJ (2007) Anti-inflammatory properties of dietary flavonoids. Nutricion hospitalaria 22: 287-293.
- Rahimi R, Ghiasi S, Azimi H, Fakhari S, Abdollahi M (2010) A review of the herbal phosphodiesterase inhibitors; future perspective of new drugs. Cytokine 49: 123-129.
- Francis SH, Corbin JD (1988) Purification of cGMP-binding protein phosphodiesterase from rat lung. Methods in enzymology 159: 722-729.
- Sebkhi A, Strange JW, Phillips SC, Wharton J, Wilkins MR (2003) Phosphodiesterase type 5 as a target for the treatment of hypoxia-induced pulmonary hypertension. Circulation 107: 3230-3235.
- Carpenter TC, Stenmark KR (2001) Hypoxia decreases lung neprilysin expression and increases pulmonary vascular leak. American journal of physiology Lung cellular and molecular physiology 281: 941-948.
- Sturlan S, Baumgartner M, Roth E, Bachleitner-Hofmann T (2003) Docosahexaenoic acid enhances arsenic trioxide-mediated apoptosis in arsenic trioxide-resistant HL-60 cells. Blood 101: 4990-4997.
- Casey DP, Madery BD, Curry TB, Eisenach JH, Wilkins BW, et al. (2010) Nitric oxide contributes to the augmented vasodilatation during hypoxic exercise. The Journal of physiology 588: 373-385.
- Kukreja RC, Salloum FN, Das A (2012) Cyclic guanosine monophosphate signaling and phosphodiesterase-5 inhibitors in cardioprotection. Journal of the American College of Cardiology 59: 1921-1927.
- Schumacker PT (2011) Lung cell hypoxia: Role of mitochondrial reactive oxygen species signaling in triggering responses. Proc Am Thorac Soc 8: 477-484.
- Green LC, Ruiz de Luzuriaga K, Wagner DA, Rand W, Istfan N, et al. (1981) Nitrate biosynthesis in man. Proc Natl Acad Sci USA 78: 7764-7768.
- Forstermann U, Munzel T (2006) Endothelial nitric oxide synthase in vascular disease: from marvel to menace. Circulation 113: 1708-1714.
- Alp NJ, McAteer MA, Khoo J, Choudhury RP, Channon KM (2004) Increased endothelial tetrahydrobiopterin synthesis by targeted transgenic GTP-cyclohydrolase I overexpression reduces endothelial dysfunction and atherosclerosis in ApoE-knockout mice. Arteriosclerosis, thrombosis and vascular biology 24: 445-450.
- Yanagisawa M, Kurihara H, Kimura S, Tomobe Y, Kobayashi M, et al. (1988) A novel potent vasoconstrictor peptide produced by vascular endothelial cells. Nature 332: 411-415.
- Hay DW, Hubbard WC, Undem BJ (1993) Endothelin-induced contraction and mediator release in human bronchus. British journal of pharmacology 110: 392-398.
- Sarada SK, Titto M, Himadri P, Saumya S, Vijayalakshmi V (2015) Curcumin prophylaxis mitigates the incidence of hypobaric hypoxia-induced altered ion channels expression and impaired tight junction proteins integrity in rat brain. Journal of neuroinflammation 12: 113.
- Wilson SH, Simari RD, Lerman A (2001) The effect of endothelin-1 on nuclear factor kappa B in macrophages. Biochemical and biophysical research communications 286: 968-972.
- Maggiorini M, Brunner-La Rocca HP, Peth S, Fischler M, Bohm T, et al. (2006) Both tadalafil and dexamethasone may reduce the incidence of high-altitude pulmonary edema: A randomized trial. Annals of internal medicine 145: 497-506.
- Fischler M, Maggiorini M, Dorschner L, Debrunner J, Bernheim A, et al. (2009) Dexamethasone but not tadalafil improves exercise capacity in adults prone to high-altitude pulmonary edema. American journal of respiratory and critical care medicine 180: 346-352.
- Patir H, Sarada SK, Singh S, Mathew T, Singh B, et al. (2012) Quercetin as a prophylactic measure against high altitude cerebral edema. Free radical biology & medicine 53: 659-668.
- Barker KR, Conroy AL, Hawkes M, Murphy H, Pandey P, et al. (2016) Biomarkers of hypoxia, endothelial and circulatory dysfunction among climbers in Nepal with AMS and HAPE: A prospective case-control study. Journal of travel medicine 23: 005.
Citation: Tripathi A, Kumar B, Kumar M, Kaur P, Sagi SSK (2020) Quercetin Prophylaxis: A Novel Approach to Prevent Hypoxia-Mediated Increase in Oxidative Stress, Pde-5 Activity and Transvascular Leakage in the Lungs of Rats. J Pulm Med Respir Res 6: 041.
Copyright: © 2020 Tripathi A, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.

