
Recellularization of Canine Placental Extracellular Matrix: Mesenchymal Stem Cells Applied to Tissue Bioengineering
*Corresponding Author(s):
Maria Angelica MiglinoDepartment Of Surgery, School Of Veterinary Medicine And Animal Science, University Of São Paulo, Avenue Prof. Dr. Orlando Marques De Paiva, 87, Cidade Universitária, Butantã, São Paulo, SP, CEP: 05508-270, Brazil
Tel:+55 1130917690,
Fax:+55 1130917690
Email:miglino@usp.br
Abstract
The use of decellularized biological matrices in tissue bioengineering has been widely studied, since the supply of tissue / organs for transplantation is low, in exchange for the high need. Decellularized placental matrices from humans and animals have been shown to be an efficient tool to reduce the demand for this problem due they have a rich extracellular matrix composition that, when cultured with stem cells, demonstrate effective cell / matrix interaction. In this study, we used decellularized canine placentas for recellularization with mesenchymal stem cells derived from fetal canine bone marrow and canine dental pulp. It was demonstrated by immunofluorescence analysis that the placental matrices cultured with two cell types for 7 days obtained satisfactory results, evidencing the interaction of the cells by the positive expression of CD90+ and CD105+ mesenchymal stem cells markers in the extracellular matrix. We also demonstrated by the scanning electron microscopy techniques the adhesion of the cells to placental scaffolds. Our results can conclude that the recellularization of placental scaffolds with mesenchymal stem cells were effective and could evidence a new approach for possible application studies of these scaffolds in small damage areas that need repair applied to tissue regenerative medicine.
Keywords
Biological scaffolds; Bone marrow cells; Decellularization; Dental pulp cells; Regenerative medicine
ABBREVIATIONS
ECM: Extracellular Matrix
VEGF: Vascular Endothelial Growth Factor
MSCs: Mesenchymal Stem Cells
CBMC: Canine Bone Marrow
CDPC: Canine Dental Pulp
SDS: Sodium Dodecyl Sulfate
PBS: Phosphate Vuffered Saline
UV: Ultraviolet Light
α-MEM: Minimum Essential Medium Eagle-Alpha Modification
FBS: Fetal Bovine Serum
SEM: Scanning Electron Microscope
INTRODUCTION
Biological biomaterials obtained from organs and tissues decellularization techniques have received expansive emphasis on tissue bioengineering [1-5]. The decellularization aims remove the cells from the tissue and maintain the three-dimensional structure of your extracellular matrix and their bioactivity, resulting in a acellular biological scaffolds [2,5-8].
Acellular biological scaffolds, also known as extracellular matrix (ECM), may be derived from animal species (xenologous origin) and have been widely studied in regenerative medicine [9-14].
Usually the use of decellularized biological scaffolds appears as a promising tool in order to eliminate the scarce supply of tissues and organs for transplants [1]. Decellularization of a three dimensional matrix scaffold (i.e., animal placentas) enables the removal of cellular and immunogenic components from an organ/tissue while preserving the ECM with its native micro and macroscopic anatomy [15].
Placentas represents an interesting alternative for obtaining acellular scaffolds to be used in tissue bioengineering [3,10,16]. The characteristics that make the placenta a source of decellularized biomaterial are: high sample supply, rich diversity of extracellular matrix proteins, can be collected without damage to the donor, and a complete defined vascularization [17].
Decellularized placentas from different species (humans and animals) including the canine placenta demonstrate be useful as biological scaffolds (tissue or as a hydrogel), corroborating for tissue bioengineering [3,18-23]. Due the ECM properties preserved after removal of cellular content, decellularized placentas support cell migration and proliferation, as well as cell / matrix interaction [3,20,24]. Mouse placental scaffolds cultured with mouse embryonic fibroblasts demonstrated a tri-dimensional repopulation and cell-matrix adhesion [23].
Decellularized canine placentas when applied in vitro in interaction with the endothelial progenitor cells derived from the yolk sac transduced with Vascular Endothelial Growth Factor (VEGF), demonstrate interaction with the ECM for recellularization of the tissue [25].
In this study, we examined whether the use of decellularized canine placentas to recellularize with Mesenchymal Stem Cells (MSCs) derived from Canine Bone Marrow (CBMC) and Canine Dental Pulp (CDPC) for 7 days in culture. Cell adhesion capacity / matrix interaction was investigated for possible use as a small graft applied to the tissue engineering.
MATERIALS AND METHODS
Canine placental decellularization and recellularization
Placentas of bitches with 35 days of gestation were used. The placentas were Sterile in 0.1% Sodium Dodecyl Sulfate (SDS) for 15 days under stirring in a Sterile environment and washed in Triton X-1001% [20]. The decellularized placentas were passed in a critical point LEICA EM CPD 300, for pre-sterilization of the samples (Figures 1a-1c). They were then washed in 1X Phosphate Buffered Saline (PBS) with 0.5% antibiotic (penicillin-streptomycin) and left in ultraviolet (UV) light for 10 minutes (Figure 1d). The sterility of the decellularized placentas were tested in culture medium with Minimum Essential Medium Eagle with 10% Fetal Bovine Serum (FBS) and in cubated at 37°C with 5% CO2 for 24 hours (Figures 1e and 1f), as standardized by our group [25]. A number of 5 x 104 CBMC and CDPC were plated on non-adherent dishes (Sarstedt) for 7 days in culture (Figure 1g). The cell lines used come from a cell bank standardized by the group [26].
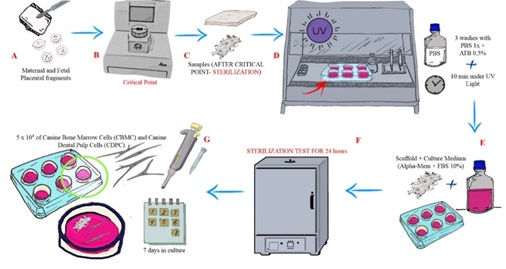
Figure 1: Experimental design of canine placenta decellularization and recellularization process.
Immunofluorescence cells and recellularized scaffold
Non-adherent plates containing samples of placentas recellularized with CBMC / CDPC for 7 days were fixed with 4% paraformaldehyde. Supports were washed with PBS + 0.5% Tween and incubated with the primary antibody fibronectin (Abcamab2413), CD105 (Invitrogen MA5-11854) and CD90 FITC (Abcamab124527) at a dilution of 1: 200, at 37°C for 1 hour. The samples were then washed in PBS + 0.5% Tween and incubated for 1 hour at 4°C with secondary antibody Alexa Fluor 594 (Thermo FisherA30008) and Alexa Fluor 488 (Thermo FisherA-11094). Plates were incubated with DAPI for nuclear staining. They were analyzed in the Confocal Microscope - Olympus Fluo View 1000 (FV1000).
Scanning electron microscopy
The decellularized and recellularized placentas were fixed in 4% paraformaldehyde. They were dehydrated in increasing concentrations of alcohol, dried at critical point (LEICA EM CPD 300) and glued with carbon tape in metal (sputter coating) with gold metalizer. Subsequently they were photographed in a Scanning Electron Microscope (SEM) LEO 435VP.
RESULTS
Sterile placental fragments for recellularization assay were performed in 7 days, by suspension in anon-adherent dishes using two types of Mesenchymal Stem Cells (MSC): CBMC and CDPC. The placental scaffolds demonstrated chemotaxis by the cells types, favoring cell/matrix interaction.
Positive expression for the CD90 marker in the cytoplasm of canine bone marrow mesenchymal stem cells and canine dental pulp cells was demonstrated (Figure 2).
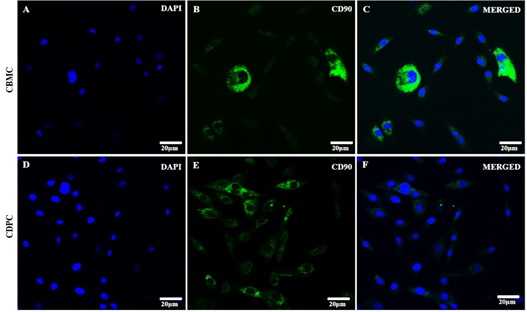
Figure 2: Immunofluorescence of CBMC and CDPC. A-C: canine bone marrow cells; D-F: canine dental pulp cells (DAPI - cell nuclei; CD90 cytoplasmatic marker).
By the immunofluorescence assay, it was possible to confirm the adhesion of CBMC and CPDC in the placenta scaffold cultured for 7days (Figure 3). These when in contact with the scaffolds demonstrated high expression of fibronectin (Figures 3c, 3g, 3k and 3o), a structural protein from ECM (preserved in the decellularization process) that helps to the cell/matrix adhesion. In addition, the interaction of the cells with scaffolds demonstrated a positive expression of mesenchymal stem cells markers: CD90+ (Figures 3b, 3d, 3j and 3l) and CD105+ (Figures 3f, 3h, 3n and 3p).
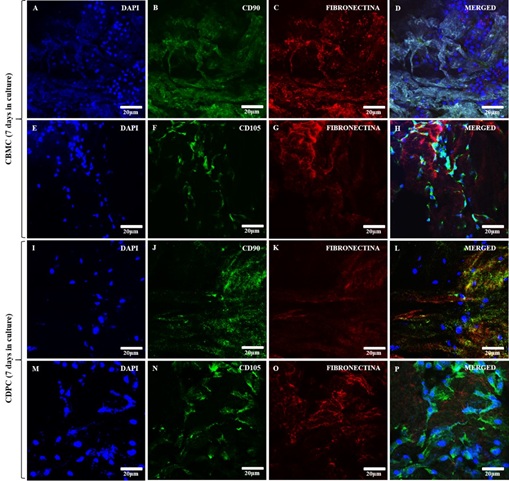
Figure 3: Immunofluorescence of recellularized canine placental scaffolds with CBMC and CPDC for 7 days in culture. (A, E) and (I, M): DAPI immunostaining (blue) for canine bone marrow cells and canine dental pulp cells, respectively; B and J (CD90) F and N (CD105): Green immunostaining for canine bone marrow cells and canine dental pulp cells, respectively. C, G, K and O: Red immunostaining for fibronectin in the placental extracellular matrix; D, H, L and P: merged.
The recellularization of canine placenta scaffolds with CBMC and canine bone marrow cells and canine dental pulp cells was also observed by scanning electron microscopy confirming that there was an interaction between the extracellular matrix and cells in culture in vitro for 7 days (Figure 4). Besides MSCs attached to placental ECM scaffolds preserved their collagens structure morphology.
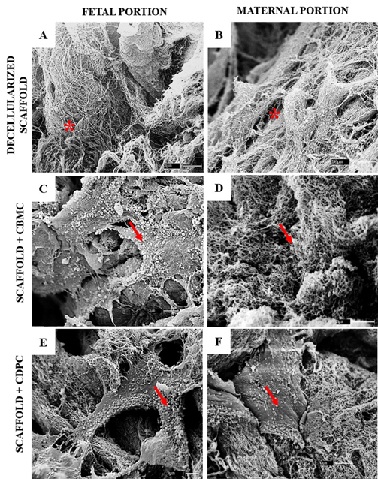
Figure 4: Scanning Electron Microscopy of scaffolds of recellularized placentas with CMOC and CPDC for 7 days of culture. A and B: canine placenta decellularized fetal and maternal portions, respectively (* observe absence of cell nuclei); C and D: canine placenta recellularized with CBMC; E and F: canine placenta recellularized with CDPC; (→ observe cell / matrix interaction).
DISCUSSION
Placentas decellularization techniques of different species have been standardized in recent years. Proteins and the three-dimensional architecture of the extracellular matrix are preserved, applied to recellularization process and used as small fragments tissue or hydrogel for regeneration [17-20,22,23,25,27-29].
Previously, the decellularization of canine placentas of 35 days gestation was standardized. In the cellular removal process, we obtained satisfactory results regarding the quantification of the desired gDNA for an acellular biological scaffold. Furthermore, after the decellularization process, canine placentas decellularization demonstrated the preservation of their ECM proteins such as protein structures (collagens type I, III and IV), proteoglycans and adhesion glycoproteins (fibronectin and laminin). Such constitutes ECM molecules directly influence the role of cell adherence to the scaffolds [20].
MSCs have been used in regenerative medicine since they are multipotent stem cells with due to their ability to regenerate tissue from mesenchymal origin. Besides, their capability of suppressing immune responses, anti-inflammatory, immunomodulatory and paracrine effects thus reduces transplantation rejection in clinical applications [30]. In addition, Papanikolaou (2019) reviews results in experimental studies using diverse sources of MSC for use in regenerative medicine [31].
We cultured bone marrow and dental pulp cells on the 3D decellularized canine placentas scaffolds in non-adherent plaques, according the recellularization process as already observed in the culture with progenitor cells from the canine yolk sac transduced with vascular endothelial growth factor [25], which directly influences the migration / adhesion of cells on the scaffold.
Mesenchymal stem cells derived from bone marrow has multipotential capacity to differentiate [32]. These, when applied in tissue engineering for regenerative medicine, evidence their role as a pluripotent stem cell because they have the role of bioactive macromolecules [33,34].
Against this background, our results showed that the biological scaffold derived from placenta decellularization assay cultured for 7 days with MSCs had positive expression of mesenchymal markers CD90+ and CD105+, demonstrating the cell / matrix interaction in the process of recellularization. These data contributed to the recellularized scaffolds with the MSCs being promising sources for use in regenerative medicine [32,35-38].
According to results obtained by human cells derived from bone marrow were efficient in the recellularization process of the small intestine [39], an organ with ECM components similar to canine placenta (collagen, laminin and fibronectin), in addition to the vascularization present in both organs. Cell differentiation in decellularized lungs was observed when cultured for recellularization assay using MSCs derived from mouse bone marrow, suggesting the capacity of these cells to modulate the environments of cells/matrix [40].
Moreover, dental pulp stem cells have characteristics similar to MSC, including the auto-renewal capacity and the potential of multiline differentiation [41]. These still have high plasticity, being able to differentiate in neurons, adipocytes and osteoblasts [42] and demonstrate efficacy in therapeutic treatment in dogs with chronic spinal cord injury [26]. DPSC have been widely used as potentially novel therapeutic agents in regenerative medicine due our multipotency and immunosuppressive effects [43].
In conclusion we demonstrated that the recellularization of canine placentas with mesenchymal stem cells derived from canine bone marrow and dental pulp were successful. The decellularized placenta samples used in this study for recellularization demonstrated that the native tissue structure preserved ECM components corroborated for cell/matrix interaction. Considering MSCs have been suggested for recellularization in decellularized scaffolds [44], these results provide a basis for the development of new approaches, since mesenchymal stem cells derived from bone marrow and dental pulp may evidence a therapeutic potential for future applications in tissue bioengineering in vivo.
ACKNOWLEDGEMENTS
The authors are thankful to Advanced Center of Image Diagnosis (CADI-FMVZ-USP) facility and staff for technical support and Coordination for the Improvement of Higher Education Personnel (CAPES).
FUNDING
This work was supported by FAPESP (São Paulo Research Foundation - Financial Support: 2014/50844-3).
ETHICAL APPROVAL
All animal procedures were performed in accordance with the National Institutes of Health Guide for the Care and Use of Laboratory Animals and approved by the Ethics Committee (nº 6611181016) from School of Veterinary Medicine and Animal Science of São Paulo University (FMVZ-USP, São Paulo, SP, Brazil).
CONFLICT OF INTEREST
The authors declare no conflict of interest.
AUTHOR’S CONTRIBUTION
Gustavo Sá Schiavo Matias and Paula Fratini conceived and designed the research. Gustavo Sá Schiavo Matias, Vitória Frias Batista and Paula Fratini conducted the experiments. Gustavo Sá Schiavo Matias, Ana Claudia Oliveira Carreira, Maria Angelica Miglino and Paula Fratini analyzed the data. Gustavo Sá Schiavo Matias and Paula Fratini wrote the manuscript. Maria Angelica Miglino for funding. All authors read and approved the manuscript.
REFERENCES
- Crapo PM, Gilbert TW, Badylak SF (2011) An overview of tissue and whole organ decellularization processes. Biomaterials 12: 3233-3243.
- Gilbert TW (2012) Strategies for tissue and organ decellularization. Journal of Cellular Biochemistry 7: 2217-2222.
- Schneider KH, Aigner P, Holnthoner W, Monforte X, Nürnberger S, et al. (2016) Decellularized human placenta chorion matrix as a favorable source of small-diameter vascular grafts. Acta Biomaterialia 29: 125-134.
- Keane TJ, Badylak SF (2014) Biomaterials for tissue engineering applications. Seminars in Pediatric Surgery 3: 112-118.
- Scarrit ME (2015) A review of cellularization strategies for tissue engineering of whole organs. Front Bioeng Biotechnol 3: 43.
- Sabetkish S, Kajbafzadeh A-M, Sabetkish N, Khorramirouz R, Akbarzadeh A, et al. (2015) Whole-organ tissue engineering: Decellularization and recellularization of three-dimensional matrix liver scaffolds. J Biomed Mater Res A 4: 1498-1508.
- Keane TJ, Swinehart IT, Badylak SF (2015) Methods of tissue decellularization used for preparation of biologic scaffolds and in vivo relevance. Methods 84: 25-34.
- Arenas-Herrera JE, Ko IK, Atala A, Yoo JJ (2013) Decellularization for whole organ bioengineering. Biomed Mater 8: 014106.
- Badylak SF, Vorp DA, Spievack AR, Simmons-Byrd A, Hanke J, et al. (2005) Esophageal Reconstruction with ECM and Muscle Tissue in a Dog Model. J Surg Res 1: 87-97.
- Flynn L, Semple JL, Woodhouse KA (2006) Decellularized placental matrices for adipose tissue engineering. J Biomed Mater Res A 2: 359-369.
- Hong JW, Lee WJ, Hahn SB, Kim BJ, Lew DH (2010) The Effect of Human Placenta Extract in a Wound Healing Model. Annals of Plastic Surgery 1: 96-100.
- Kim JK, Kim TH, Park SW, Kim HY, Kim Sh, et al. (2010) Protective Effects of Human Placenta Extract on Cartilage Degradation in Experimental Osteoarthritis. Biol Pharm Bull 6: 1004-1010.
- Jung J, Lee H-J, Lee JM, Na K-H, Hwang S-G, et al. (2011) Placenta extract promote liver regeneration in CCl4-injured liver rat model. Int Immunopharmacol 8: 976-984.
- Choi JS, Kim JD, Yoon HS, Cho YW (2013) Full-Thickness Skin Wound Healing Using Human Placenta-Derived Extracellular Matrix Containing Bioactive Molecules. Tissue Eng Part A 19: 329-339.
- Badylak SF, Taylor D, Uygun K (2011) Whole-Organ Tissue Engineering: Decellularization and Recellularization of Three-Dimensional Matrix Scaffolds. Annu Rev Biomed Eng 1: 27-53.
- Brigido SA, Carrington SC, Protzman NM (2018) The Use of Decellularized Human Placenta in Full-Thickness Wound Repair and Periarticular Soft Tissue Reconstruction: An Update on Regenerative Healing. Clin Podiatr Med Surg 1: 95-104.
- Hopper RA, Woodhouse K, Semple JL (2003) Acellularization of human placenta with preservation of the basement membrane: a potential matrix for tissue engineering. Ann Plast Surg 6: 598-602.
- Leonel LCPC, Miranda CMFC, Coelho TM, Ferreira GAS, Caãada RR, et al. (2018) Decellularization of placentas: establishing a protocol. Braz J Med Biol Res 51: 6382.
- Barreto R da SN, Romagnolli P, Mess AM, Miglino MA (2018) Decellularized bovine cotyledons may serve as biological scaffolds with preserved vascular arrangement. J Tissue Eng Regen Med 4: 1880-1888.
- Matias G de SS, Rigoglio NN, Carreira ACO, Romagnolli P, Barreto R da SN, et al. (2018) Optimization of Canine Placenta Decellularization: An Alternative Source of Biological Scaffolds for Regenerative Medicine. Cells Tissues Organs 4: 217-225.
- Kakabadze Z, Kakabadze A, Chakhunashvili D, Karalashvili L, Berishvili E, et al. (2018) Decellularized human placenta supports hepatic tissue and allows rescue in acute liver failure. Hepatology 5: 1956-1969.
- Zhang X, Xiao S, Liu B, Miao Y, Hu Z (2019) Use of extracellular matrix hydrogel from human placenta to restore hair-inductive potential of dermal papilla cells. Regen Med 8: 741-751.
- Barreto RSN, Romagnolli P, Fratini P, Mess AM, Miglino MA (2019) Mouse placental scaffolds: a three-dimensional environment model for recellularization. J Tissue Eng 204173141986796.
- Keatch RP, Schor AM, Vorstius JB, Schor SL (2012) Biomaterials in regenerative medicine: engineering to recapitulate the natural. Curr Opin Biotechnol 4: 579-582.
- Fratini P, Rigoglio NN, Matias G de SS, Carreira ACO, Rici REG, et al. (2018) Canine Placenta Recellularized Using Yolk Sac Cells with Vascular Endothelial Growth Factor. Biores Open Access 1: 101-106.
- Prado C, Fratini P, de Sá Schiavo Matias G, Bocabello RZ, Monteiro J, et al. (2019) Combination of stem cells from deciduous teeth and electroacupuncture for therapy in dogs with chronic spinal cord injury: A pilot study. Res Vet Sci 123: 247-251.
- Jung J, Choi JH, Lee Y, Park JW, Oh IH, et al. (2013) Human Placenta-Derived Mesenchymal Stem Cells Promote Hepatic Regeneration in Ccl4 -Injured Rat Liver Model Via Increased Autophagic Mechanism. Stem cells 31: 1584-1596.
- Lobo SE, Leonel LC, Miranda CM, Coelho TM, Ferreira GA, et al. (2016) The Placenta as an Organ and a Source of Stem Cells and Extracellular Matrix: A Review. Cells Tissues Organs 4: 239-252.
- Klama?Bary?a A, Rojczyk E, Kitala D, ?abu? W, Sm?tek W, et al. (2020) Preparation of placental tissue transplants and their application in skin wound healing and chosen skin bullous diseases - Stevens-Johnson syndrome and toxic epidermal necrolysis treatment. Int Wound J 2: 491-507.
- Murphy MB, Moncivais K, Caplan AI (2013) Mesenchymal stem cells: Environmentally responsive therapeutics for regenerative medicine. Exp Mol Med 45: 54.
- Papanikolaou IG, Katselis C, Apostolou K, Feretis T, Lymperi M, et al. (2017) Mesenchymal Stem Cells Transplantation following Partial Hepatectomy: A New Concept to Promote Liver Regeneration-Systematic Review of the Literature Focused on Experimental Studies in Rodent Models. Stem Cells Int 2017: 7567958.
- Dominici M, Le Blanc K, Mueller I, Slaper-Cortenbach I, Marini F, et al. (2006) Minimal criteria for defining multipotent mesenchymal stromal cells. The International Society for Cellular Therapy position statement. Cytotherapy 8: 315-317.
- Caplan AI (2007) Adult mesenchymal stem cells for tissue engineering versus regenerative medicine. J Cell Physiol 213: 341-347.
- Muraglia A, Cancedda R, Quarto R (2000) Clonal mesenchymal progenitors from human bone marrow differentiate in vitro according to a hierarchical model. J Cell Sci 113: 1161-1166.
- Dissanayaka WL, Zhu X, Zhang C, Jin L (2011) Characterization of Dental Pulp Stem Cells Isolated from Canine Premolars. J Endod 37: 1074-1080.
- Wenceslau CV, Miglino MA, Martins DS, Ambrósio CE, Lizier NF, et al. (2011) Mesenchymal Progenitor Cells from Canine Fetal Tissues: Yolk Sac, Liver, and Bone Marrow. Tissue Eng Part A 17: 2165-2176.
- Ponnaiyan D, Jegadeesan V (2014) Comparison of phenotype and differentiation marker gene expression profiles in human dental pulp and bone marrow mesenchymal stem cells. Eur J Dent 8: 307-313.
- Munévar JC, Gutiérrez N, Jiménez NT, Lafaurie GI (2015) Evaluation of two human dental pulp stem cell cryopreservation methods. Acta Odontol Latinoam 2: 114-121.
- Patil PB, Chougule PB, Kumar VK, Almström S, Bäckdahl H, et al. (2013) Recellularization of acellular human small intestine using bone marrow stem cells. Stem Cells Transl Med 2: 307-315.
- Crabbé A, Liu Y, Sarker SF, Bonenfant NR, Barrila J, et al. (2015) Recellularization of Decellularized Lung Scaffolds Is Enhanced by Dynamic Suspension Culture. PLoS One 10: 0126846.
- Chalisserry EP, Nam SY, Park SH, Anil S (2017) Therapeutic potential of dental stem cells. J Tissue Eng 8: 2041731417702531.
- Miura M, Gronthos S, Zhao M, Lu B, Fisher LW, et al. (2003) SHED: Stem cells from human exfoliated deciduous teeth. Proc Natl Acad Sci U S A 100: 5807-5812.
- Kanjevac T, Gustafson C, Ivanovska A, Ravanetti F, Cacchioli A, et al. (2019) Inflammatory Cytokines and Biodegradable Scaffolds in Dental Mesenchymal Stem Cells Priming. Curr Stem Cell Res Ther 14: 320-326.
- Wagner DE, Bonvillain RW, Jensen T, Girard ED, Bunnell BA, et al. (2013) Can stem cells be used to generate new lungs? Ex vivo lung bioengineering with decellularized whole lung scaffolds. Respirology 18: 895-911.
Citation: Matias GSS, Batista VF, Carreira ACO, Miglino MA, Fratini P (2020) Recellularization of Canine Placental Extracellular Matrix: Mesenchymal Stem Cells Applied to Tissue Bioengineering. J Stem Cell Res Dev Ther 6: 035.
Copyright: © 2020 Gustavo Sá Schiavo Matias, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.

