
Reciprocal Relation of Fetuin-A and Beta Crosslaps with Bone Health
*Corresponding Author(s):
Syeda Sadia FatimaDepartment Of Biological And Biomedical Sciences, Aga Khan University, Stadium Road, Karachi, Pakistan
Email:sadia.fatima@aku.edu
Abstract
MethodsTotal of 115 females of ages between 20 to 60 years were recruited in this cross-sectional study from Jinnah Postgraduate Medical Centre, Karachi. They were grouped as A & B on the basis of Bone Mass Density (BMD) T score > -1 and < -1, respectively. Anthropometric measurements were recorded and BMD was calculated by ultrasound bone densitometer (considering T-score ≥ -1 as normal). Serum was analyzed for bone minerals, Vitamin D, CTX and Fetuin-A. Data was analyzed statistically by SPSS 21, Mann-Whitney U test and Spearman’s correlation (r) were applied where p value < 0.05 was considered significant.
ResultsThe complete cohort showed normal calcium levels while a low level of vitamin D was observed (P > 0.05). Interestingly, both serum Fetuin-A and CTx levels were found high in group B as compared to group A (p < 0.001). Serum Fetuin (r = -0.718, p < 0.001) and CTx (r = -0.756, p < 0.001) depicted negative correlation with BMD % and BMI, while their levels were positively associated to each other (r = 0.481, p < 0.001).
DiscussionLow values of BMD T-score (less than -1) are associated with high levels of Fetuin-A in our female population. Raised Fetuin-A in the presence of low vitamin D and high CTx, suggests that serum Fetuin-A does not reflect increased bone turnover. Further experiments are required to validate the role of Fetuin-A in bone mineralization.
Keywords
INTRODUCTION
Osteoporosis is known to cause over 8.9 million fractures annually [2]. Pakistan also faces increasing rates of osteoporosis, with currently 9.91 million people suffering from the disease; the number is predicted to increase to 11.3 million by 2020 and 12.91 million by 2050. Moreover, population of Pakistan is also challenged with an endemic of Vitamin D Deficiency (VDD). Conferring to a couple of studies conducted in Karachi, prevalence of VDD is 70-97% in healthy asymptomatic individuals [3].
Fetuin-A, also known as Alpha 2-Heremans-Schmid (AHSG) Glycoprotein is a non-collagenous protein that is stored in mineralized bone and teeth. It is derived from the hepatocytes and acts as a strong inhibitor of ectopic mineralization [4,5]. It is cleaved from a single chain precursor and its mature form contains two polypeptide chains [6]. Fetuin-A contains a cystatin-like protein domain and is responsible for inhibiting calpain, papain, cathepsin, and caspases by acting on cysteine peptidases. This enables Fetuin-A to be a role player in a number of physiological and pathological processes. Most importantly cystatin domain-1 has a high affinity for calcium-rich minerals. Studies reveal that Fetuin-A binds most strongly to mineralized bone by binding calcium and phosphorus together to form complexes [7]. Hence, it is a potent inhibitor of metastatic calcification [8]. It has also been hypothesized that Fetuin-A works by promoting calcification within bone and inhibiting calcium precipitation within serum [9]. Moreover, remineralization of demineralized bone has also been proven to be possible within serum containing Fetuin-A, and not with the one deficient of it, leading to a conclusion that Fetuin-A has a direct effect on promoting bone mineralization [7].
The degradation products of collagen type I reflect the activity of osteoclasts, and can be assessed by measurement of telopeptides (CTx and NTx) and hydroxyproline or matrix proteins such as bone sialoprotein [10,11]. The most abundant form of collagen in the bone is Type I collagen which renders serum CTx (Carboxy- terminal Telopeptide) to be the most sensitive and specific test for bone resorption [10]. CTx is derived from type I collagen, which is the only type that is affected by osteoclasts and hence a true estimate of bone resorption can be obtained from this [12].
We hypothesize a link between Fetuin-A, CTx and bone health. Our study aims to investigate bone health of healthy females, by employing serum levels of Fetuin-A and CTx and their relationship with BMD percentage.
MATERIAL AND METHODS
Data about the subjects’ name, age, weight, height, physical activity, exposure to sun, and veil status were assembled by the primary investigator in a predesigned questionnaire. Anthropometric measurements were noted in standing posture and with light clothes, using Stadiometer (ZT -120 Health Scale, made in China). BMD of calcaneus (heel) bone (being a portable tool) was measured by ultrasound bone densitometer (OstepsysSonost 3000 Bone Densitometry) where T-score ≥ -1 was considered normal). Grouping was done as group A & B on the basis of bone mass density T score > -1 and < -1, respectively. All patients with BMD < 1 (either osteoporotic or at risk of osteoporotic) were recruited in group B.
About four ml of blood was collected in sterile Venoject tubes. Serum was separated after centrifuging them at 2000xg for 5 minutes and then was aliquoted in small volumes and stored at -80ºC until further use. The frozen serum samples were thawed right before analysis of calcium, alkaline phosphatase, Vitamin D, CTX and Fetuin-A. Calcium and alkaline phosphatase were assessed by enzymatic colorimetric kits available by Roche. Serum Fetuin was analyzed by Enzyme Linked Immuno-Sorbent Assay kit method (Cat No.RD191037100 provided by BioVendor), while CTx was performed by the Elecsys 2010 analyzer (Roche Diagnostics).Vitamin D was estimated by commercially available ELISA kits (kit cat # KAP197; kit cat # KAP2281 by DIA source Immunoassays S.A. Belgium respectively). The following reference range for vitamin D levels were considered; deficient <10 ng/ml, insufficient = 10 - 29 ng/ml, sufficient = 30 - 100 ng/ml and toxic = >100 ng/ml [13].
Data of continuous variables i.e. biophysical (age, height, weight, BMI, waist circumference, hip circumference, waist hip ratio, body fat, BMD) and biochemical parameters (calcium, alkaline phosphatase, vitamin D, CTx and Fetuin-A) were statistically analyzed by employing SPSS (version 21; SPSS Inc., Chicago, IL, USA) and calculated as mean ± Standard Deviation (SD). Comparison of means was done by utilizing Mann-Whitney U test. Correlation between serum CTx, Fetuin-A and BMD percentage was established by Spearman’s coefficient of correlation (r). The p-values <0.05 were considered significant.
RESULTS
| Variables |
Group A (BMD T score ≥ -1) Mean ± SD (n=56) |
Group B (BMD T score < -1) Mean ± SD (n=59) |
P value |
| Age (years) | 38.6 ± 6.28 | 40.47 ± 7.53 | > 0.05 |
| Weight (Kg) | 72.6 ± 16.23 | 67.10 ± 12.32 | > 0.05 |
| Height (m) | 1.5 ± 0.03 | 1.6 ± 0.04 | > 0.05 |
| BMI (Kg/m2) | 28.9 ± 5.88 | 26.14 ± 4.5 | 0.021 |
| Calcium (mg/dL) | 8.8964 ± 0.55 | 8.84 ± 0.48 | > 0.05 |
| Alkaline PO4 (IU/L) | 205.32 ± 76.57 | 216.20 ± 93.57 | > 0.05 |
| Vitamin D (ng/mL) | 16.22 ± 9.41 | 16.90 ± 12.86 | > 0.05 |
| Bone Mass Density (%) | 90.86 ± 8.9 | 67.46 ± 7.78 | < 0.001 |
| Fetuin-A (mg/L) | 45.15 ± 12.39 | 70.97 ± 10.49 | < 0.001 |
| CTX (ng/mL) | 0.19 ± 0.10 | 0.49 ± 0.15 | < 0.001 |
The complete cohort showed normal calcium levels (mean 8.86 ± 0.51 mg/dL) thus no substantial difference was observed between the groups. With low levels of serum vitamin D (mean 16.56 ± 11.13 ng/ml), alkaline phosphatase (mean 210.5 ± 85.07 IU/L) was seen to be very high in all study subjects. BMI was found to be decreased in group B (p = 0.021). The serum CTx levels were detected high in group B as compared to group A (p < 0.001) but interestingly serum Fetuin A levels were also revealed to be raised in individuals with low BMD T-score (p < 0.001).
Fetuin A was represented as negatively correlated with BMD percentage (r = -0.718, p < 0.001) and BMI (r = - 0.348, p< 0.001). Furthermore, CTx (r = -0.756, p < 0.001) depicted negative correlation with BMD percentage while at the same time its levels were positively associated to Fetuin-A (r = 0.481, p < 0.001) (Figures 1a - 1d).
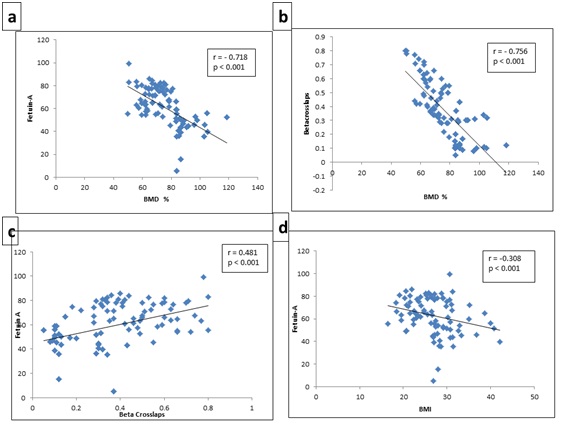
Figure 1: Correlation between (a) BMD% and Fetuin-A; (b) BMD% and Beta Crosslaps; (c) Beta Crosslaps and Fetuin-A; (d) BMI and Fetuin-A.
DISCUSSION
Studies on relation of Fetuin-A and CTx with BMD percentage are limited particularly in the Pakistani population which is transparently vitamin D deficient though does not exhibit any noticeable signs of declined bone health. This provides an area to investigate the link between bone health markers (vitamin D, Fetuin-A, CTx) and Bone health (BMD). To establish this link, we estimated the levels of vitamin D, Fetuin-A, CTx and BMD and studied their correlation. The present study revealed high serum levels of alkaline phosphatase, CTx and quite interestingly of Fetuin-A of individuals with low BMD percentage. However, calcium levels were indifferent in the overall study cohort (Table 1). In this part of the world, considering the cultural and ethnic backgrounds, comparative low levels of vitamin D are now considered as normal, as depicted by Arya et al., [14] and Roy et al., [15] in their studies. This generalized hypovitaminosis D could be a result of the genetic makeup of this population [16]. Further detailed genetic studies might be able to provide a causal relationship.
Increase in BMI directs towards presence of more adipose tissue in the body. Increase adiposity leads to the inflammatory condition in the body. Fat accumulation was observed to over-express Fetuin-A mRNA in the liver of rats after inducing obesity; either by causing tissue insensitivity to insulin, by increasing fat metabolism or by limiting glucose clearance [17]. Furthermore, Stefan et al., indicated in a human study that fatty liver is associated with high plasma Fetuin-A levels [18], this has been further proven by other studies [19]. However, Lavebratt et al., for the first time reported negative association between Fetuin-A levels and fatness; this is consistent with our findings [20]. Study utilizing mediation analysis, suggested evidence of a causal relationship between the AHSG gene and BMD through fetuin-A and BMI mediators [21]. However, further genetic studies should be conducted to validate this fact in our population.
According to WHO criteria, individuals with BMD T-scores < -2.5 are osteoporotic and the ones between < -2.5 and -1 are at a high risk of developing osteoporosis, while T-scores > -1 are considered to be normal [2]. Our study depicts increased bone resorption in osteopenic subjects as suggested by the low BMD and high CTx levels [22]. CTx being an authentic bone resorption marker suggests that he bone is having osteoclastic activity going on for the release of minerals like calcium in the blood [23]. Previous studies have also endorsed CTx to be raised when the BMD decreases establishing it a reliable marker for bone health [24].
Another interesting finding is that of Fetuin-A being high in individuals with decreased BMD percentage. According to a study conducted on elderly females, Fetuin-A levels are associated with bone mass and bone resorption. Fetuin-A’s is a multi-functionality protein and leads to govern a number of mechanisms in the body by cytokine activation. It might have an effect on osteoclastic activity of the bone as it is strongly associated with CTx [25]. However, raised levels of Fetuin-A have been associated with higher BMD suggesting fetuin-A to be promoting bone mineralization in elderly women [7].
The biphasic response of Fetuin-A i.e., its ability to promote or inhibit calcification in different conditions has thus become a focus for researchers for its use as a potential bone marker of various bone disorders diagnostics [9]. However, regulation of Fetuin-A is not very well explored. As both the markers are responsible for bone resorption, we further need to investigate that which of the two proteins is responsible for decreasing bone health in our population or both are working in synergism. Small sample size and evaluation of an inflammatory marker stands as a limitation of our study.
Physical activity acts as a powerful rehabilitation tool for osteoporotic patients. Vibration exercises strengthen the muscles and bones against the vibrating platform. Whole body vibration intensifies the level of hormones, averting osteoporosis. Exercises based on piezoelectric theory, produce pressure induced bone formation by the variation of electrical potential, thus acting as a stimulant of the process of bone formation [26].
CONCLUSION
REFERENCES
- Li M, Li Y, Deng W, Zhang Z, Deng Z, et al. (2014) Chinese bone turnover marker study: reference ranges for C-terminal telopeptide of type I collagen and procollagen I N-terminal peptide by age and gender. PLoS One 9: e103841.
- World Health Organization Scientific Group (2007) Assessment of osteoporosis at the primary health care level: summary report of a WHO Scientific Group. World Health Organization , Geneva, Switzerland.
- Foundation I O. p. 3.
- Fink HA, B?žková P, Garimella PS, Mukamal KJ, Cauley JA, et al. (2015) Association of fetuin-A with incident fractures in community-dwelling older adults: The Cardiovascular Health Study. J Bone Miner Res 30: 1394-1402.
- Mori K, Emoto M, Inaba M (2011) Fetuin-A: a multifunctional protein. Recent Pat Endocr Metab Immune Drug Discov 5: 124-146.
- Jahnen-Dechent W, Heiss A, Schäfer C, Ketteler M (2011) Fetuin-A regulation of calcified matrix metabolism. Circ Res 108: 1494-1509.
- Ix JH, Wassel CL, Bauer DC, Toroian D, Tylavsky FA, et al. (2009) Fetuin-A and BMD in older persons: the Health Aging and Body Composition (Health ABC) study. J Bone Miner Res 24: 514-521.
- Nimitphong H, Sritara C, Chailurkit LO, Chanprasertyothin S, Ratanachaiwong W, et al. (2015) Relationship of vitamin D status and bone mass according to vitamin D-binding protein genotypes. Nutr J 14: 29.
- Toroian D, Price PA (2008) The essential role of fetuin in the serum-induced calcification of collagen. Calcif Tissue Int 82: 116-126.
- Eastell R, Hannon RA (2008) Biomarkers of bone health and osteoporosis risk. Proc Nutr Soc 67: 157-162.
- Seibel MJ (2005) Biochemical markers of bone turnover: part I: biochemistry and variability. Clin Biochem Rev 26: 97-122.
- Zaitseva OV, Shandrenko SG, Veliky MM (2015) Biochemical markers of bone collagen type I metabolism. Ukr Biochem J 87: 21-32.
- Magnuson W G Dietary Supplement Fact Sheet: Vitamin D. skin. 1: 2. https://ods.od.nih.gov/factsheets/list-all/VitaminD/
- Arya V, Bhambri R, Godbole MM, Mithal A (2004) Vitamin D status and its relationship with bone mineral density in healthy Asian Indians. Osteoporos Int 15: 56-61.
- Roy DK, Berry JL, Pye SR, Adams JE, Swarbrick CM, et al. (2007) Vitamin D status and bone mass in UK South Asian women. Bone 40: 200-204.
- Palacios C (2006) The role of nutrients in bone health, from A to Z. Crit Rev Food Sci Nutr 46: 621-628.
- Lin X, Braymer H, Bray G, York D (1998) Differential expression of insulin receptor tyrosine kinase inhibitor (fetuin) gene in a model of diet-induced obesity. Life sci 63: 145-153.
- Stefan N, Hennige AM, Staiger H, Machann J, Schick F, et al. (2006) Alpha2-Heremans-Schmid glycoprotein/fetuin-A is associated with insulin resistance and fat accumulation in the liver in humans. Diabetes Care 29: 853-857.
- Yilmaz Y, Yonal O, Kurt R, Ari F, Oral A Y, et al. (2010) Serum fetuin A/?2HS-glycoprotein levels in patients with non-alcoholic fatty liver disease: relation with liver fibrosis. Ann Clin Biochem 47: 549-553.
- Lavebratt C, Wahlqvist S, Nordfors L, Hoffstedt J, Arner P (2005) AHSG gene variant is associated with leanness among Swedish men. Hum Genet 117: 54-60.
- Sritara C, Thakkinstian A, Ongphiphadhanakul B, Chailurkit L, Chanprasertyothin S, et al. (2014) Causal relationship between the AHSG gene and BMD through fetuin-A and BMI: multiple mediation analysis. Osteoporos Int 25: 1555-1562.
- Dar FJ, Iqbal R, Ghani F, Siddiqui I, Khan AH (2012) Bone health status of premenopausal healthy adult females in Pakistani females. Arch Osteoporos 7: 93-99.
- Teitelbaum SL (2000) Bone resorption by osteoclasts. Science 289: 1504-1508.
- Bjarnason NH, Christiansen C (2000) Early response in biochemical markers predicts long-term response in bone mass during hormone replacement therapy in early postmenopausal women. Bone 26: 561-569.
- Chailurkit L, Kruavit A, Rajatanavin R, Ongphiphadhanakul B (2011) The relationship of fetuin-A and lactoferrin with bone mass in elderly women. Osteoporos Int 22: 2159-2164.
- Weber-Rajek M, Mieszkowski J, Niespodzi?ski B, Ciechanowska K (2015) Whole-body vibration exercise in postmenopausal osteoporosis. Prz Menopauzalny 14: 41-47.
Citation: Alam F, Nihal S, Abbas A, Abdullah M, Shah S, et al. (2016) Reciprocal relation of Fetuin-A and Beta Crosslaps with bone health. J Orthop Res Physiother 2(1): 024.
Copyright: © 2016 Faiza Alam, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.

