
Research Trend and Literature Review on Identification, Effects and Minimization of Disinfection by Products (DBPs) with Focus on Chlorination as Disinfection Treatment
*Corresponding Author(s):
Sayali ApteDepartment Of Civil Engineering, Symbiosis Institute Of Technology, Symbiosis International, Deemed University, Pune, India
Email:sayali.apte@sitpune.edu.in
Abstract
The Disinfection by Products (DBPs) are formed due to the reaction between the disinfectant and naturally occurring organic matter in water. The DBPs being carcinogenic compounds that can cause variety of cancers and affect the human health critically [1]. The paper presents a detailed literature review on DBPs identification, its effect and alternate treatment system for DBP removal and minimization. The literature review is supported by bibliometric analysis for understanding the extent of research till date and the research trend this domain. The SCOPUS data analysis indicates the highest number (878) of documents recorded for the DBP trihalomethane indicating more research has been incorporated for trihalomethane. Chu W contributed maximum documents in field of DBP and chlorination while [2] has maximum number of citations for his research work on new generation DBPs. China and United States are two leading countries in research area of DBP and chlorination.
Due to diverse characteristics of DBPs their identification is difficult and ultimately the removal or minimization is a challenge. It is observed from the detailed literature review that few innovative techniques like Gas Chromatography/electron capture detector, GC/ECD; Gas chromatography/mass spectrometry, GC/MS; High- performance liquid chromatography/mass spectrometry, HPLC/MS for identification and removal of DBP has been used recently. The available literature suggests many theoretical alternative treatment systems for reduction, removal and minimization of DBPs like Granular Activated Carbon (GAC) and Response Surface Methodology (RSM) based on adsorption technology. The literature review indicates further study need to be executed through a lab-scale model which would actually use this treatment methods and reinforce the utility of these methods in real life scenario. A need of research on removal or minimization of DBPs by modifying and/or advancing the Current treatment systems.
Keywords
Disinfection, water treatments, Disinfection by Products, DBP identification, effects of DBPs, Minimization and removal of DBPs.
Introduction
The process of disinfection of water is mainly carried out using Ultraviolet light, Ozonation and Chemical disinfection i.e., chlorination. The carcinogenic by product formed during the process of disinfection due to the reaction between the disinfectant and the naturally present organic matter in water, are called as Disinfection By Products (DBPs). Chlorination is one of most widely used disinfection method due to its low cost and provision of residual effect than the ultraviolet light and ozonation treatment [3] The DBPs Formed during the process of chlorination of water depends on the compounds used for chlorination. For example, if chlorine liquid is used as disinfectant trihalomethanes (THMs), chlorophenols, chlorate, alkanic acids etc. are formed as by product while chlorite; chlorates are formed when chlorine dioxide is used as disinfectant. Chloramine acids, nitrite, nitrate and aldehydes are formed when chloramine is used as disinfectant. Chloramine is efficient at decreasing the concentrations of the DBPs like trihalomethanes and haloacetonitriles in potable water than chlorination, concluded that chlorine dioxide (ClO2) prior to chlorination is valid for regulation of Trihalomethanes (THMs), haloacetic acid (HAA) and haloacetonitriles (HAN) in both pure and polluted waters, but creation of chlorite is the concern and halonitromethanes (HNMs) and haloketones (HKs) are not efficiently restricted by ClO2 pre-oxidation [4]. Ultraviolet light when used as disinfectant, the DBPs are also formed during reaction between the organic matter and ultraviolet rays. The potential of DBP formation is approximately more in ultraviolet light disinfection than in chlorination [5]. In ozonation disinfection is carried out by radical-type chain reactions, which consume ozone along with the reaction of ozone with dissolved organic matter.
While ozonation treatment itself creates DBPs, like aldehydes and ketones, and also increases the concentration of bacterial nutrients by converting non-biodegradable organic matter to more biodegradable compounds [6] and affects human health [7] In ozonation disinfection, the organohalogenic DBPs formed are bromoform, monobromine etc. while iodate and bromate are inorganic DBPs and ketons, aldehydes, ketoacids, carboxylic acids are non-halogenic DBPs, examined the impact of ozonation on formation the DBP. When pre ozonation is applied versus non- ozonated samples, trihalomethane formation potential is found to increase by 10% to 30%. A small increase in haloacetic acid (HAA) creation is observed in the ozonated illustrations. Both the ozonation and ultraviolet light converts some of the bromides in water to bromate, which is harmful to human health [8].
The natural organic matter (NOM) present in water is a very complex combination of organic compounds and it is present in both groundwater and surface water in the form of particulate, colloidal and dissolved organic matter. From the perspective of human health, NOM does have very little or insignificant impact on the well-being, but during the water disinfection treatments, NOM reacts with disinfectant used during disinfection processes and produces Disinfection-by-Products (DBPs). The DBPs affects the human health severely due to their carcinogenic property and control of NOM and DBP is difficult which required multidisciplinary effort by collaboration of chemists, engineers and toxicologists [9]. According to the water characteristics and the properties of NOM, different types of DBPs are formed as an outcome of water disinfection method which ultimately affects human health [10, 11]. Many researchers are working on the identification effect and minimization of DBPs formed in the process of water disinfection the present paper provides an overview of the extent of research in the area of DBPs thorough a detailed literature review supported by bibliometric data. The SCOPUS database analysis provides a direction and basis to the literature review as per the overall research trend. The combination of bibliometric analysis and literature review provides an overall scenario of research in the domain, which is the novelty of the present paper. The available literature is classified into various sections covering identification, effects and minimization of DBPs. The Chlorination is widely used treatment method for water disinfection worldwide. As per the SCOPUS database (figure 1) it observed that literature available in field of chlorination and DBPs is more than UV and ozone treatment methods. The present paper discusses the available literature for water disinfection and DBP’s with special focus on DBPs formed during chlorination treatment. The literature review is carried out considering the trend of the research in the area and based upon the SCOPUS data analysis.
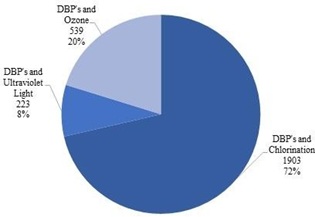 Figure 1. Number of DBPs related documents in Chlorination, UV and Ozone
Figure 1. Number of DBPs related documents in Chlorination, UV and Ozone
Source: www.scopus.com (data retrieved on 27th March 2022)
Literature Review
SCOPUS is one of the largest abstract and citation database of peer-reviewed literature such as scientific journals, books and conference proceedings. It provides a complete overview of the world's research output in the fields of science, technology, medicine, social sciences, and arts and humanities. In the present paper the SCOPUS database is used for understanding the extent of research and latest research trend in the area of DBP’s and chlorination to achieve a more comprehensive and effective literature review. The analysis of literature includes broad subject areas in which the research is conducted, authors with highest citations working in the domain and major countries contributing to the research in the form of research funding and the impact of the funding on the research contribution. A detailed bibliometric analysis in field of DBPs and chlorination as disinfection technology is discussed through year wise analysis, country-wise analysis, subject-wise analysis, top funding agencies, top authors contributing in the field and top cited publications. Based on the research trends and data analysis on the available literature, a detailed literature review is carried out. The literature review is categorized as identification of DBPs, effects of DBPs on human health and related issues and minimization or removal techniques of DBPs.
Year wise, country wise, subject wise and publication type wise analysis of documents for chlorination Treatment
As chlorination treatment is used widely due to its low cost and residual effect maximum extensive studies are accounted related DBPs generated by chlorination disinfection treatment and hence detailed bibliometric analysis of chlorination is carried out in this section. The research publication data for keyword set “Disinfection by products” and “Water” and “Chlorination” and “Disinfection”, recorded total 6783 documents on Scopus from year 2000 to 2023 (figure 2).
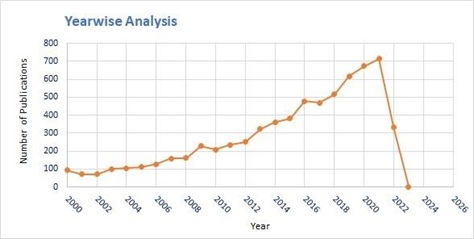 Figure 2. Year wise analysis of documents for keywords “Disinfection by products” and “Water” and
Figure 2. Year wise analysis of documents for keywords “Disinfection by products” and “Water” and
“Chlorination” and “Disinfection”
Source: www.scopus.com (data retrieved on 27th March 2022)
From year 2000 the trend of publications in the field of DBPs generation by chlorination treatment is increasing. For keywords “Disinfection by products” and “Water” and “Chlorination” and “Disinfection”, 94 articles are recorded in the year 2000 and trend goes on increasing till 713 publications documented in year 2021 (figure 2). From the figure 2, it is observed that the trend of publication is increasing and can be inferred that more research is being carried out in this domain but still there is a wide scope of research in area of DBPs formation, effect and minimization due to chlorination.
The figure 3 shows the country-wise publication for DBP formation by chlorination treatment. It is observed that China has maximum publication for DBP generation by chlorination. Total 2376 documents are published by China from year 2000 to 2023. While United States, Canada, Spain and Australia documented 1712, 464, 354 and 298 research documents respectively (figure 3).
 Figure 3. Number of publications from different countries related to DBP formed by chlorination
Figure 3. Number of publications from different countries related to DBP formed by chlorination
Source: www.scopus.com (data retrieved on 27th March 2022)
Figure 4 shows the top five subject areas contributing in field of DBPs due to disinfection of water by chlorination treatment. The maximum number of research publications related to the DBP and Chlorination are published in the subject area of Environmental Science (figure 4).
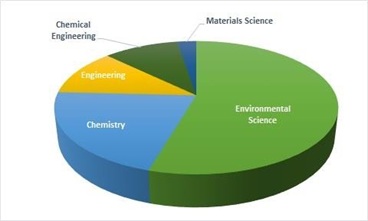 Figure 4. Subject wise analysis of documents
Figure 4. Subject wise analysis of documents
Source: www.scopus.com (data retrieved on 27th March 2022)
The top funding agencies promoting the research in the area of DBP formation due to chlorination treatment is the National Natural Science Foundation of China followed by Fundamental Research Funds for the Central Universities, National Science Foundation and Ministry of Science and Technology of the People's Republic of China. The data shows that China has highest funding in this research domain and same is reflected in the research publications (figure 5).
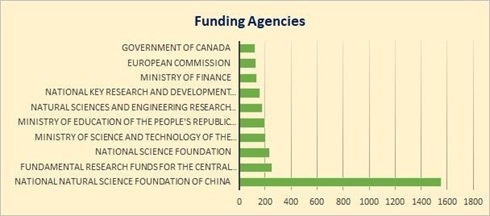 Figure 5. Top 10 funding agencies
Figure 5. Top 10 funding agencies
Source: www.scopus.com (data retrieved on 27th March 2022)
As per the SCOPUS database, figure 6 shows the authors contributing significantly in the field of DBPs due to chlorination. From the data, one can get the detailed idea about top researchers with highest publications and highest citations working in the area of DBP. Author Chu W. contributed maximum number of publications in research area of DBPs while highest citations recorded by the publication named “Occurrence of a new generation of disinfection by-products” [12]. Top 10 highest citated research publications are reviewed which gives idea about the extent of research in the field.
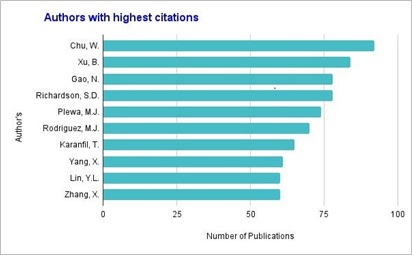 Fig. 6. Authors with highest publications
Fig. 6. Authors with highest publications
Source: www.scopus.com (data retrieved on 27th March 2022)
The citations are supposed to reflect the impact of the research or its quality. One of the indicators in research quality is the number of citations to various publication. Table 1 discusses about top 10 publications with maximum citations.
The higher number of citations indicates more impactful and significant research in the domain. The top 10 highest citation publications for the identification, effect and minimization of DBPs are given in Table 1. The highest cited publications are observed to be review articles. The paper mainly focuses on actual experimental work on DBP identifications, effects and minimization techniques. The highest cited research entitled “Occurrence of a new generation of disinfection by-products” contributing in field of DBPs has total 1186 citations.
The top ten most cited experiment-based papers addressed in table 1 in the field of DBP and chlorination are also discussed in detailed in literature review section under classification of identification, effects and minimization of DBPs sections.
From SCOPUS database, most recognized DBPs such as Trihalomethane, Chlorite, Chloral hydrate, Haloketones, halogenated acetic acids (HAA’s), Chlorinated phenols and Halogenated acetonitrile’s are searched to know the extent of research for particular type of DBP from year 2000 to 2022. From the search it is observed that the highest number of publications recorded for DBP Trihalomethane (figure 7). This analysis illustrate that more research has been carried out for Trihalomethane DBP. After trihalomethane, chlorite and chloral hydrate contributed maximum number of publications as 143 and 118 respectively (figure 7).
 Table 1. Publications with highest citations
Table 1. Publications with highest citations
Dissolved Organic Matter (DOM) is a part of NOM that including soil particles, plant residues, soluble components secreted by living organisms, comprising of bacteria, algae, and plants, also produces DBPs after disinfection of water which is indirectly affects the human health. DOM is considered as major precursor for formation of DBP [4, 13].
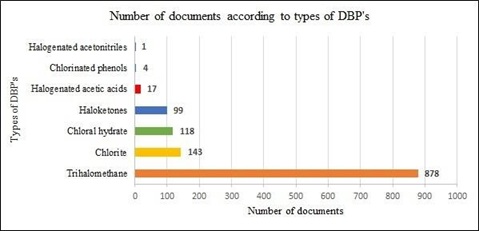 Figure 7. Number of documents according to types of
Figure 7. Number of documents according to types of
Source: www.scopus.com (data retrieved on 27th March 2022)
The DBPs affects human health badly if present in the water in large amount even after the treatment [14]. The type of DBP formed, depends upon various factors like type of the disinfectant used, concentration of the disinfectant used, pH, temperature of the raw water and the concentration and type of NOM present in water (figure 8). Different types of DBPs are formed for different characteristics of water and type of disinfectant used [15]. Till date more than 600 DBPs are identified [16] but still research is going on as there are many unknown by products that has to be identified.
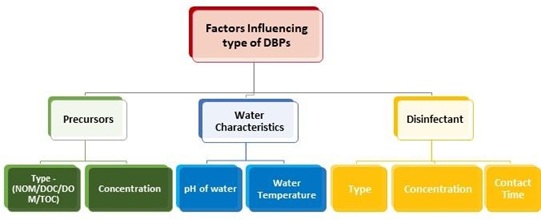 Figure 8. Factors Influencing DBP (Disinfection by Product) formation
Figure 8. Factors Influencing DBP (Disinfection by Product) formation
The research studies related to Chlorinated phenols and Halogenated acetonitrile’s are very less as compared to other DBPs. The most studied DBPs and the effect of these DBPs on human health are discussed in Table 2.
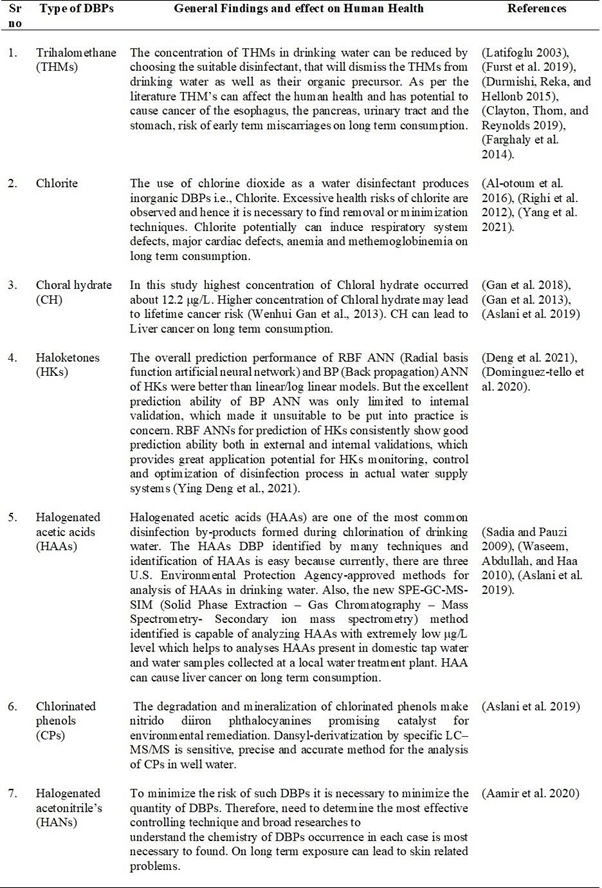 Table 2. Types of DBPs and effects on human health
Table 2. Types of DBPs and effects on human health
Many researchers endeavoured to find out the new disinfection technologies for minimization or removal of these DBPs [17]. Few researchers commented that the Electro-chlorination process of water disinfection is one of alternative that minimizes DBPs [18]. The first DBP is identified initially in 1974 [19] and till date several types of other DBPs are recognized. The distinct DBPs are formed according to the different characteristics of the water in diverse regions. These DBPs are carcinogenic compounds that causes different types of cancers and affect the human health critically [20]. There is need to work further on the removal or minimization of these DBPs by changing or advancing the current treatment systems [14, 21]. While studying the microbial characteristics, disinfection methods for water disinfection, it is significant to analyse the physico-chemical result of disinfection technique on the characteristics of water. The literature review carried out in present paper supported by bibliographic data focuses on the DBPs identification, its effects and minimization or removal (figure 9).
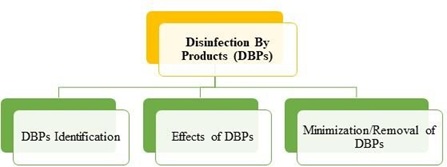 Figure 9. literature review classification
Figure 9. literature review classification
During late 1900’s it is discovered that, the disinfection application is commonly associated with the generation of disinfection by-products (DBPs) which can induce additional public health dilemmas [22] starting a research in the field of identification, effects and minimization of these DBPs. In 1974 the first DBP Trihalomethanes (THM’s) is identified [14] after use of these treatment systems for treating drinking water for many decades. Later on, many different types of DBPs are identified from 1974 till 2018 [23] and further research is still going on to find out new DBPs. The type and extent of DBPs formed depends on many factors such as pH, temperature of a water, type and concentration of a disinfectant used affects the DBPs formation. This indicates the need to work further with more efficient detection techniques is one of the top cited experimental works and found out new DBP named N- nitrosodimethylamine using DMA (dimethylamine) as model precursor. More detailed study of model required for applicability in water and wastewater treatments [24] is second most cited document, discussed that, many treatment plants follow alternative disinfectant than chlorine to reduce the recognized DBPs. But prior studies informed that while reducing the identified DBPs, formation of unknown DBPs has observed [25] is one of the highest cited experimental works amongst top cited publications. The paper discusses the quantitative occurrence of known DBPs from 12 selected water treatment plants situated in United States. The study identifies 28 new DBPs which provides a new insight in area of DBP study. Weinberg et al discusses about the future health effects of known DBPs on basis of quantity of DBPs produced and toxicity level of DBP. The health effects of newly identified DBPs need to study to understand the toxicity extent and future health effects [26] performed experiments to identify the DBPs from eight selected triazines of different combinations in drinking water by using ultra performance liquid chromatography-quadrupole-time of flight- tandem mass spectrometry (UPLC-Q-ToF-MS/MS). Out of eight triazines, 3 triazines formed DBPs after reacting with the hypochlorite in water. The experiments also concluded that at different strengths of triazines and hypochlorite presented that, scope of the reaction depended on their relative concentrations. But still further identification of these by products in terms of their toxicity level should be identified with help of advanced identification techniques or detectors to assess potential risks of contact through chlorinated drinking water [27] also identified a polar Br-DBPs using a new method, ancestor ion scan with ultra- performance liquid chromatography/electrospray ionization triple quadrupole mass spectrometry. And “black box” from the record of “humic substances in addition of bromide and chlorine” across the output of “haloacetic acids plus trihalomethanes” is opened to a substantial extent [28] are also top most cited articles discussing that the DBPs can minimized by different types of techniques (discussed in detail in section 2.3) but before minimization and removal of DBPs it is most important to find out the determination of DBPs qualitatively and quantitatively. For proper analysis of DBPs need to investigate high end equipment’s that interpret close characteristics of DBPs [29]. Detailed and extensive study regarding type and structural characteristics of substitutes present in water and their corresponding DBP formation need to investigate to have great controlled on DBP formation and its effects. [12] identified unregulated DBPs, involving nitrogenous DBPs in lab. Reactions and distribution taps. In the ground examination, 9 tap locations receiving water from full-scale chlorinated surface water drinking water treatment plants on two continents are found. In this study the chlorinated by products are identified using gas chromatography-mass spectrometry functioned in scan mode (50 -300 amu) [30] identified DBPs using GC_GC-qMS (Gas chromatography) and Quantitative structure activity relationship is used to estimate the toxicity data, using a computer-based technique. Overall, 170 volatile and semi-volatile DBPs are recognized. A precision of the composite detection is governed by assessing 47 recognized composites. Around 90% (41 of the 47) of the composites that are instinctively recognized are precise. The results confirm that GC_GC-qMS coupled with a QSAR model is an influential and quick nontargeted screening method for composites. The process and consequences deliver novel thought for recognition and ordering of DBPs [31]. Investigation of DBPs is usually executed by time- and solvent-intensive sample formulation approaches such as liquid–liquid and solid phase extraction. But [10], established a novel method i.e., headspace gas chromatography with micro-electron capture detection and utilized for the investigation of THMs in drinking water. Objective of this effort is to build a new direct headspace method for fast investigation of THMs that eliminated or diminished the essential for previous time-consuming sample formulation. The study concluded that the new method is effectively established to decrease or eliminate distinctive sample creation steps, such as salt addition, pre-concentration and filtration, to examine examples clearly from source with nominal exterior. In that way as the DBP identification techniques will get advanced then it will be easy to detect the DBPs and ultimately can work further rapidly for their removal [32].
Guang Huang, 2017 reported the detection of N-chlorinated dipeptides as novel DBPs in potable water by means of corresponding high-resolution quadrupole time-of-flight (QTOF) and quadrupole ion-trap mass spectrometry method [33]. Analysed the intensity of THMs in the water source in Perlis, Malaysia. The water samples are gathered from end-user tap water near the water treatment plant (WTP) situated in Perlis. The THMs are analysed using a Gas Chromatography-Mass Spectrometry (GC/MS). The document displays that THM creation is maximum in the tap water provided by Water Treatment Plant Timah Tasoh, and the lowermost quantity of THMs is in tap water provided by WTP Kuala Sungai Baru [34], reviewed a different technique used for identification of the DBPs from last decade and commented on which technology is suitable for identification of particular DBPs. Often used techniques for DBPs detection and determination mainly include TOX (Total organic halogen), GC-ECD (Gas chromatography - Electron capture detection), GC-MS (Gas chromatography-mass Spectrometry) , UPLC-MS/MS , and GC- HRMS (GC equipped with high-resolution mass spectrometry). From this review authors concluded that the Total organic halogen (TOX) study is frequently used as a substitute measurement to measure both known and unknown DBPs in potable water. CI (Chemical Ionization), should be included for the detection of formerly overlooked DBPs. While all other GC associated procedures are appropriate for the recognition of volatile and semi volatile DBPs, while LC-MS is an perfect tool for examining polar DBPs or thermally volatile DBPs [35] examined bibliometric analysis of study tendencies on DBPs in potable water from year 1975 to 2018. The bibliometric review article on DBPs in drinking water, highlights the publication numbers, well cited papers, study areas, authors, leading nations and organizations. Study also explains about the DBPs identified from year 1974 to 2018. In 1974 the first DBP i.e., Trihalomethane is identified, after that in 1984 chlorite, 1986 – haloacetonitriles, 1989-haloaceticacids, 1990- aromatic DBPs and many more and recently in year 2017 performic acid is identified. According to the study he concluded that the most studied DBP among several others is THMs. Amongst the recognized DBPs, some evolving classes deficient evaluation of their poisonousness and hazard due to longstanding disclosure and hence considering the complication of DBPs in actual waters, the possible hazards of exposure to mixtures, new DBPs should be examined quantitatively and qualitatively. It is essential to remove the DBPs from drinking water to minimize the harmful effects of DBPs on human health, but before removing of the DBPs, identification is very important [36] investigated the effect of increasing chlorine dose on the formation of DBP’s. The study concluded that when aromatic index value of DBP is greater than 0.5 then firstly decrease and then increase in amount of DBP observed during increasing the chlorine dosage. For DBPs with aromatic index value less than 0.5 shows that increase in DBPs as increase in chlorine dosage [37].
Till date haloquinone chloroimides (HQCs) DBPs are not identified because of analytical method limitations. In this study investigated new methodology to identify five types of haloquinone chloroimides (HQCs). The new DBPs named 2,6-dichloroquinone-4-chloroimide (2,6-DCQC), 2,6-dibromoquinone-4-chloroimide (2,6-DBQC), 2- chloroquinone-4-chloroimide (2-CQC), 3-chloroquinone-4-chloroimide (3-CQC), and 2,6-dichloroquinone-3-methylchloroimide (2,6-DCMQC) were identified in this study [38].
Types of DBPs
The characteristics and compositions of NOM present in raw water varies significantly according to the change in water characteristics (figure 1) and ultimately influences the DBP formation. The produced DBPs are classified as regulated and Unregulated DBPs. The DBPs with known characteristics which may be controlled or minimizes are termed as Regulated DBPs. Whereas DBPs which are newly generated due to reaction between unknown NOM and disinfectant and difficult to regulate are known as unregulated DBPs [37, 39]. DBPs categorized on the basis of their chemical structure are Aromatic and Non-aromatic DBPs [22]. The DBPs with planar cyclic structure that obeying Huckel’s Rule are considered as an aromatic DBPs. The DOC (Dissolved Organic Matter) which has a complex composition is a major precursor for formation of such aromatic DBPs [40] is one of the highest cited experimental works and studied the factors affecting formation of DBPs and concluded that substitute present in water with aliphatic structures leads to maximum formation of trihalomethane DBP [13], classified the different types of DBPs on basis of molecular size and hydrophobicity of precursors and concluded that formation of DBPs may reduce by optimizing the treatment processes for removal of both hydrophobic and hydrophilic organic matter [24] analyzed dissolved organic matter (DOM) by high resolution mass spectrometry (HRMS) for northeastern China water sample. The changes in water sample after chlorination are investigated and concluded that DOM and chlorinated products are main source of DBPs formation [41].
The DBPs have been identified and classified under different type according to their composition and characteristics. The classification of few of well-known DBPs is shown in figure 10. Further study on identification of DBPs may lead to detection of different DBPs with several characteristics and will be added under new classification group [41].
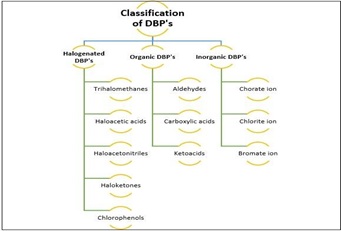 Figure 10. General Classification of DBP's
Figure 10. General Classification of DBP's
The identification of DBPs in the past (around 1900) using prior methods was difficult, time-consuming and inefficient and was hectic to identify different types of DBPs. But now the novel DBPs identification techniques explained above would help a lot for further study of DBPs effects and minimization.
Effects of DBPs on Human Health
Kirsten Waller et al., 1997 studied the effect of DBPs on spontaneous abortion. The investigations carried out on 5,144 pregnant women’s and concluded that out of four types of trihalomethanes, only exposure to bromodichloromethane leads to spontaneous abortion [34].
Organization among the incorporation of chlorinated portable water in surplus with risk of bladder and rectal cancer obeyed by mortality has been described in some epidemiological studies [14] is also considered under the category of top cited articles that discusses about the health effects of DBPs observed in human beings are different than the health effects on animals detected at laboratory which is major concern to study further. Compared the formation of DBPs using different types of disinfectants like chlorine, chloramine, both with and without preozonation, and chlorine dioxide. The study discussed that use of alternate disinfectant may reduce the one type of DBP but at same time some other DBP formed [39,32]. Reviewed an organized literature and meta-analysis of epidemiological investigations including primary reviewed statistics on the alliance of entire trihalomethane contact and well-being consequences interrelated to embryonic growth and prematureness. The data analyzed for the 4 bad birth outcomes: low birth weight (LBW), term low birth weight (term LBW), preterm delivery, and small for gestational age (SGA). The study concluded that there is little effect of THMs exposure on fetal growth and prematurity but THMs exposure affects small for gestational age (SGA) [42].
Reviewed a detailed literature related to the health effects of Trihalomethanes on human health. The study explained that the Trihalomethanes has carcinogenic property which causes various types of cancers. The review also explained about the relationship of THM’s on birth effect and commented that there is no confirm evidence that THM’s affects the pregnancy but may leads to liver and kidney damage in case of animals therefore more detailed study required. The paper also focused on the effect of trihalomethane on genotoxicity i.e., mutagenicity as well as DNA damage. The long-term exposure to THMS may lead to DNA damage [43].
Wen-Cheng Cao et al., 2016, examined the relationship among blood markers of delayed gravidity communicate to trihalomethanes (THMs) in portable water and embryonic growth and gestational phase. The outcomes of learning recommended that raised parental THM contact may harmfully affect embryonic growth [12], examined the birth defect study after exposure to DBPs and developed a multi- DBPs regression model. Also carried out the study based on cardiovascular defects (CVDs) to assess HAA revelation and another to evaluate bromoform exposures and this is the first study. The findings of the study concluded from epidemiological and toxicological studies that the DBPs exposures increases the risk of some cardiovascular defects (CVDs) and Ventricular septal defects (VSDs) [15] studied a prior literature resulted that DBPs are carcinogenic and causes cancer but in this study, author explored detailed literature and sorted out the type of cancer caused by particular type of DBP. And findings of study concluded that out of all the DBPs Bromodichloromethane and MX (Mutagen X) DBP are most affecting risk factors and liver and kidney are the furthermost general target organs for toxicity poisonousness by DBPs [22] assessed the THMs level of in municipal drinking by collecting the THM data from 2005–2018 of 26 European countries covering 75% of the population. The study concluded that, THMs levels exceeded than standard limit in drinking water of nine counties out of twenty-six countries and might lead to a significant numeral of bladder cancer instances and became a burden on EU countries. But this burden would be prevented by enhancing treatment of water, disinfection, and supply, among other actions, without negotiating the microbiological characteristic of potable water, [17] discussed about formation, identification and removal techniques of DBPs through prior studies. After reviewing 52 research studies, concluded that health risk associated with DBPs can be reduced only by following the guidelines strictly related to DBPs [44] concluded that strict regulations, legislation, and guidelines should followed before discharging pollutants is most important to protect environment, water, and public health [45].
From the literature it inferred that the DBPs are carcinogenic and affects the human health. Also, many research papers concluded that the long-time exposure to DBPs causes cancer. One of the studies concluded that Bromodichloromethane and MX (Mutagen X) DBP are most harmful DBPs and mostly causes liver and kidney failure. From many research articles it has been proved that DBPs affects the health of pregnant women’s and ultimately leads to abortion. Overall DBPs causes several diseases including various types of cancers.
When different NOMs reacts with several types of disinfectants, the generated DBPs also have diverse affects central nervous system, kidney related problems and affects reproduction and hence it is necessary to minimize the DBPs from treated water. Disinfection by Products affects the human health causing mild and severe effects. DBPs affects human health due to their carcinogenic property. Bladder cancer, effects on gravidity consequences such as embryonic loss, fetal development, pre delivery and inherited malformation are some severe effects that causes due to long exposure to DBPs. While risk of asthma, breathless, eczema, and other breathing outcomes amongst children joining swimming pools are mild effects of the DBPs [46].
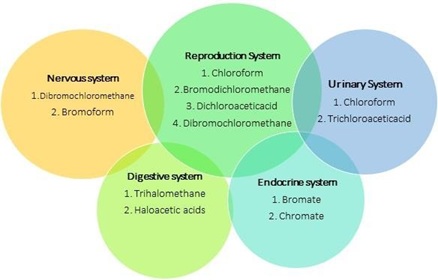 Figure 11. Different systems of body affected by particular type of DBPs
Figure 11. Different systems of body affected by particular type of DBPs
characteristics than prior one. The diverse properties of DBPs are due to various factors (figure 1) and ultimately result into different types of diseases that affects different parts of human body. Figure 11 shows different types of DBPs that affect specific body part or system of human body. These DBPs are already been identified in prior studies and characteristics are well known that can be helpful to decide the medication treatment. But if any new DBP affects human then it’s been very difficult to give treatment, without knowing the exact properties of newly produced DBP. Therefore, the further study related to the identification of new DBPs is necessary to know their characteristics in advance to avoid the extreme ill effects on human health [47].
Minimization / Removal of DBPs
It is prerequisite to disinfect untreated drinking water as it causes many waterborne diseases. But DBPs get created after the disinfection treatment and that also affects human health badly and hence it is urgent need to minimize or remove these DBPs by changing the treatment system. After identification and effect of DBPs from last decades, now it is more important to study further for DBPs minimization. Minashree and Sunil Kumar Gupta, 2019 investigated a new technology i.e., Response Surface Methodology to disinfect drinking water and minimizes the DBPs production even after the treatment. In 2019, authors succeeded in minimizing one DBP that is Trihalomethane that is first identified in 1974 [48]. And hence now it is most important to study further for minimization of new DBPs. In this section, literature regarding to the new techniques required for minimization or removal of DBPs after disinfection is discussed. The organic chloramines and DBPs are generated during the process of UV disinfection and can be controlled using combination of UV/chlorination treatment. The formation of DBPs after disinfection can also controlled by ozone-low pressure ultraviolet light followed by chlorination process [49].
UV/advanced reduction process also helps to minimize the chlorate formed after disinfection. The UV/persulphate process and UV-LED Based Advanced Oxidation Processes are some recent and advanced ultraviolet light-based process for minimization of DBPs [16,25].
The prior studies discussed that the disinfection of water using ozone treatment also generate DBPs after the disinfection treatment. In recent study concluded that ozonation treatment increases chlorophenylacetonitrile Some of the by products like bromate can be reduced by advanced ozone treatment and ozone combined free active chlorine system [50] is one of the highest cited experimental works and discovered that WWTP (Waste Water Treatments plant) discharges can be a source of a wide range of halogenated and nonhalogenated DBPs of health concern which is important for growing water reclamation programs in arid portions of the United States and elsewhere. Studies concluded that water rich in brominated and iodinated characteristics lead to generate most toxic DBPs and hence more focus on checking and knowing the characteristic of water before treatment is important reviewed articles related to the bromide and iodide elimination techniques from potable water founts as bromide and iodide formed DBPs after treating with chemicals. Bromide and iodide elimination methods has been generally grouped into 3 types, viz.; membrane techniques, electrochemical and adsorptive methods. Reverse osmosis (RO), nanofiltration (NF) and electrodialysis membrane (MCI) techniques are studied under membrane methods. While electrochemical methods considered are electrolysis, capacitive deionization and membrane capacitive deionization. And under adsorptive methods, layered double hydroxides, impregnated activated carbons, carbon aerogels, ion exchange resins, aluminum coagulation and soils are evaluated. After analyzing all the techniques through review articles, this study concluded that Membrane techniques (RO, NF, MCI) are noted to get admirable halide elimination capability, but these methods may be costly and consumes more energy. Electrochemical method (electrolysis, CDI and MCDI) can also have effective halide elimination capabilities, unlike the membrane methods. From this study it is suggested that adoption technique would be the promising area of research as it has better ability of minimizing the DBPs and also the technique is cost efficient [51] Reviewed applications of nanotechnology in field of water and waste water treatments.
From the detailed review xiaolei concluded that use of nanotechnology in field of water treatments has great revolutionary results. But due to some temporary challenges like cost, environmental hazard, the research including nanomaterials is at experimental stage only. Very few pilot stations are installed and able to make it commercialize which can be overcome by collaboration between research institutes and industries, government and stakeholders.
Recently in year 2019 & 2020 many researchers focuses on minimization of DBPs through new disinfection technologies. Recently in 2019 Minashree Kumari & Sunil Kumar Gupta investigated a response surface methodology for removing the THMs. THMs is the first DBP that has been found out in 1974 and now in 2019, researcher succeeded in removing one DBP i.e., THM’s. Response surface methodology (RSM) is a multivariate statistical tool and it is based on adsorption technique. Authors developed a RSM model and validate results with experimental results. Analytical study of experiments is carried out using qualitative and quantitative examination of TTHMs utilizing phase separation liquid-liquid extraction technique. Study concluded that the experimental results matched with the developed RSM model. Generally acid pH is noticed efficient for elimination but this analysis discovered that entire elimination of THM is achieved at pH near to neutral [52] performed a laboratory scaled Chlorination process afterward every phase in the drinking water treatment procedure to examine the result of each method process to the formation of DBPs. And tests inspected that the suspended ion exchange had a great ability to eliminate dissolved organic carbon (DOC) and use of granular activated carbon (GAC), proficiently reduces the oxidative stress and genotoxic of DBPs. Discussed the results regarding to the identification and minimization of the DBPs through a case study. In this study, sample water from potable water treatment works, located in West of Flanders in Belgium is used for investigations. The DBPs is identified using a headspace-trap method. This case study concluded that the activated carbon filtration is effective to remove the HAAs and ITHMs formed during intermediate chlorination in this water treatment plant [53].
As disinfection treatment used to kill the disease-causing pathogens but after the process of chemical treatment, organic matter combined with chemicals and formed DBPs that also affects the human health. And hence, also commented through a review article that, DBPs removal is urgent need finally in this COVID – 19 situations as removal of DBPs is related to the focusing on the biological characteristics of water. The review also commented that the use post-disinfection may reduce the formation of DBPs. Secondly author concluded that use of physical treatment would be better option rather to treat water chemically for minimization of byproducts [54] discussed that the electro-disinfection has several advantages over chlorination disinfection as it is cost effective, safe and in-situ generation of chlorine reduces the risk of chlorine storage and transportation but has doubts regarding to the formation of DBPs. And hence in this study Djamel Ghernaout performed different electro-chemical setups to optimize the electrochemical treatment for minimum DBPs formation. From the investigations it concluded that the formation of perchlorates reduces when, the electrolyte separates the anode and cathode by a proton exchange membrane. The study also concluded that the employing of a Ti/IrO2 anode reduces by product formation. If electro-chemical treatment used with graphite intercalation compound (GIC) adsorbents it gives better results [55]. Developed and discussed about a novel decontamination method to regulate halogenated DBPs in potable water, also based on Anion-exchange resin adsorption followed by electrolysis process and compared with chlorination treatment. The results concluded that the new developed process might considerably reducing the levels of TOX and regulated DBPs like THMs and HAAs.
Recorded new developments in operating with DBPs formation in electrochemical devices via review article. The study determined that the use of DSA dimensionally stable anodes in electro disinfection process can neutralize microbes in water and reduces the formation of DBPs. Also, the concentrations and poisonous effects of DBPs could greatly reduce by employing granular activated carbon post-treatment.
Moreover, further research on secure multi-barrier techniques, like distillation and membrane processes, remain to be suggested, tested, and industrially encouraged. Hence there is huge scope to work further in water treatment area for DBPs removal as recently in year 2020, reviews articles commented on removal of DBPs but very few lab -scale investigations are performed [56].
The prior studies discussed above, inferred that the DBP generation can be restricted by adopting different physical methods like adsorption, Response Surface Methodology, Granular Activated Carbon, instead of chemical treatments. Minimization of DBPs, using physical treatments are applied once the carcinogenic DBPs are formed after the chemical treatments. In this process, the structure or chain of the by-product formed after the chemical treatment is breakdown, in further physical treatment. The breakdown of DBP structure or minimization of DBP after the chemical treatment is more difficult [57].
Control on NOMs in water before disinfection, confine the formation of DBPs and also it is easier than the DBP minimization after disinfection. This is also another way for removal and minimization of DBPs but for that extensive study related to the characteristics and composition of different types of NOMs is required as NOM formation in water varies according to the change in geological, environmental factors. Thus, instead of minimizing the DBPs after disinfection it will be better to restrict the NOMs present in water before disinfection once enormous data get collected on different NOMs [58] investigated a multi-objective optimization framework for minimizing the chlorine dosage to reduce DBP formation and maximizing the residual chlorine for safe and lead-free water. The study performed using multi-objective genetic optimization algorithm in an integrated MATLAB-EPANET-MSX platform.
In this study compared the removal efficiency of pollutants present in hospital wastewater for Constructed Wetlands and tube settler. The study conclude that the pollutant removal efficiency of constructed wetland is more than tube settler [59]
Conclusion
The present literature review focuses on identification, effect and minimization or removal of Disinfection By Products (DBPs) formed due to the reaction between naturally occurring organic matter in water and disinfectants. The DBPs are the by products that are formed during the process of disinfection and these by products have carcinogenic properties that also affects the human health. The long-term exposure to DBPs causes birth defects and may lead to abortion, the consumption of drinking water containing Trihalomethane may lead to DNA damage known as genotoxicity i.e., mutagenicity. This indicates an urgent need to identify, remove or reduce the DBPs concentration from treated water.
As discussed in the paper, there are methods for qualitative and quantitative detection of DBPs for easy, quick and accurate detection. The analysis of DBPs requires high end equipment that interpret close characteristics of DBPs. Detailed and extensive study regarding type and structural characteristics of substitutes present in water and their corresponding DBP formation need to investigate to have great control on DBP formation and its effects. These detection methods make it easy to work further for the removal of these by-products. Many researchers accounted their perspectives through review articles for minimization of the DBPs and commented that use of post disinfection and use of Granular Activated Carbon (GAC) method will be beneficial for DBPs minimization. Electro-disinfection method of in-situ chlorination may also minimize the DBPs concentration than chlorination treatment. Minashree and Sunil Kumar Gupta, 2019 investigated a new technology i.e., Response Surface Methodology (RSM) based on adsorption technology and successfully remove the one DBPs i.e., THMs commented that DSA dimensionally stable anodes in electro disinfection process can neutralize microbes in water and reduces the formation of DBPs. Many review articles suggested that the use of physical methods like adsorption will be better option to be incorporated after disinfection treatment to reduce the DBPs. Moreover, further research on safe multi-barrier methods, like distillation and membrane processes, remain to be suggested, tested, and industrially encouraged. Few review articles published in 2019 and 2022 focuses on DBPs removal or minimization techniques and suggests many alternative treatment systems for reduction of DBPs but further study need to be executed through a lab-scale model to derive the treatment system to be used in real scenario.
The paper additionally emphasizes on the ten most cited experiment-based research papers and the latest articles in the domain of DBP and chlorination under the review classification as identification, effects and minimization of DBPs. It can be concluded from the literature review that the research in the field of DBP’s needs further investigations on the health effects of newly identified DBPs and there is a need to study and understand the toxicity extent and future health effects of the newly identified DBPs. Few research papers concluded that water rich in brominated and iodinated characteristics lead to generate most toxic DBPs and hence more focus on checking and knowing the characteristic of water before treatment is important. The paper can be useful to know the extent of the research in field of DBP and gives clear idea to work further on areas which has not covered in the prior and present studies.
Conflict of interest
On behalf of all authors, the corresponding author states that there is no conflict of interest.
Data Availability Statement
All data collected and analyzed during the study are included in this published article
Acknowledgment
The present work is a part of the project named PAVITR funded by European Union’s Horizon 2020 research and Innovation program under grant agreement No 821410.
References
- NadeemA, Khan, Ahmed S, Husain A, Changani F (2020) Chlorination Disinfection By Products in Municipal Drinking Water a Review
- Jalil Ab, Faizal M,Hamidin N, Nagoor Gunny AAA, Kamarudzaman AN (2018) Identification of Trihalomethanes (THMs) Levels in Water Supply A Case Study in Perlis Malaysia.E3S Web of Conferences.
- Wahab A, JungB,Sivasubramanian R Batchelor B, etal. (2016) Chlorate Reduction by Dithionite UV Advanced Reduction Process International Journal of Environmental Science and Technology 14: 123-134.
- Al-otoum F, Al-ghouti MA, Ahmed TA, Abu-dieyeh M, Ali M (2016) Chemosphere Disinfection By-Products of Chlorine Dioxide Chlorite Chlorate and Trihalomethanes Occurrence in Drinking Water in Qatar. J of Chemosphere 164: 649-56.
- Alexandrou LD,Meehan BJ, Morrison PD, AH Jones O (2017) A New Method for the Fast Analysis of Trihalomethanes in Tap and Recycled Waters Using Headspace Gas Chromatography with MicroElectron Capture International Journal of Environmental Research and Public health.
- Hassan A, Hosseini MS, Mohammadi S, Naghavi-Behzad M (2019) DrinkingWater Disinfection By Products and Their Carcinogenicity a Review of an Unseen International Journal of Cancer Management.
- Barrett SE, Krasner SW, Amy GL (2000) Natural Organic Matter and Disinfection By Products Characterization and Control in Drinking Water An Overview. ACS Symposium Series761: 2–14.
- BonacquistiTP(2006)A Drinking Water Utilitys Perspective on Bromide Bromate and Toxicology.
- BondT, Goslan EH, Parsons SA, Jefferson B (2011)Treatment of Disinfection By-Product Environmental Technology 32: 1-25.
- Tom B, Mokhtar Kamal NH, Bonnisseau T, Templeton MR (2014) Disinfection By-Product Formation from the Chlorination and Chloramination of Amines. Journal of Hazardous Materials 278:288-96.
- Boucherit A, Moulay S, Ghernaout D, AlGhonamy Al, Ghernaout B, et al. (2015) New Trends in Disinfection By-Products Formation upon Water Treatment.
- Rikke B, Bahi N, Lope De Alda MJ, Farré M, Fernandez JM, et al. (2009) identification of disinfection by products of selected triazines in drinking water by lc-q-tof-ms/ms and evaluation of their toxicity.Journal of mass spectrometry 44(3):330-37.
- Cao WC, Zeng Q, Luo Y, Chen HX, Miao D et al. (2016) Blood Biomarkers of Late Pregnancy Exposureto Trihalomethanes in Drinking Water and Fetal Growth Measures and Gestational Age in a Chinese Cohort Environmental Health Perspectives 124: 536-541.
- Nhamo C, Marais S, Moyo W, Mbali M, Limakatso C, et al. (2020)Contemporary Issues on the Occurrence and Removal of Disinfection Byproducts in Drinking Water - A Journal of Environmental Chemical Engineering.
- Chen B, Zhang C, Wang L,Yang J, Sun Y (2021) Removal of Disinfection by products in drinkingwater by flexible reverse osmosis?: Efficiency comparison fates influencing factors and mechanisms.
- Min C,Chung H, Yoon J (2003) Disinfection of Water Containing Natural Organic Matter by Using Ozone-Initiated Radical Reactions. 69: 2284-2291.
- Junghoon C, Richard L Valentine (2002) Formation of N -Nitrosodimethylamine ( NDMA ) from Reaction of Monochloramine? A New Disinfection by-Product. 36:817-824.
- Wenhai C,Gao N, Yin D, SW Krasner, Templeton NR et al. (2012)TraceDetermination of 13 Haloacetamides in Drinking Water Using Liquid Chromatography TripleQuadrupole Mass Spectrometry with Atmospheric Pressure Chemical Ionization. Journal of Chromatography A 1235:178-781.
- Chu, Li D, Gao N, Templeton MR, Tan C et al. (2014)The Control of Emerging Haloacetamide DBP Precursors with UV / Persulfate Water Research.
- Clayton GE, Thorn RM, Reynolds DM (2019) Comparison of Trihalomethane FormationUsing ChlorineBased Disinfectants Within a Model System? Applications Within Point-of-Use Drinking Water Treatment.
- Deng Y, Zhou X, Shen J, Xiao G, Hong H, et al. (2021) New Methods Based on Back Propagation (BP) and Radial Basis Function (RBF) Arti Fi Cial Neural Networks for Predicting the Occurrence of Haloketones in Tap ” Sci Total Environ 10: 772.
- Dominguez-Tello A, Dominguez-Alfaro A, Gómez-Ariza JL, Arias-Borrego A, García-Barrera T (2020) “EFf ervescence- Assisted Spiral Hollow- Fi Bre Liquid-Phase Microextraction of Trihalomethanes , Halonitromethanes ,Haloacetonitriles and Haloketones in Drinking ” J Hazard Mater 5:397.
- Dubey S, Deepak G, Sharma YC, Bux F (2020) The Occurrence of Various Types of Disinfectant By-Products (Trihalomethanes, Haloacetic Acids, Halo acetonitrile) in Drinking Water. LTD.
- Bujar DH, Reka AA, Hellonb TG (2015) Disinfection of Drinking Water and Trihalomethanes?: A Review Disinfection of Drinking Water and Trihalomethanes?: A Review.(November).
- Evlampidou I, Ribera LF, Rueda DR, Lavedan EG, Costet N, et al. (2020) Trihalomethanes in Drinking Water and Bladder Cancer Burden in the European Union.” Environmental Health Perspect 128: 1-14.
- Farghaly AM, Ahmed AM, Gad AA, Hashem MA (2014) A Study for Producing Drinking Water with Safe Trihalomethanes Concentrations. 807-818.
- Feder PI, Ma ZJ, Teuschler LK, Schenck KM, Simmons JE, et al. (2009) Evaluating Sufficient Similarity for Disinfection By-Product (DBP) Mixtures: Multivariate Statistical Procedures.” J Toxicol Environ Health A 72: 468-81.
- Furst KE, Coyte RM, Wood M, Vengosh A, Mitch WA (2019) Disinfection Byproducts in Rajasthan, India: Are Trihalomethanes a Su Ffi Cient Indicator of Disinfection Byproduct Exposure in Low-Income Countries?. Environ Sci Technol 12007–12017.
- Gan W, Guo W, Mo J, He Y, Liu Y, et al. (2013) Science of the Total Environment The Occurrence of Disinfection By-Products in Municipal 670 Drinking Water in China’s Pearl River Delta and a Multipathway Cancer Risk Assessment. Sci Total Environ 447: 108-15.
- Yiqun G, Ma S, Guo X, Chen B, Jassby D 2018. AC Indirect Photolysis. Elsevier Ltd.
- Ghernaout D (2018) International Journal of Advanced and Applied Sciences Disinfection and DBPs Removal in Drinking Water Treatment?: A Perspect for a Green Tech 5:108-117.
- Djamel G, Elboughdiri N (2020) Disinfection By-Products: Presence and Elimination in Drinking Water.” OALib 07: 1-27.
- Ghernaout D, Alghamdi A, Ghernaout B (2019) Microorganisms Killing: Chemical Disinfection vs. Electrodisin app eng 3: 13-19.
- Ghernaout D, Elboughdiri N (2020) Disinfection By-Products (DBPs) Control Strategies in Electrodisinfection. OALib 7: 1-14.
- Djamel G, Elboughdiri N (2020) Foresight Look on the Disinfection By-Products Formation. OALib 7:1-17.
- Djamel G, Elboughdiri N (2020) Strategies for Reducing Disinfection By-Products Formation during Electrocoagulation.
- Djamel G, Elboughdirib N, Alghamdi A, Ghernaout B (2020) Trends in Decreasing Disinfection By-Products Formation during Electrochemical Technologies. OALib 7: 1-17.
- Michael G, Kopplin PS, Stavklint H, Susan D, Hertkorn RN, Bastviken D (2014) Changes in Dissolved Organic Matter during the Treatment Processes of a 692 Drinking Water Plant in Sweden and Formation of Previously Unknown Disinfection ” 693 Environmental Science and Technology 48: 12714-22.
- James G, Bennett J, Patelarou E, Smith RB, Toledano MB, et al. (2010) Exposure to Disinfection By-Products, Fetal Growth, and Prematurity: A Systematic Review and Meta-Analysis. Epidemiology 21: 300-313.
- https://www.canada.ca/en/health-canada/programs/consultation-organic-matter-drinking-water/document.html
- Hu Chen-yan, Si-cheng Ren, Yi-li Lin (2021) Kinetics of Diatrizoate Degradation by Ozone and the Formation of Disinfection by Products in the Sequential Chlorination.” 11: 560-571.
- Guanghui H, ReckhowDA (2007) Comparison of Disinfection Byproduct Formation from Chorine and Alternative Disinfectants. 41:1667-1678.
- Guang H, Jiang P, Xing-Fang Li (2017) Mass Spectrometry Identification ofN-Chlorinated Dipeptides in Drinking Water. Analytical Chemistry 89: 4204-4209.
- Iarc (1991) IARC Monographs on the Evaluation of Carcinogenic Risks to Humans-Volume 52 - Chlorinated Drinking-Water; Chlorination by Products; Some Other Halogenated Compounds; Cobalt and Cobalt Compounds.Evaluation 553.
- Jiang J, Jiarui H, Xiangru Z (2020) Nonhalogenated Aromatic DBPs in Drinking Water Chlorination: A Gap between NOM and Halogenated Aromatic DBPs. Environmental Science and Technology 54: 1646-1656.
- Jung YJ, Kang JW, Page MA, Phillips MJ, Mariñas BJ, et al. (2007) Control of Disinfection andHalogenated Disinfection Byproducts by the Electrochemical Water Science and Technology 5 55: 213-219.
- Khan Nadeem A, Viola V, Sergij V, Borys B, Mika S, et al. (2021) HospitalEffluent Guidelines and Legislation Scenario around the Globe?: A Critical Rev Journl of Environmental Chemical Engineering 9: 105874.
- Kitis M, Karanfil T, Kilduff JE (2004) The Reactivity of Dissolved Organic Matter for Disinfection By-Product Formation. Turkish Journal of Engineering and Environmental Sciences 28:167-179.
- Minashree K, Kumar SG 2019 Response Surface Methodological (RSM) Approach for Optimizingthe Removal of Trihalomethanes (THMs) and Its Precursor’s by Surfactant Modified Magneic Nanoadsorbents (SMNP) - An Endeavor to Diminish Probable Cancer Risk. Scientific Reports 9: 111.
- AysegulL (2003) Indoor and Built Environment Formation of Trihalomethanes by the Disinfection of Drinking Water 12: 1.
- Chunmei L, Wang D, Li N, Luo Q, Xu X, et al. (2016) Identifying Unknown By-Products in Drinking Water Using Comprehensive Two-Dimensional Gas Chromatograph Quadrupole Mass Spectrometry and in Silico Toxicity Assessment. Chemosphee 163: 535-543.
- Liu C, Yan-LingDengab1Xiao-QiongYuancPan-PanChenabYuMiaoab et al. (2022) Exposure to disinfection by-products and reproductive hormones among women: Results fromthe Tongji Reproductive and Environmental (TREE) study. Environmental Research 209: 112863.
- Liang JK, Song ZM, Wu QY, Hu HY, et al. (2022) Effects of chlorine dose on the composition and characteristics of chlorinated disinfection by products in reclaimed water. Science of the Total Environment 82410: 153739.
- Liang L, Singer PC (2003) Factors Influencing the Formationand Relative Distribution of Haloacetic Acids and Trihalomethanes in Drinking Water. 37: 2920-2928.
- Lin, Qiufeng, Feilong Dong, Cong Li, and Junkui Cui (2021) Disinfection byproduct Formation from Algal Organic Matters after Ozonation or Ozone Combined with Activated Carbon Treatment with Subsequent Chlorination. Journal of Environmental Sciences 104: 233-241.
- Lin Q, Dong F, Miao Y, Li C (2020) Removal of Disinfection By-Products and Their Precursors during Drinking Water Treatment Processes. Water Environ Res 92: 698-705.
- Liu X, Li C, Yang M, Tan C, Chu W (2020) The Occurrence, Characteristics, Transformation and Control of Aromatic Disinfection by Products: A Water Research 184:116076.
- Liu Z, Ye T, Xu B, Tian-yang Z, Li M, et al. (2022) Formation and Control of Organic Chloramines and Disinfection By-Products during the Degradation of Pyrimidines and Purines by UV / Chlorine Process in Water. Chemosphere 286: 131747.
- Lopez-prieto IJ, Park M, Azadiaghdam M, Pan H, Sara LJ, et al. (2021) Formation and Control of Disinfection By-Products from Iodinated Contrast Media Attenuation through Sequential Treatment Processes of Ozone-Low Pressure Ultraviolet Light Followed by Chemosphere 278:130394.
Citation: Apte S, Shinde SR, Khare K. (2022) Analyses of the Agricultural Convergence towards Environmental Conditions. J Environ Sci Curr Res 5: 035.
Copyright: © 2022 Sayali Apte, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.

