
Respiratory Complications in Patients with Ehlers-Danlos Syndrome: A Systematic Review
*Corresponding Author(s):
Christina ParducciNYIT College Of Osteopathic Medicine, New York, United States
Tel:+ 8454909245,
Email:cparducc@nyit.edu
Abstract
The Ehlers-Danlos Syndromes (EDS) are genetic connective tissue disorders that are currently categorized into 14 subtypes. Symptoms of each subtype overlap, with some distinct manifestations. The majority of EDS cases are either hypermobile, vascular, or classic. The pulmonary issues of this syndrome are widely under-researched despite the significant impact these symptoms have on the EDS patients’ prognosis and mortality. The purpose of this study was to conduct a qualitative systematic review of the effects of the respiratory complications seen in EDS. This study did not limit the scope of the subtypes investigated. An electronic search was conducted on PubMed and OVID. Publication dates were limited to the last 10 years and non-English abstracts were excluded. Two reviewers conducted a literature search which yielded a total of 25 records. After the application of our eligibility criteria, 10 manuscripts were included in this systematic review. One manuscript was obtained for inclusion by manually checking references. This systematic review demonstrated the significance of recurrent hemoptysis as a presenting symptom of EDS, specifically vascular EDS. Recurrent hemoptysis was shown to have a strong relationship to the development of a pneumothorax, especially when patients were not diagnosed with EDS until a histological examination of lung tissue was performed. Obstructive sleep apnea was also found to be another major aspect of EDS that may affect the patient’s quality of life. Overall, EDS is a systemic disease and thus, multi-organ management should be considered. Depending on the presenting symptoms and severity of the disease, recommendations for interventions in patients with respiratory complications varied.
Introduction
Ehlers-Danlos Syndrome is an inherited connective tissue disorder that is a result of mutations in the genes that code for collagen synthesis. EDS was first described in 400 BC by Hippocrates but it was not until the early 1900’s that Edvard Ehlers and Henri-Alexandre Danlos realized it was a distinct pathology with symptoms that were often misinterpreted [1]. The two dermatologists began to recognize those afflicted with EDS based on their physical presenting signs such as hyperextensibility and inflammatory skin lesions [1]. Since then, the significance of EDS has been shown to be more than skin deep, with issues ranging from cardiovascular to gastrointestinal. Classification of EDS continues to this day as more research is devoted to the syndrome with a 14th rare subtype being found in 2018 [2]. Heritability varies with each subtype as do clinical symptoms. The majority of the subtypes can be recognized through pedigrees, showing an autosomal dominant or recessive pattern yet there are cases of de novo mutations in those with no familial history [3]. Not only is heritability variable but so are the signs, symptoms, and severity in any given patient.
The diagnosis of these syndromes relies almost entirely on a physician’s clinical diagnostic skills thus making recognition of the syndrome heavily dependent on education and experience. This diagnostic dilemma has made it increasingly difficult for patients with such a rare disease to get a swift diagnosis and treatment [4]. Though there is no known cure, there are numerous treatment options and standards of care to guide a clinician’s management of these patients [5]. Research has shown that patient education can drastically delay the course of the syndrome and decrease patient morbidity [6]. We hypothesized that some of the most fatal threats to these patients are detectable with appropriate education. We further believe that a significant fraction of the complications that these patients face is due to respiratory issues go unnoticed. This is a compound issue that has grown to be problematic not only due to the rarity of the syndrome but the under-researched topic of their respiratory complications and the effect they have on mortality.
Methods
The purpose of this systematic review was to identify current literature that described respiratory complications in Ehlers-Danlos patients. The electronic search strategy was completed in December 2020.PubMed and OVID search engines were used to search the National Library of Medicine’s MEDLINE database. The Pubmed search criteria used were “Ehlers-Danlos Syndrome” and “respiratory complications” using the AND function. The OVID search criteria used were “Ehlers-Danlos Syndrome” and “respiratory complications'' using the AND function. The inclusion of related terms function in the search was disabled. Publication dates were limited to the last 10 years.
Our predetermined search strategy yielded twenty-five manuscripts. Reasons for exclusion based on search criteria are further demonstrated in figure 1. Two duplicates were removed from our initial search leaving twenty-three abstracts to be screened. The abstract screening was conducted by two reviewers. One non-English paper was removed based on our abstract eligibility criteria. Full manuscripts were then reviewed by the same two reviewers who applied a manuscript eligibility criterion. Articles that focused on a connective tissue disorder or specialty were not considered. Letters to the editor were also excluded. After application of this criterion, eight manuscripts were eliminated leaving a total of ten manuscripts from our initial search that had adequate data for this systematic review. One additional manuscript was obtained for inclusion by manually checking references. These results are displayed in figure 1.
Quality assessment of case reports and review articles was performed by two reviewers according to the Joanna Briggs Institute. Each study was classified into the following groups: Low risk of bias if all quality criteria were judged as “present”, moderate risk of bias if one or more key domains were “unclear” and high risk of bias if one or more key domains were “absent”. The same criteria were applied to research articles using the National Institute of Health’s Study Quality Assessment tools. These results are displayed in table 1.
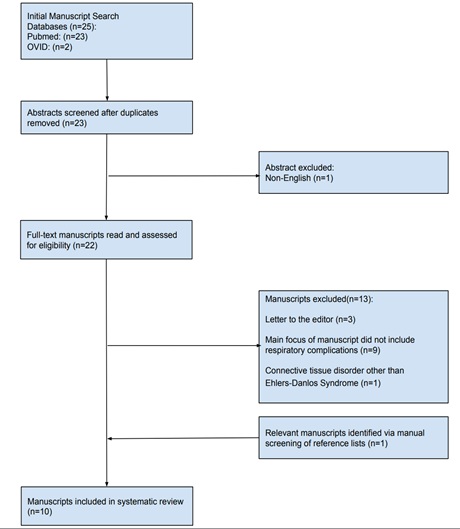 Figure 1: Flow chart of the study selection process.
Figure 1: Flow chart of the study selection process.
Results
Hematoma
Data revealed one study that focused on hematomas in patients with EDS. Nine patients were included due to pulmonary complaints including hemoptysis and recurrent pneumothoraces [7]. Only one had been diagnosed with vEDS before examination. Histological examination of lung tissue revealed several changes related to the fragility of tissue caused by vEDS. Hematomas varied in size, the largest being 50mm, and in composition. Some were surrounded by active bleeding; others had cavities or collagen deposition. Fibrous nodules were also examined and revealed ossification or bone marrow formation. Emphysematous changes were seen in eight of the nine patients. Ossification, as well as emphysematous complications, were seen in several other studies [7,8].
Hemoptysis
Though it was discussed as a presenting symptom of vEDS in many papers, two case reports focused on hemoptysis as the main point of interest[9]. One case report discussed a male who presented with recurrent hemoptysis at 16 years old and was diagnosed with EDS. The patient died at 20 years old after going into cardiopulmonary arrest. An autopsy revealed red circular lesions on the surface of both lungs and areas of parenchymal hemorrhage. Histological examination revealed old thrombi and fibrous nodules surrounding metaplastic bone. Similarly, another case report found an 18-year-old female with hemoptysis of 6 months developing a pneumothorax. CT scan showed focal emphysema, organized hemorrhage, and dense fibrous nodule containing ossifications. She was diagnosed with vEDS and responded well to tranexamic acid [8].
Obstructive sleep apnea
Our search yielded two manuscripts on Obstructive Sleep Apnea (OSA). A parallel cohort study was conducted on adults that proposed the harmful effects of OSA, particularly cardiovascular complications, as seen in the well-studied Marfan's population would be similar in the EDS population [10]. The study included 100 participants in a control group that was matched to an equal number of EDS counterparts based on age, sex, height, and weight. The participants were mostly Caucasian (98.5%) and female (82%). Beighton’s score did not correlate with OSA severity. The study projects that 25% of patients with EDS have OSA, regardless of BMI, and meet the criteria for traditional treatment. Results were significant for OSA contributing to daytime sleepiness, higher resting heart rates and lower quality of life. Another study considered OSA in EDS children and was performed before the 2017 guidelines so the naming reflects this [11]. This prospective project, consisting mainly of hypermobile type (III) females and Caucasians, was designed as a case-control study that one-to-one matched children and teens with EDS to healthy controls. Significant findings showed obstructive sleep apnea and hypopnea were more common in the EDS group whilst the frequency of central sleep apneas was similar between groups. Furthermore, the higher a child's obstructive apnea-hypopnea index, which is used as a diagnostic tool for OSA, the lower they scored their perception of their general health. Daytime sleepiness is also negatively correlated with general health perception. Potential predictors of OSA development, such as craniofacial abnormalities due to EDS, were investigated yet no relationship was found.
Pneumothorax
Our search revealed a variety of research on pneumothoraces. One paper focused on the genetics of developing a spontaneous pneumothorax, focusing on three main causes: connective tissue disorders, abnormal lung architecture, and mutations in tumor suppressor genes [12]. Vascular EDS was highlighted as having an autosomal dominant inheritance pattern with pulmonary complications including cavitary lesions, cysts, ossified fibrous nodules and hemopneumothoraces. EDS causes a mutation in COL3A1 leading to tissue integrity issues along with impaired vessel repair which is theorized to lead to fibrous pseudotumors and pulmonary vasculature injury. The manuscript reveals that vEDS is the only subtype that leads to pneumothorax and may be the first presenting symptom of the syndrome. Most patients were diagnosed genetically (80%) vs clinically (16%). This manuscript provides an algorithm for practitioners to use based on their examination; it guides their plan on when to refer to a geneticist and how to counsel the patient. Standard treatment for pneumothoraces has been successful.There is still debate on if management should include CT imaging for first-time pneumothoraces though official guidelines are against it. The manuscript reveals that vEDS is the only subtype that leads to pneumothorax and may be the first presenting symptom of the syndrome.
Another manuscript from our search revealed the first case of bilateral pneumothorax in a vEDS patient [13].A 16-year-old male with a history of colonic perforation and atopic dermatitis presented with chest pain. Physical examination showed no hyperextensibility, a normal respiratory exam, and a normal ophthalmological examination. The right axilla showed internal bleeding. Chest x-ray revealed bilateral apical free air spaces, diffuse ground-glass opacities, and pleural effusions. Catheterization of the intrathoracic space revealed a bloody pleural effusion confirming suspensions of intrapulmonary hemorrhage. The patient was treated and discharged in stable condition. Clinicians suspected EDS due to the patient's history of pneumothorax, hemorrhage, and colonic perforation. Mutation analysis revealed COL3A1 a point mutation and cultured skin fibroblasts showed a type III collagen alpha 1 production of 12.3% of normal. The patient was subsequently diagnosed with EDS. Similarly, another case report revealed a 24-year-old male with a history of recurrent pneumothoraces and bloody sputum [14]. Apical bullae were discovered during his first pneumothorax hospitalization, and subsequent nodules were discovered during repeated visits for pneumothoraces in the following months. Biochemical analysis of skin biopsy confirmed a diagnosis of EDS. Total Pleural Covering technique (TPC) was used as a less invasive approach due to the increased friability of pleura tissue secondary to EDS. Regenerated oxidized cellulose mesh sheets were used to overlay the visceral pleura while fat pads secured with fibrin glue were chosen over sutures to repair defects. Studies suggest that this technique can decrease the recurrence of pneumothoraces via pleural thickening. This was the first report of TPC being used in a patient with vEDS [14].
Lung Transplantation
One manuscript discovered in our search revealed the first case of a patient with COPD secondary to EDS that received a bilateral lung transplant [15]. A 29-year-old woman with vEDS experienced hypermobility problems since the age of 5 and was diagnosed with early-onset emphysema at 12 years old. At 26 years of age, she was referred to a geneticist based on chest CT showing extensive emphysema. The patient was diagnosed with vEDS based on molecular and biochemical analysis. She quickly deteriorated over a year while on the lung transplant list and required venovenous extracorporeal membrane oxygenation to bridge transplantation [15]. Disseminated intravascular coagulopathy occurred after 3 days of support and required transfusion [15]. Lung transplantation was successful after prolonged weaning and almost 50 days of hospitalization [15]. Histopathology of patients’ lungs showed a scarcity of elastin in the alveolar walls, hemosiderin deposits, and areas of hemorrhage [15].
Aspiration
One case report found that the management of a concurrent EDS issue, laxatives for constipation, led to the development of ARDS-like exogenous lipoid pneumonia due to neurological decline [16]. A 58-year-old male with EDS had presented to the RICU with rapid onset of ARDs with a history of cognitive and motor deficits in the days prior. Furthermore, the patient had a partially thrombosed aneurysm in the basilar artery causing dysphagia [16]. All of these factors lead to a small amount of petroleum jelly that was only being used for a week, to be aspirated and caused a life-threatening lipoid pneumonia [16].
Quality assessment
After applying our validated quality assessment tools, we found 7 manuscripts to have a low risk of bias, 1 to have a moderate risk, and 2 to have a high risk. These results are displayed in table 1.
|
Publication Year |
Title |
Conclusion |
Specific Respiratory Complications |
Citation |
Bias Risk |
|
2010 |
Pleuropulmonary pathology of vascular Ehlers-Danlos syndrome: Spontaneous laceration, hematoma and fibrous nodules |
Spontaneous laceration to lung tissue in vEDS patients is a key feature of pulmonary complications leading to hematomas, hemorrhaging, fibrous nodules, emphysema blebs and organizing pneumonia. |
-Hematoma -Emphysema -Fibrous Nodules with ossification |
Kawabata Y, Watanabe A, Yamaguchi S, Aoshima M, Shiraki A, Hatamochi A, Kawamura T, Uchiyama T, Watanabe A, Fukuda Y. Pleuropulmonary pathology of vascular Ehlers-Danlos syndrome: Spontaneous laceration, haematoma and fibrous nodules. Histopathology. 2010 Jun; 56 (7):944-50. doi: 10.1111/j.1365-2559.2010.03574.x.Epub 2010 May 24. PMID: 205002297. |
Low |
|
2018 |
Pulmonary Fibrous Nodule with Ossifications May Indicate Vascular Ehlers-Danlos Syndrome with Missense Mutation in COL3A1 |
18 year old female presented with 6 months of hemoptysis and resulted in the development of a pneumothorax, leading to EDS diagnosis. The patient responded to tranexamic acid for hemoptysis. |
-Hemoptysis -Pneumothorax - Emphysema -Fibrous nodules with ossification |
Berezowska S, Christe A, Bartholdi D, Koch M, von Garnier C. Pulmonary Fibrous Nodule with Ossifications May Indicate Vascular Ehlers-Danlos Syndrome with Missense Mutation in COL3A1. Am J Respir Crit Care Med. 2018 Mar 1;197(5):661-662. DOI: 10.1164/rccm.201709-1963IM. PMID: 29323927. |
Low |
|
2012 |
Respiratory complications of Ehlers-Danlos syndrome type IV |
Pulmonary hemorrhage due to EDS caused the sudden death of a 20-year-old male. EDS is important to consider as a possible cause of death in those with arterial rupture. |
-Hemoptysis -Pulmonary hemorrhage -Pneumothorax -Ossified nodules |
Hatake K, Morimura Y, Kudo R, Kawashima W, Kasuda S, Kuniyasu H. Respiratory complications of Ehlers-Danlos syndrome type IV. Leg Med (Tokyo). 2013 Jan;15(1):23-7. DOI: 10.1016/j.legalmed.2012.07.005. Epub 2012 Aug 30. PMID: 22940417. |
Low |
|
2017 |
Obstructive sleep apnoea and quality of life in Ehlers-Danlos syndrome: a parallel cohort study |
Obstructive sleep apnea is a relevant complication of Ehlers-Danlos syndrome and should be considered as a contributing factor in patient symptoms such as fatigue. |
-Obstructive sleep apnea -Hypopneic events |
Gaisl T, Giunta C, Bratton DJ, Sutherland K, Schlatzer C, Sievi N, Franzen D, Cistulli PA, Rohrbach M, Kohler M. Obstructive sleep apnoea and quality of life in Ehlers-Danlos syndrome: a parallel cohort study. Thorax. 2017 Aug; 72(8):729-735. doi: 10.1136/thoraxjnl-2016-209560. Epub 2017 Jan 10. PMID: 28073822. |
Low |
|
2018 |
Obstructive Sleep Apnoea in Children and Adolescents with Ehlers-Danlos Syndrome |
The frequency of obstructive sleep apnea in EDS children has been underestimated. It significantly increases disease burden including but not limited to chronic fatigue, quality of life, and pain symptoms. |
-Obstructive sleep apnea -Hypopneic events |
Stöberl AS, Gaisl T, Giunta C, Sievi NA, Singer F, Möller A, Rohrbach M, Kohler M. Obstructive Sleep Apnoea in Children and Adolescents with Ehlers-Danlos Syndrome. Respiration. 2019; 97(4):284-291. doi: 10.1159/000494328. Epub 2018 Nov 28. PMID: 30485858. |
Moderate |
|
2019 |
The Genetics of Pneumothorax |
Pneumothorax is only seen in vascular EDS and can be a presenting symptom. It is treated with standard protocol. Provides an algorithm to guide practitioners on management. |
-Pneumothorax -Cavitary lesions -Ossified nodules -Cysts / blebs / bullae |
Boone PM, Scott RM, Marciniak SJ, Henske EP, Raby BA. The Genetics of Pneumothorax. Am J Respir Crit Care Med. 2019 Jun 1; 199(11):1344-1357. DOI: 10.1164/rccm.201807-1212CI. PMID: 30681372; PMCID: PMC6543724. |
High |
|
2015 |
Ehlers-Danlos Syndrome Type IV with Bilateral Pneumothorax |
First case report of bilateral pneumothorax due to vascular EDS. |
-Bilateral pneumothorax - Calcified nodules -Ground glass opacities -Intrapulmonary hemorrhage - Pleural effusion |
Nakagawa H, Wada H, Hajiro T, Nagao T, Ogawa E, Hatamochi A, Tanaka T, Nakano Y. Ehlers-Danlos Syndrome Type IV with Bilateral Pneumothorax. Intern Med. 2015;54(24):3181-4. DOI: 10.2169/internalmedicine.54.4947. Epub 2015 Dec 15. PMID: 26666608. |
Low |
|
2014 |
Total pleural covering technique for intractable pneumothorax in a patient with Ehlers-Danlos syndrome |
The total pleural covering technique is beneficial for vascular EDS patients with recurrent pneumothorax. |
-Pneumothorax -Hemo-sputum |
Kadota Y, Fukui E, Kitahara N, Okura E, Ohta M. Total pleural covering technique for intractable pneumothorax in a patient with Ehlers-Danlos syndrome. Gen Thorac Cardiovasc Surg. 2016 Jul; 64(7):425-8. DOI: 10.1007/s11748-014-0504-9. Epub 2014 Dec 16. PMID: 25512090. |
Low |
|
2014 |
Bilateral lung transplantation in a patient with Vascular Ehlers-Danlos syndrome |
Extracorporeal Life Support (ECLS) technology has been shown to improve outcomes in patients with end-stage lung disease as a bridge to lung transplantation. |
-Chronic obstructive pulmonary disease -Respiratory arrest -Lung failure |
García Sáez D, Mohite PN, Zych B, Sabashnikov A, Moza A, Carby M, Simon AR. Bilateral lung transplantation in a patient with Vascular Ehlers-Danlos syndrome. Ann Thorac Surg. 2014 May; 97(5):1804-6. DOI: 10.1016/j.athoracsur.2013.07.132. PMID: 24792277. |
Low |
|
2016 |
When Acute Respiratory Distress Syndrome is not ARDS |
Low amounts of oil-based laxatives and neurological impairment as seen in progressive EDS increase ARDS likelihood. |
-ARDS-like exogenous lipoid pneumonia |
Tonelli, R., Andreani, A., Castaniere, I., et al. When Acute Respiratory Distress Syndrome is not ARDS. Lung 194, 865-866 (2016). https://doi.org/10.1007/s00408-016-9917-9 |
High |
Table 1: Inclusion Chart of Search: This chart includes a complete list of the manuscripts that made it through our selection process.
Discussion
This is the first systematic review to look at the pulmonary complications associated with EDS. The majority of respiratory cases involved EDS patients with the vascular subtype, with one paper asserting that only those with vEDS will experience pneumothoraces [12]. A diagnosis of EDS was often overlooked in those with respiratory issues until the histological examination was considered. This was seen in multiple manuscripts that discussed the ossification of fibrous nodules, also known as pseudotumors. Pseudotumors are defined by an inefficient repair mechanism in patients with EDS and have been seen in patients as young as 5 months old [8]. The causality of pseudotumors in the lung parenchyma was not clearly defined, though it was postulated to be due to the dysfunction of tissue repair seen in EDS. Looking at literature outside the scope of EDS, patients that have diseases that predispose them to form ossified pulmonary nodules also have the propensity to develop pneumothoraces. Research has shown a possible relationship between pneumothorax and pseudotumors. One study demonstrated that subjects with Diffuse Pulmonary Ossification (DPO), a rare disease where patients develop bilateral ossifications, often have recurrent pneumothoraces [17]. There has been little research done on EDS and DPO as both are rare diseases. The pathophysiology of the pseudotumors and their relationship with recurrent pneumothoraces among patients with EDS warrants further investigation. This would be specifically relevant to the management of the vascular subtype which our review demonstrated has the most likely occurrence of these two comorbidities.
Obstructive sleep apnea was linked to debilitating symptoms of EDS such as fatigue, generalized pain and lower quality of life. It is thought that the reason OSA is seen in the hypermobile population is because of increased nasal resistance [11,18]. It is a highly prevalent comorbidity that is often underestimated in this community, particularly in children [11]. A recent paper stated that the prevalence of OSA among people with EDS is 39%. In addition, it claimed that “those with EDS or [Marfan syndrome] are six times more likely to have OSA than those without a hypermobility disorder” [18]. Physicians that are not experts on the hypermobile population should be aware of the peripheral and central risks of OSA despite the thin frame that often accompanies these patients. Further studies on the effects of OSA and if traditional treatments are effective in children should be conducted.
The population that is studied for EDS is limited. The majority of clinical research studies had predominantly females and Caucasians. Furthermore, there are few clinical studies done on this population and most of our search yielded case reports. The few clinical trials had a small sample size while case reports often included one to two subjects. This is understandable as EDS is extremely rare and the ability to conduct such studies is limited.
Multidisciplinary management was encouraged in some studies to promote earlier diagnoses of patients. Geneticists and radiologists proved vital to the discovery of EDS and its management. For instance, the treatment of pneumothorax in patients with EDS is the same as in a healthy patient. Yet management of pneumothorax imaging has been debated. A study proposed that a first-time pneumothorax should entail a CT scan as it can detect the diffuse cystic lung changes seen in EDS and may lead to early diagnosis and is a cost-effective way of possibly preventing future hospitalizations [12]. Current guidelines on pneumothorax protocol are against this type of management.
Limitations
Though the majority of our search yielded results with a low risk of bias, our search may have been limited by search terms. Respiratory complications were chosen to include the entire respiratory tract and we did not want to be limited to just the lungs by choosing a more restrictive word such as pulmonary. Including a greater number of search words or more diverse terms may have improved our results. Furthermore, we were limited in our search engine use. OVID yielded the same results as PubMed so no contribution was made by using OVID as an additional search engine.
Conclusion
Though rare, there are a variety of respiratory complications associated with EDS. These should be considered along with a further awareness about Ehlers-Danlos presentation such as hypermobility, predispositions to bleeding, and tissue fragility. More uncommon symptoms of EDS such as those discussed in our systematic review should also be taken into consideration when creating a differential. Education of general practicing physicians and clinical research that may be more accessible through experts could help shed more awareness on the syndrome. Ehlers-Danlos is a multi-system disease that requires a team of doctors to manage. Precautions must be taken in the management of these patients and prompt diagnosis is of the utmost importance.
Conflicts
No funding or conflict of interest.
References
- Parapia LA, Jackson C (2008) Ehlers-Danlos syndrome - a historical review. British Journal of Haematology141: 32-35.
- Blackburn PR, Xu Z,Tumelty KE, Zhao RW, Monis WJ, et al. (2018) Bi-allelic Alterations in AEBP1 Lead to Defective Collagen Assembly and Connective Tissue Structure Resulting in a Variant of Ehlers-Danlos Syndrome. Am J Hum Genet 102: 696-705.
- Malfait F, Francomano C, Byers P, Belmont J, Berglund B, et al. (2017) The 2017 international classification of the Ehlers-Danlos syndromes. Am J Med Genet C Semin Med Genet175:8-26.
- Grahame R (2001) Time to take hypermobility seriously (in adults and children). Rheumatology 5: 485-487.
- Riley B (2020) The many facets of hypermobile ehlers-danlos syndrome. J Am Osteopath Assoc120:30-32.
- Miklovic T, Sieg VC (2020) Ehlers Danlos Syndrome. StatPearls.
- Sáez DG, Mohite PN, Zych B, Sabashnikov A, Moza A, et al. (2014) Bilateral lung transplantation in a patient with Vascular Ehlers-Danlos syndrome. Ann Thorac Surg 97: 1804-1806.
- Hatake K, Morimura Y, Kudo R, Kawashima W, Kasuda S (2013) Respiratory complications of Ehlers-Danlos syndrome type IV. Leg Med (Tokyo) 15: 23-27.
- Berezowska S, Christe A, Bartholdi D, Koch M, von Garnier C (2018) Pulmonary fibrous nodule with ossifications may indicate vascular ehlers-danlos syndrome with missense mutation in COL3A1. Am J Respir Crit Care Med 197: 661-662.
- Gaisl T, Giunta C, Bratton DJ, Sutherland K, Schlatzer C, et al. (2017) Obstructive sleep apnoea and quality of life in Ehlers-Danlos syndrome: A parallel cohort study. Thorax 72: 729-735.
- Stöberl AS, Gaisl T, Giunta C, Sievi NA, Singer F, et al. (2017) Obstructive sleep apnoea in children and adolescents with ehlers-danlos syndrome. Respiration 97: 284-291.
- Boone PM, Scott RM, Marciniak SJ, Henske EP, Raby BA (2019) The Genetics of Pneumothorax. Am J Respir Crit Care Med 199: 1344-1357.
- Nakagawa H, Wada H, Hajiro T, Nagao T, Ogawa E, et al. (2015) Ehlers-danlos syndrome type IV with bilateral pneumothorax. Intern Med 54: 3181-3184.
- Kadota Y, Fukui E, Kitahara N, Okura E, Ohta M (2016) Total pleural covering technique for intractable pneumothorax in patient with Ehlers-Danlos syndrome. General Thoracic and Cardiovascular Surgery 64: 425-428.
- GSáez DG, Mohite PN, Zych B, Sabashnikov A, Moza A, et al. (2014) Bilateral lung transplantation in a patient with Vascular Ehlers-Danlos syndrome. Ann Thorac Surg 97: 1804-1806.
- Tonelli R, Andreani A, Castaniere I, Fantini R, Mengoli C, et al. (2016) When acute respiratory distress syndrome is not ARDS. Lung 194: 865-866.
- Gao Y, Egan AM, Moua T (2020) Dendri form pulmonary ossification complicated by recurrent spontaneous pneumothorax: Two case reports and a review of the literature. Respir Med Case Rep 23: 101067.
- Sedky K, Gaisl T, Bennett DS (2019) Prevalence of obstructive sleep apnea in joint hypermobility syndrome: A systematic review and meta-analysis. J Clin Sleep Med 15: 293-299.
Citation: Parducci C, Edimo C, Hasan S, Wong S, Riley B (2021) Respiratory Complications in Patients with Ehlers-Danlos Syndrome: A Systematic Review. J Pulm Med Respir Res 7: 066.
Copyright: © 2021 Christina Parducci, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.

