
Results of Selective Intra-Arterial Infusions of Mesenchymal Stem Cells in Two Children with Rasmussen Encephalitis
*Corresponding Author(s):
Verónica Cantarín-ExtremeraNeuropediatrics, Hospital Universitario Niño Jesús, Ave, Menéndez Pelayo, 65.28009, Madrid; Member Of The Clinical Group Linked (GCV14/ER/6) To The Networked Biomedical Research Centre For Rare Diseases (CIBERER), Carlos III Health Institute, Madrid, Spain
Tel:+34 915035900481,
Fax:+34 915744669
Email:veronica.cantarin@salud.madrid.org / verocantarin@hotmail.com
Abstract
Aim: Rasmussen’s Encephalitis (RE) is a devastating pediatric syndrome. Patients receive different therapies, and eventually undergo functional hemispherectomy as the only treatment to achieve complete control of epileptic seizures. New therapies are needed to improve the prognosis of this rare disease. Since cytotoxic T lymphocytes have an active role in the pathogenic process of RE, we evaluated the feasibility of Mesenchymal Stem Cells (MSCs) for patients with RE based on the known immunomodulatory potential of these cells.
Methods: We present two patients with RE and refractory epilepsy. They were treated with antiepileptic drugs, steroids, immunoglobulins and plasmapheresis, but the epilepsy and cognitive declination did not stop. Both patients underwent a video-electroencephalographic record, complete laboratory tests, seizures diary and a neuropsychological testing prior to the first administration of MSC and after that. Bone-marrow-derived MSCs were culture expanded and prepared to study of the axis CXCR3- CXC-chemokine ligand 10 (CXCL10) in MSCs chemotaxis. After confirming fulfillment the situation of the patients, 4 doses of MSCs, 30×106 cells each were administered targeting the affected hemisphere by selective intra-arterial infusion.
Results: The administrations were well-tolerated, and no adverse effects were seen after each infusion. A decrease in the frequency of generalized and focal seizure episodes was found in the first 2 weeks after each infusion. A significative improvement in Conners Continuous Performance Test was documented after the first infusion too.
Conclusion: Though preliminary, this strategy appears to be safe and feasible in patients with RE. Given the transient improvement provided by short-lived MSCs, it could be a treatment option in acute deteriorations such a status epilepticus or repetitive seizures
Keywords
Cellular therapy; Neuroinflammation; Rasmussen Encephalitis; Refractory epilepsy
INTRODUCTION
Rasmussen encephalitis (RE) is a rare devastating childhood disease characterized by intractable epilepsy, epilepsia partialis continua, progressive hemiparesis, and unilateral hemispheric atrophy with a clear cognitive deterioration that may appear before the onset of neurological symptoms. The progression of the symptoms to significant neurological impairment usually occurs within months to a few years. RE causes are unknown and no standardized medical treatment protocol currently exists for patients. Antiepileptic Drugs (AED) are not effective and despite data supporting a beneficial effect of early immunosuppressive and immunomodulatory interventions, surgery with hemispheric disconnection is the treatment of choice to try to avoid mental retardation, dementia, and death [1-3]. Therefore, new therapies are needed to improve the prognosis of this rare disease.
MSCs are a multipotent, non-hematopoietic class of progenitor cell that can be isolated from a variety of tissues, and are been investigated as a potential alternative therapeutic approach in degenerative and inflammatory disorders such as Crohn’s disease, Graft-versus-Host Disease (GvHD), Systemic Lupus Erythematosus (SLE), multiple sclerosis, fibrosis and arthritis [4,5]. The rationale for using MSCs in chronic intractable diseases of the Central Nervous System (CNS) partly relies on their ability to dampen inflammation, inhibit pathogenic immune responses, and release neuroprotective factors. Preclinical data show that MSC trophic properties and bioactive substances seem to effectively suppress neuroinflammation, decrease local lesions, and reduce the symptoms of neurological functional deficits and may slow or halt the development of irreversible disabilities [6-10].
Cytotoxic T lymphocytes have an active role in the pathogenic process of RE [11]. We have recently reported that the CXCR3-CXCL10 axis has a role in recruiting pathogenic T lymphocytes into the brains of these patients [12]. Based on their immunomodulatory properties and ability to respond to chemokine gradients, we have evaluated the feasibility of MSCs for patients with RE.
PATIENT AND METHODS
Clinical cases
Patient 1
A 12 years-old female was referred with the diagnosis of RE by Electroencephalogram (EEG), clinical and radiological criteria. She started two years earlier with partial seizures and was diagnosed of temporal lobe epilepsy. Seizures were not controlled with AEDs and steroids. Epilepsia partialis continua with focal to bilateral tonic-clonic episodes followed, developing light cognitive declination (Figure 1 (continuous line)). She showed an intellectual level below the mean (IQ = 70), with slight difficulties, and without significant discrepancies in all intellectual indices: Verbal Comprehension (VCI = 82), Perceptual Reasoning (PRI = 71), Working Memory (WMI = 75) and Processing Speed (PSI = 79). Alterations in some non-verbal skills (line orientation, nonverbal reasoning) and verbal tasks (recognition vocabulary, verbal comprehension and Word fluency / semantic association) were recorded. Alterations were also recorded in verbal and visual mnesic capacities (more acute the visual), in attentional processes and cognitive executive functions. In summary, both alterations associated with the functioning of the dominant hemisphere for language and non-dominant were recorded. The patient was left-handed, a Wada test showed complete dominance for the language of the left hemisphere. She suffered a left hemiparesis in the context of generalized epileptic seizures too. Monthly cycles of immunoglobulin and daily steroids were added to her pharmacological therapy with no success, the patient continued with focal and generalized secondary seizures daily or every other day. Based on the capacity of MSCs to modulate T-cell mediated autoimmune diseases, we proposed the use of autologous marrow-derived MSCs, targeting the affected hemisphere by selective intra-arterial infusion.
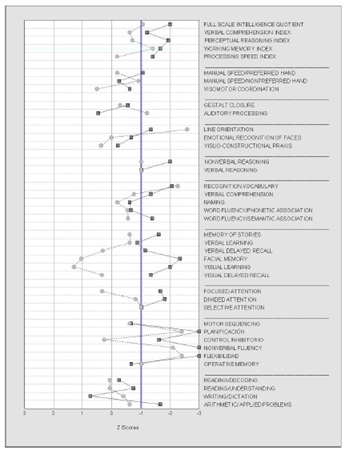 Figure 1: Neuropsychological profile from our patients expressed in Z scores. Scores below -1 are considered as deficit (Patient 1 continuous line; Patient 2 dashed line).
Figure 1: Neuropsychological profile from our patients expressed in Z scores. Scores below -1 are considered as deficit (Patient 1 continuous line; Patient 2 dashed line).
Patient 2
An 11 years-old female was referred to our hospital for re-evaluation of RE. She was diagnosed at 7 years-old as suffering Status Epilepticus (SE), focal, generalized epileptic seizure episodes, followed by epilepsia partialis continua with cognitive declination ((Figure 1 (dashed line)). The patient showed an intellectual level within the average (IQ=84) with normal values in intellectual indices of Verbal Comprehension (VCI=91), Perceptual Reasoning (PRI=90) and Processing Speed (PSI=97), and slight difficulties in the index of Working Memory (WMI=97). She presented alterations in verbal tasks (verbal auditory processing and recognition vocabulary) and nonverbal skills (line orientation). The processes of abstract reasoning, memory, attention and executive functions were suitably preserved. As in the previous case, although with milder and less generalized difficulties, there are both alterations associated with the functioning of the dominant and non-dominant hemispheres. She was right-handed without family history of left-handed. The syndrome was catalogued as RE. She was treated with AED drugs, steroids, immunoglobulins and plasmapheresis, but the epilepsy and cognitive declination did not stop. She had a firts time hemispherectomy surgery on March 2011 and second time on November 2011. Pathology studies of the biopsies confirmed a stage 1 in the first surgery and a stage 3 in the second one. The patient did not improve after surgery but continued with focal motor seizures every day and impaired awareness. She then received autologous marrow-derived MSCs, in a protocol like that of patient 1.
The local Research Ethics Board and the Spanish Medicine Agency (AEMPS) approved each patient's treatment in an individualized basis, and informed consent was obtained from each participant.
MSC production
Bone marrow MSCs were obtained from the iliac crest of patients. Briefly, mononuclear cells were obtained by Ficoll gradient centrifugation (400 g, 25 min, 20 °C) and cultured in complete Dulbecco’s modified Eagle’s medium (DMEM, Gibco, Carlsbad, CA) supplemented with 10% fetal bovine serum (FBS, HyClone, Logan, UT) and 1% penicillin–streptomycin (P/S, Gibco). The medium was replaced after 48 hours. Cells were maintained at 37 °C and 5% CO2. MSC production complied with the principles of Good Manufacturing Practice (GMP) in an AEMPS-approved clean room.
MSC administration
Cell infusions were performed through selective internal carotid angiography of the affected hemisphere, using a transfemoral approach. We used a 4F catheter positioned in the cervical section of the internal carotid artery, and infussed the cell suspension during 10 minutes. The catheter was retrieved after the procedure and the femoral access compressed. No complications were reported, neither neurological nor at the access point.
Clinical assessment
Both patients underwent a video-electroencephalographic record prior to the first administration of MSC as well as complete laboratory tests (blood count, coagulation, biochemistry including: hepatic, renal and ionic profile, and C reactive protein). A report of seizures was established daily, initiated after signing the informed consent and finishing after the last administration.
Each patient remained hospitalized for 24 hours to monitor the possibility of any acute adverse effect after MSC infusion. Upon discharge, the family was contacted by phone every 48 hours during the first week, and subsequently, once a week, to determine subacute or deferred adverse effects, and number of seizures, fever, headache or neurological symptoms different from the usual one. Blood tests were repeated and the seizure diary was checked once a month. A Conners Continuous Performance Test (CPT-3), computerized assessment of time perception to assess aspects of attention, was performed before the first MCS infusion, and twice afterwards by the patient 1.
Pathology studies
Brain material obtained after surgery of patient 1 who underwent hemispherectomy for treatment of Rasmussen encephalitis was fixed in 10% neutral formalin. Paraffin-embedded section were cut at 3 μm and stained for hematoxylin-eosin. Several polyclonal and monoclonal antibodies were used for immunohistochemical studies.
Chemotaxis assay
Human MSCs were placed in the upper chambers of 6.5 mm diameter 8 μm pore transwell systems (HTS Transwell-24 Well Plate, Corning, MA). The chemotactic stimulus in the lower chamber was human recombinant CXCL10/IP-10 (R&D Systems) at 100 ng/ml. The anti-CXCL10 (R&D Systems, 200 ng/ml) and anti-CXCR3 (R&D Systems, 200 ng/ml) antibodies served as control. All conditions were done in triplicates. Plates were incubated for 6 h at 37 °C 5% CO2. Migrating cells were stained with crystal violet and counted under microscopy. Results show Chemotaxis Units, an arbitrary measure of MSCs counted per microscopic field.
RESULTS
The axis CXCR3-CXCL10 in MSC chemotaxis
T lymphocytes are recruited into the affected brain areas of RE patients through the CXCR3-CXCL10 axis. We tested whether human MSCs could respond to the same cue. We did standard transwell assays using primary human MSCs and found that these cells responded to the chemotactic gradient of human recombinant CXCL10 (Figure 2). The effect was abolished by means of inhibitory antiCXCL10 or antiCXCR3 antibodies. Therefore, human MSCs may target the damaged brain tissues of these patients through the same mechanisms that use pathogenic T lymphocytes [12].
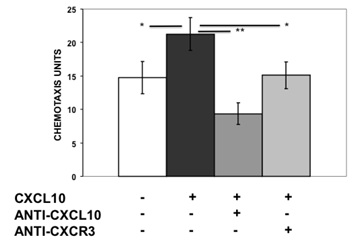 Figure 2: Chemotactic response of MSC to CXCL10. MSC cultures were performed in standard transwell assays in triplicates. Mean ± standard error of arbitrary chemotactic units. * p<0.05; ** p<0.01.
Figure 2: Chemotactic response of MSC to CXCL10. MSC cultures were performed in standard transwell assays in triplicates. Mean ± standard error of arbitrary chemotactic units. * p<0.05; ** p<0.01.
Clinical results
Patient 1
After approval by the Spanish Medicine Agency, autologous MSCs were prepared under Good Manufacture Procedure conditions as explained elsewhere [12]. MSCs were infused through catheterization of the carotidal right system (Figure 3). The patient received 4 doses of MSCs, 30×106 cells each. The administrations were well tolerated, and no acute or long-term adverse effects were seen after infusions. A decrease in the frequency of generalized seizure episodes was found within the first 2 weeks after the first 2 administrations and the patient was seizure-free during 20 days for the first time in 2 years (Figure 4). The third dose was delayed, and we documented that the frequency of seizures returned to pre-MSC levels. A 3rd and a 4th doses were then provided, upon which generalized seizures decreased and eventually faded. The patients still had an epilepsia partialis continua. A CPT-3, computerized assessment of time perception to assess aspects of attention, was performed before the first MCS infusion, and twice afterwards. A significative improvement was documented after the first infusion, regaining pretherapy scores before the 3rd one (Figure 5). Five months after the first MSC infusion and one after the last one, the patient underwent epilepsy surgery. Histological analysis of the damage brain confirmed the typical findings corresponding to a stage 2 RE. No signs of tissue damage were found, nor could the presence of MSCs be demonstrated. The patient received a second surgery 7 months later, due to progressive disease.
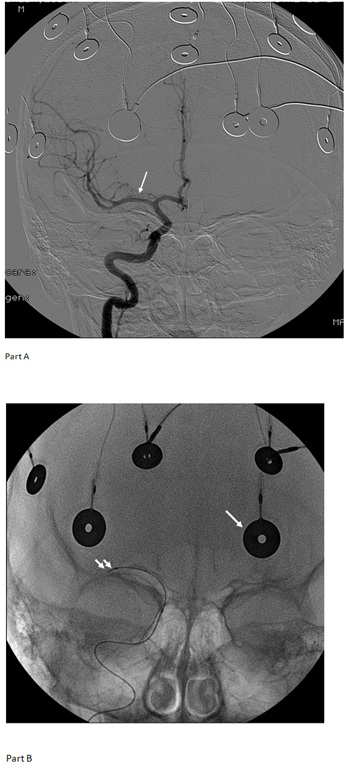 Figure 3: Part A: Digital substraction arteriography of right internal carotid artery. From cerebral medial artery (CMA) (arrow) it was insused. Part B: Anterior-posterior skull radiography, superficial electrodes of EEG (white arrow) microcatheter was positioned in CMA (double arrow).
Figure 3: Part A: Digital substraction arteriography of right internal carotid artery. From cerebral medial artery (CMA) (arrow) it was insused. Part B: Anterior-posterior skull radiography, superficial electrodes of EEG (white arrow) microcatheter was positioned in CMA (double arrow).
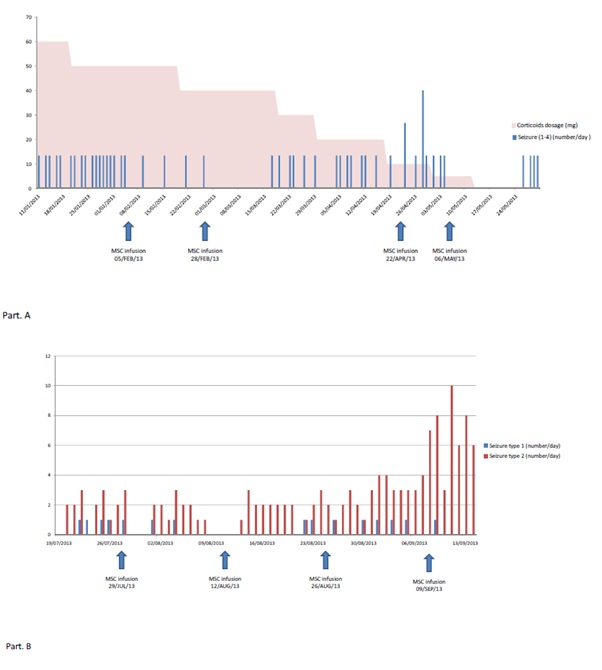
Figure 4: Part A: Patient 1. Number of focal to bilateral tonic-clonic seizures by day. You can see the corticoid dosage over time. Part B: Patient 2: She suffered two types of seizures (Type 1: focal awere motor seizure; Type 2: focal impaired awareness motor seizure).
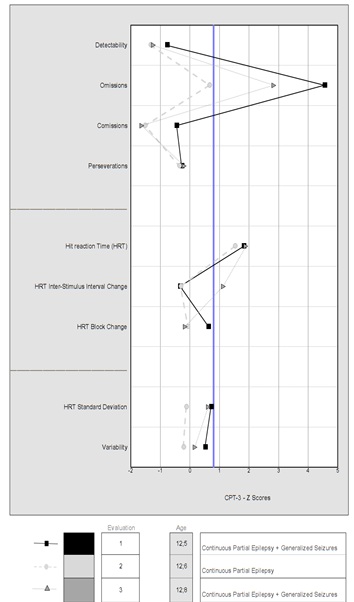 Figure 5: The results of patient 2 are presented in the Conners Continuous Performance Test (CPT-3). Evaluations performed with the same antiepileptic drugs. The first evaluation was before the first infusion. The second one was done a month later. In this, there was an improvement in the pattern associated with the disappearance of generalized seizures. In the third, the improvement disappears (reappearance of the seizures). In all three evaluations, the patient presented a continuous partial epilepsy.
Figure 5: The results of patient 2 are presented in the Conners Continuous Performance Test (CPT-3). Evaluations performed with the same antiepileptic drugs. The first evaluation was before the first infusion. The second one was done a month later. In this, there was an improvement in the pattern associated with the disappearance of generalized seizures. In the third, the improvement disappears (reappearance of the seizures). In all three evaluations, the patient presented a continuous partial epilepsy.
Patient 2
The patient received 4 doses of MSCs (two administrations with a dosage of 30x106 and two with 45x106). The administrations were well tolerated, and no adverse effects were seen after infusions. A complete control in the frequency of seizure episodes was found in the first 2 weeks after the first 2 infusions, reappearing afterwards (Figure 4B). A 3rd and 4th infusions did not have any effect, and the family decided to stop the treatment. She had a new surgery later. The anatomo-phatological study showed findings of stage 3-4 RE, with no signs of tissue damage related to the cellular therapy. A summary of the treatment protocol is shown in the figure 6.
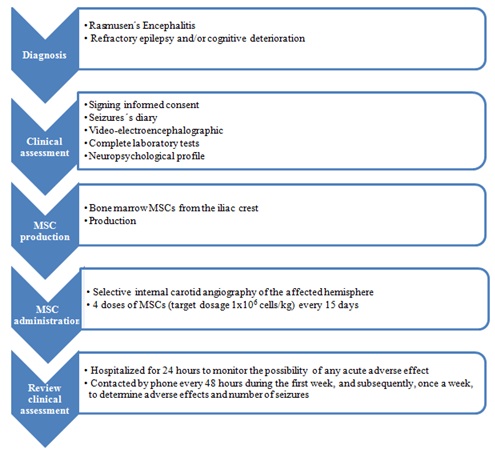 Figure 6: Summary of the treatment protocol.
Figure 6: Summary of the treatment protocol.
Pathology studies
Patient 1 surgical specimen was studied after therapy and diagnosed as RE stage 2. In the cortex we found moderate chronic inflammation, with progression to panlaminar distribution: meninges, in superficial and deep neuronal layers of the cerebral cortex and white matter, with neuronophagia, decrease of neuronal population, activated microglia, reactive astrocytosis. Immunocytochemical studies, using astroglial and microglial markers, consistently demonstrated the concomitant presence of CD3, CD8 T lymphocytes and microglial reactions (CD68). NeuN positive neurons were significantly decreased.
DISCUSSION
In this study, we present an initial dataset related to the safety and preliminary clinical efficacy of MSCs-based treatment for symptomatic RE, an example of immune-mediated epilepsy. RE typically affects previously normal children, causing severe deficits. There is no curative treatment for this disease. Although a number of promising medical treatments have emerged (tacrolimus, rituximab, natalizumab, adalimumab), none have yet been proven to control RE in the long term, nor provide adequate seizure relief to all patients [2,3,13,14]. Hemispherotomy, when possible, is widely considered to be only real ‘cure’ for RE, although at a high toll in terms of neurocognitive capacities for patients in their early years of life.
We tested MSCs for these patients based on the capacity of these cells to suppress immune-mediated tissue damages. The main pathological feature of RE is brain inflammation of one hemisphere, dominated by clones of cytotoxic CD8 T cells causing tissue damage [3]. Owens et al., reported findings of a pronounced Th1 response in the early symptomatic phase of the disease, with high levels of interferon-g (IFN-γ) mRNA in RE brain tissue. IFN-γ induces the production of CXC-chemokine ligand 9 (CXCL9) by microglia, and of CXCL10 by microglia and astrocytes, and promotes IL-1-induced synthesis of CC-chemokine ligand 5 (CCL5) by astrocytes, which recruit T cells [15]. Further, IFN-γ has been shown to induce bursting of hippocampal pyramidal neurons in vitro, providing a possible link between T cells and epileptogenesis [16,17]. Microglia release of IL-1β in RE is critical for epileptogenic mechanisms [18]. Medicines that target all these mediators will have a positive impact on RE therapy.
Increasing information on the immunomodulatory capacities of human MSCs have provided grounds for their use in different diseases of immune origin [4,5]. In vivo studies involving the transplantation of MSCs into animals or humans with inflammatory diseases demonstrated strongly suppressive effects of MSCs on the activation and proliferation of T cells and on T cell-mediated inflammatory responses upon exposure to a combination of cytokines. MSCs activated by IFNγ combined with either Tumour Necrosis Factor (TNF) or IL-1 (proteins present in the ER environment), produce chemokines which recruit T cells to the proximity of MSCs. Subsequently, MSCs suppress the proliferation and activity of T cells in their vicinity by expressing Indoleamine 2,3-Dioxygenase (IDO). The immunosuppression by MSCs is also mediated by the production of cytokines that inhibit the proliferation and function of pro-inflammatory immune cells, such as T helper 1 (Th1) and/or Th17 cells, pro-inflammatory macrophages, neutrophils, NK cells and B cells, and enhance the numbers of anti-inflammatory immune cells, including anti-inflammatory macrophages, regulatory T cells and regulatory B cells. Anti-inflammatory immune cells can further suppress the activity and functions of pro-inflammatory immune cells and subsequently promote tissue repair [10,19-21]. In addition, MSCs inhibit microglial proliferation and their release of proinflammatory molecules. Combined to the immunomodulatory properties, it has also been proposed that MSCs recruit and stimulate local progenitor cells that eventually repair the damaged tissue [9,22]. In the brain, MSCs induce the release of neuroprotective molecules by microglia and increase their phagocytic capability through the release of soluble factors [10].
Given the localized nature of RE and our previous experience with intra-arterial administration of MSCs within the brain vasculature [23], we chose this route to enhance the effects of the cell therapy. We did not have any acute adverse effect, evidencing that the procedure is feasible and repeated infusions are possible. Others have used the intravenous or the intrathecal routes for administration of MSCs in neurological diseases, with excellent safety profiles [7,10,24]. We did not find any side effects in the long-term follow up of these two patients, for as long as 5 years.
The clinical outcome of the patients showed transient positive results with a decrease in the frequency of generalized seizure episodes, and a seizure-free period for the first time in months of AED therapy. Moreover, patient 1 improved her CPT-3 scores after MSC infusion. The effects were not permanent, suggesting that MSCs did not persist and pointing to the half-life of the molecules secreted by the MSCs as responsible for the transient improvement. In the future, preconditioning, genetic and epigenetic manipulation of MSCs are key strategies to get better survival, proliferation and function of MSCs, increasing neural cell differentiation, immunomodulation, or other functions [25].
We did not find surviving MSCs in the brain tissues studied after surgery in patient 1, one month after the 4th MSCs infusion, supporting the clinical findings described and in accordance with previous reports on the lack of long-term engraftment and survival of administered human MSCs [26]. The images of multinucleated CD68 positive intravascular cells suggest phagocytoses of rests of MSCs by macrophages, which may explain the fate of infused MSCs. Patient 1 received MSCs earlier during her disease compared to patient 2 and had a better response in terms of seizure-free time after each of the various doses. It is tempting to speculate that the effectiveness of MSCs in RE may depend on stage of disease, preferably in the initial moments when more inflammation is present.
There are few trials of MSCs for treating patients with epilepsy, and none specifically for immune-mediated epilepsies. Hlebokazov et al., published a phase I open label study of 22 adults patients with refractory epilepsy secondary to infections, brain trauma or perinatal brain injury, treated with MSCs that were cultured under neurogenic differentiation inducing conditions [27].
To our knowledge, our experience is the first one on the use of MSCs in pediatric patients with RE. No major conclusion can be drawn from two clinical cases and only transient results but the safety of the procedure may be underscore. Our experience warrant further studies in the setting of a clinical trial.
AUTHOR’S CONTRIBUTION
All authors have contributed to this manuscript, reviewed and approved the current form of the manuscript to be submitted. All authors take full responsibility for the data, the analyses and interpretation, and the conduct of the research; full access to all of the data; and the right to publish any and all data.
CONFLICT OF INTEREST
None of the authors has any conflict of interest to disclose.
REFERENCES
- Varadkar S, Bien CG, Kruse CA, Jensen FE, Bauer J, et al. (2014 )Rasmussen’s encephalitis: Clinical features, pathobiology, and treatment advances. Lancet Neurol 13: 195-205.
- Granata TAF (2013) Rasmussen encephalitis. In: I O Dulac, M. Lassonde and HBS, Handb Clin Neurol (3rd Edn), Elsevier BV 111: 511-519.
- Takahashi Y, Yamazaki E, Mine J, Kubota Y, Imai K, et al. (2013) Immunomodulatory therapy versus surgery for Rasmussen syndrome in early childhood. Brain Dev 35: 778-785.
- Shi Y, Wang YY, Li Q, Liu K, Hou J, et al. (2018) Immunoregulatory mechanisms of mesenchymal stem and stromal cells in inflammatory diseases. Nat Rev Nephrol 14: 493-507.
- Wang S, Zhu R, Li H, Li J, Han Q, et al. (2018) Mesenchymal stem cells and immune disorders: from basic science to clinical transition. Front Med 3: 138-151.
- Squillaro T, Peluso G, Galderisi U (2016) Clinical Trials With Mesenchymal Stem Cells: An Update. Cell Transplant 25: 829-48.
- Volkman R, Offen D (2017) Concise Review: Mesenchymal Stem Cells in Neurodegenerative Diseases. Stem Cells 35: 1867-1880.
- Sargent A, Bai L, Shano G, Karl M, Garrison E, et al. (2017) CNS disease diminishes the therapeutic functionality of bone marrow mesenchymal stem cells. Exp Neurol 295: 222-232.
- Laroni A, de Rosbo NK, Uccelli A (2015) Mesenchymal stem cells for the treatment of neurological diseases: Immunoregulation beyond neuroprotection. Immunol Lett 168: 183-190.
- Agadi S, Shetty AK (2015) Concise review: Prospects of bone marrow mononuclear cells and mesenchymal stem cells for treating status epilepticus and chronic epilepsy. Stem Cells 33: 2093-2103.
- Schwab N, Bien CG, Waschbisch A, Becker A, Vince GH, et al. (2009) CD8+ T-cell clones dominate brain infiltrates in Rasmussen encephalitis and persist in the periphery. Brain 132: 1236-1246.
- Mirones I, De Prada I, Gómez AM, Luque A, Martín R, et al. (2013) A role for the CXCR3/CXCL10 axis in rasmussen encephalitis. Pediatr Neurol 49: 451–457.
- Papetti L, Nicita F, Granata T, Guerrini R, Ursitti F, et al. (2011) Early add-on immunoglobulin administration in Rasmussen encephalitis: The hypothesis of neuroimmunomodulation. Med Hypotheses 77: 917–920.
- Lagarde S, Villeneuve N, Trébuchon A, Kaphan E, Lepine A, et al. (2016) Anti-tumor necrosis factor alpha therapy (adalimumab) in Rasmussen’s encephalitis: An open pilot study. Epilepsia 57: 956-966.
- Pardo CA, Vining EPG, Guo L, Skolasky RL, Carson BS, et al. (2004) The Pathology of Rasmussen Syndrome: Stages of Cortical Involvement and Neuropathological Studies in 45 Hemispherectomies. Epilepsia 45: 516-526.
- Owens GC, Huynh MN, Chang JW, McArthur DL, Hickey MJ, et al. (2013) Differential expression of interferon-γ and chemokine genes distinguishes Rasmussen encephalitis from cortical dysplasia and provides evidence for an early Th1 immune response. J Neuroinflammation 10: 1-13.
- Dheen ST, Kaur C, Ling EA (2007) Microglial activation and its implications in the brain diseases. Curr Med Chem 14: 1189-1197.
- Wirenfeldt M, Clare R, Tung S, Bottini A, Mathern GW, et al. (2009) Increased activation of Iba1+microglia in pediatric epilepsy patients with Rasmussen’s encephalitis compared with cortical dysplasia and tuberous sclerosis complex. Neurobiol Dis 34:432-440.
- Ren G, Zhang L, Zhao X, Xu G, Zhang Y, et al. (2008) Mesenchymal Stem Cell-Mediated Immunosuppression Occurs via Concerted Action of Chemokines and Nitric Oxide. Cell Stem Cell 2: 141-150.
- Costa-Ferro ZS, Souza BS, Leal MM, Kaneto CM, Azevedo CM, et al. (2012) Transplantation of bone marrow mononuclear cells decreases seizure incidence, mitigates neuronal loss and modulates pro-inflammatory cytokine production in epileptic rats. Neurobiol Dis 46: 302-313.
- Bian P, Ye C, Zheng X, Yang J, Ye W, et al. (2017) Mesenchymal stem cells alleviate Japanese encephalitis virus-induced neuroinflammation and mortality. Stem Cell Res Ther 8: 1-13.
- Fayyad-Kazan M, Fayyad-Kazan H, Lagneaux L, Najar M (2016) The potential of mesenchymal stromal cells in immunotherapy. Immunotherapy 8: 839-842.
- Carceller F, Aleu A, Casasco A, Guimaraens L, López-Pino MA, et al. (2014) Superselective intracerebral catheterization for administration of oncolytic virotherapy in a case of diffuse intrinsic pontine glioma. J Pediatr Hematol Oncol 36: 430-4322.
- Huang P, Gebhart N, Richelson E, Brott TG, Meschia JF, et al. (2014) Mechanism of mesenchymal stem cell-induced neuron recovery and anti-inflammation. Cytotherapy 16: 1336-1344.
- Sadatpoor S omid, Salehi Z, Rahban D, Salimi A (2020) Manipulated Mesenchymal Stem Cells Applications in Neurodegenerative Diseases. Int J Stem Cells.
- von Bahr L, Batsis I, Moll G, Hägg M, Szakos A, et al. (2012) Analysis of tissues following mesenchymal stromal cell therapy in humans indicates limited long-term engraftment and no ectopic tissue formation. Stem Cells 30: 1575-1578.
- Hlebokazov F, Dakukina T, Ihnatsenko S, Kosmacheva S, Potapnev M, et al. (2017) Treatment of refractory epilepsy patients with autologous mesenchymal stem cells reduces seizure frequency: An open label study. Adv Med Sci 62: 273-279.
Citation: Cantarín-Extremera V, Friera A, González-Murillo A, Melen GJ, de Prada-Vicente I, del Castillo MCF, et al. (2020) Results of Selective Intra-Arterial Infusions of Mesenchymal Stem Cells in Two Children with Rasmussen Encephalitis. J Stem Cell Res Dev Ther 6: 030.
Copyright: © 2020 Verónica Cantarín-Extremera, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.

