
Review on Molecular Epidemiology and Public Health Significance of Brucellosis
*Corresponding Author(s):
Adonyas LuelsegedCollege Of Veterinary Medicine, Addis Ababa University, Bishoftu, Ethiopia
Tel:+251 913601337,
Email:adonyas2003@gmail.com
Abstract
Brucellosis is an important livestock and human disease in many developing countries for its cause of reproductive disease, characterized by abortion, retained fetal membranes and impaired fertility. The genus Brucella currently composed of eight terrestrial species and at least two marine species. Terrestrial Brucella species include B. abortus, B. melitensis, B. suis, B. ovis, B. canis, B. neotomaei and two new species, B. microti and B. inopinata. Brucella isolated from marine mammals is B. ceti and B. pinnipedialis. Brucella species can invade epithelial cells of the host, allowing infection through mucosal surfaces, in which the outcome of infection dependent on the species of Brucella and host. Though its distribution is worldwide; yet brucellosis is more common in countries with poorly standardized animal and public health program and also bio varieties of Brucella vary with respect to geographic region. The prevalence of brucellosis depends on different risk factors including host risk factors, agent risk factors, management risk factors and occupational risk factors. Genetically, all Brucella species are highly related to each other, exhibiting sequence similarity values of 98% to 100% at nucleotide level (core genome). Despite this close genetic relatedness, the various species can be distinguished from each other by application of high resolution molecular typing tools such as polymerase chain reaction, single nucleotide polymorphism analysis and multi-locus sequence typing or multi-locus sequence in addition to assessment of phenotype and host preference. Each year half a million case of brucellosis occurs in humans around the world. Five out of nine known Brucella species can infect humans. The most pathogenic and invasive species for human are B. melitensis, B. abortus and B. canis. Understanding the Brucella species and bio variant through advance knowledge of molecular epidemiology has significant role in the elucidating the source of infection, disease transmission pattern and furthermore in designing specific control strategies through utilization of relevant vaccine in affected livestock population. Prevention and control of brucellosis can be adopted realistically through understanding of local and regional variations in animal husbandry practices, social customs, infrastructures and epidemiological patterns of the disease and species of Brucella. Hence, this seminar paper attempted to highlight the molecular epidemiology and public health significance of brucellosis in livestock and human populations.
Keywords
INTRODUCTION
Brucellosis is one of the major common bacterial zoonosis in the world caused by organisms belonging to the genus Brucella, gram-negative, non-motile and facultative intracellular pathogens that can infect many species of animal of economic importance, such as cattle, sheep, goats, pigs and marine animals. The genus Brucella currently composed of eight terrestrial species and at least two marine species. Terrestrial Brucella species include B. abortus, B. melitensis, B. suis, B. ovis, B. canis, B. neotomaei and two new species B. microti and B. inopinata. Brucella isolated from marine mammals are B. ceti and B. pinnipedialis [2].
The disease is an old one that has been known by various names, including mediterranean fever, malta fever, gastric remittent fever, and undulant fever (because of the relapsing nature of the fever associated with the disease). Humans are accidental hosts, but brucellosis continues to be a major public health concern worldwide and is the most common zoonotic infection [3].
Human brucellosis is a zoonotic disease with a major impact on public health, even though successful eradication and control programs for domestic animals have been established in many countries around the world. The disease primarily presents as fever of unknown origin with multiple clinical signs and symptoms. Patients regularly suffer serious focal complications such as spondylitis, neurobrucellosis or endocarditis [4]. As the ultimate source of human brucellosis is direct or indirect exposure to infected animals or their products, prevention must be based on elimination of such contact. The obvious way to do this elimination of the disease from animals is often beyond the financial and human resources of many developing countries. For instant, the technical and social difficulties involved in eradicating B. melitensis from small ruminants have even taxed the resources of some developed countries. In many situations there is little alternative but to attempt to minimize impact of the disease and to reduce the risk of infection by personal hygiene, adoption of safe working practices, protection of the environment and food hygiene [5].
Presumptive diagnosis can be made by the use of several specific serological tests to making the diagnosis of Brucella antibodies, but unequivocal diagnosis requires the bacteriological demonstration of the organism. Hence, the collection and shipment of appropriate samples to the laboratory have great importance. The diagnosis of brucellosis is usually performed by a combination of methods. The identification of Brucella culture relies upon a great deal of phenotypic traits such as requirement for CO2, phage typing and metabolic tests, which among other problems involves time, bio safety, trained personnel and somewhat ambiguous results. Brucella species and biovars have been characterized by conventional phenotypic and serological methods, although such methods are not always reliable [6].
Accurate species delineation can be achieved by conventional multiplex polymerase chain reaction, single nucleotide polymorphism analysis and multilocus sequence typing or multilocus sequence analysis. Highly discriminatory multilocus variable number of tandem repeats analysis allows both species delineation and differentiation of individual isolates and thus represents a perfect first-line tool for molecular epidemiological studies within outbreak investigations [7]. To date, advanced molecular technologies have not been widely used in low income countries where brucellosis is endemic in livestock and humans. Thus, information on the prevailing Brucella species, biovars, and genotypes/strains in such areas of endemicity may shed new light on the epidemiology of Brucella infection and the species and biovars circulating [8].
Therefore, the objectives of this seminar paper are:
1. To review the molecular epidemiology of brucellosis and
2. To indicate the economic and public health significance of brucellosis.
ETIOLOGY
| Organism | Host |
| B. melitensis | Sheep, Goat and Camel |
| B. abortus | Buffalo, Cows and Camels |
| B. canis | Dog |
| B. suis | Pig |
| B. neotomaei | Rodent |
| B. ovis | Sheep |
| B. pinnipediae | Marine animals |
| B. cetaceae | Marine animals |
PATHOGENESIS
Brucella has the ability to interfere with intracellular trafficking, preventing fusion of the Brucella-containing vacuole with lysosome markers, and directing the vacuole towards a compartment that has rough endoplasmic reticulum which is highly permissive to intracellular replication of Brucella [12]. The outcome of infection is dependent on the species of Brucella and host. The Brucella species that infect livestock are host restricted. For instance B. melitensis, B. abortus, B. suis and B. ovis infect preferentially small ruminants, cattle, pigs and sheep respectively. With the exception of B. ovis, these Brucella species have zoonotic potential, with B. melitensis being the most pathogenic for humans [13].
Brucella spp lack classical bacterial virulence factors such as exotoxins, cytolysins, a capsule, fimbriae, flagella, plasmids, lysogenic phages, endotoxic lipopolysaccharide, and inducers of host cell apoptosis [14]. However, LPS plays an important role in Brucella virulence because it prevents complement-mediated bacterial killing and provides resistance against antimicrobial peptides such as defenses and lactoferrin [15]. Another important virulence mechanism of Brucella is the BvrR/BvrS two-component regulatory system, which is required for modulation of the host cell cytoskeleton upon Brucella invasion, and for regulation of the expression of outer membrane proteins, some of which are required for full virulence [16]. Cyclic β-1, 2-glucans, which are also part of the outer membrane, is also required for intracellular survival of Brucella [17]. Figure 1 shows major events in the pathogenesis of brucellosis and the host immune response.
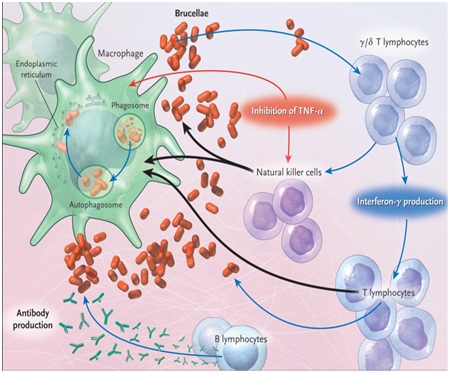 Figure 1: Major events in the pathogenesis of brucellosis and the host immune response.Source: [18].
Figure 1: Major events in the pathogenesis of brucellosis and the host immune response.Source: [18].DIAGNOSTIC METHODS OF BRUCELLOSIS
Bacteriological diagnosis
“The gold standard” for laboratory detection of Brucella and species identification is based largely on bacterial isolation and phenotypic characterization. Isolation of Brucella organisms from the suspected animal is the golden standard in terms of specificity. However, this method has a limited sensitivity, expensive, time consuming, labor-intensive and has been associated with a heightened risk of laboratory-acquired infection and has the added difficulty of being unpractical to apply on a large scale in control. Polymerase chain reaction is becoming very useful and considerable progress has been made to improve their sensitivity, specificity, and technical case and to lower costs. Nucleic acid amplification has been explored for rapid detection and confirmation of the presence of Brucella species [19].
Serological diagnosis
The standard Rose Bengal and Complement Fixation tests are the main serological tests used to detect antibodies against B. abortus and B. melitensis. Both tests have been used for several decades, proving to be successful for eradicating bovine brucellosis in some countries. Nevertheless, there is evidence that both tests are significantly less effective for the diagnosis of brucellosis in sheep and goats than in cattle [20].
Complement fixation test is a widely used confirmatory test for brucellosis. It is technically challenging because a large number of reagents must be titrated daily and a large number of controls of all the reagents is required. It is also an expensive test again because of the large number of reagents needed and because it is labor intensive. Some of the problems of CFT are few positive reactions, sometimes negative result in early stage of infections, the test is rather expensive and complicated. Other problems include the subjectivity of the interpretation of result occasional direct activation of complement by serum (anti complementary activity) and the inability of the test for use with hemolyzed serum samples. False positive results may also occur in animals infected with organisms antigenically related to Brucella [10].
ELISA is very sensitive and good for detecting latent carriers, incomplete antibodies, relatively simple and easily automated. A very good as control test in free areas and as survey testing areas where no vaccination have been performed, but complicated and cannot be carried out everywhere, severely hampered by vaccination and still too little standardized. Indirect Enzyme-Linked Immunosorbent Assay have been developed using purified smooth lipopolysaccharide as the antigen and have been reported to be at least as sensitive and specific as the combination of both RBT and CFT for the diagnosis of brucellosis in ruminants [21].
Molecular diagnosis: PCR, RFLP and MLVA
 Figure 2: Comparison of five Brucella species genomes to B. melitensis 16M by microarray.Source: [23].
Figure 2: Comparison of five Brucella species genomes to B. melitensis 16M by microarray.Source: [23]. One of the first PCR assays to differentiate among Brucella species was called abortus-melitensis-ovis-suis PCR, developed by Bricker and Halling in 1994. This PCR uses a single reverse primer, targeting the Brucella specific insertion element IS711, and four different forward primers, each specific for a given species as estimated by testing representative isolates. Species are differentiated on the basis of different PCR fragment sizes. In 2006, a new conventional multiplex PCR (Bruce-ladder), using eight primer pairs in a single reaction, was developed by García-Yoldi and colleagues [25]. Because this PCR covers all species and biovars it rapidly replaced the AMOSPCR as a diagnostic tool and is still used in many diagnostic laboratories. The most recent multiplex PCR assay to differentiate among B. suis biovars 1 to 5 (Suis-ladder) was developed in 2011 by scientists [26].
The first Multiple-Locus Variable number tandem repeat Analysis (MLVA assay) named ‘HOOF-Prints’ (hypervariable octameric oligonucleotide fingerprints), was developed by Bricker et al., in [27]. The Brucella genome contains a family of tandem repeats sharing the repeat unit ‘AGGGCAGT’. Eight highly variable such loci, present in most Brucella species, were selected for use in the HOOF-Print assay. Variations of the repeat numbers at each locus can easily be investigated by amplifying the corresponding regions and subsequent gel electrophoresis or, preferably, capillary electrophoresis, given the short repeat unit size. This selection of tandem repeats has a very high discriminatory power and can be useful for local outbreak investigations. However, it cannot provide a species assignment owing to the high level of homoplasy at these loci. With the HOOF-Print assay a reliable tool to study the relationship of human cases and outbreak dynamics became available for the first time. Indeed, high resolution markers allow the discrimination of individual strains and therefore can be used for trace-back analyses and epidemiological studies in outbreak scenarios. A high discriminatory power is desired when investigating an outbreak with very limited geographical and temporal distribution, and highly variable loci will then be preferred. However, rapidly evolving Variable-Number Tandem-Repeat (VNTR) markers often suffer from homoplasy, i.e., the appearance of the same genetic alteration in two or more branches of a phylogenetic tree. These phenomena can disrupt and confound the accurate phylogenetic placement of some isolates within an MLVA cluster and prevent accurate species-level designation [28].
None of the existing molecular tools provide adequate resolution to confidently permit epidemiological trace back in the case of accidental import or deliberate release. However, the completion of genome sequences for a B. suis and a B. melitensis strain provided an opportunity to assess the presence of tandem repeats that might facilitate the development of an MLVA scheme. Initial analysis indicated the presence of many potentially useful regions of diversity in the Brucella genomes and, indeed, during the planning stages of the present study, an MLVA scheme that utilizes eight distinct copies of an octameric repeat (the “HOOF-Prints” assay) was described [26].
Several molecular typing methods are introduced to find DNA polymorphism that is able to identify the Brucella species and biovars, among which detection of polymorphisms by PCR-RFLP has several advantages including the easy implementation, interpretation and use for large quantities of samples. In this method, by Omp25, Omp2 and Omp31 loci and all Brucella species can be differentiated and their biovars identified. Several studies use these genes to differentiate Brucella species and biovars performed around the World [29]. The genus Brucella has ten recognized species with more than 90% DNA homology. These species cause brucellosis that is of economic and public health importance in terrestrial and aquatic animals and humans [30].
RISK FACTORS FOR BRUCELLOSIS
Host risk factors
The predilection sites being the reproduction tract of male and female especially the pregnant uterus. Allatoic factors stimulate the growth of most Brucella. These factors include Erythritol, possibly steroid hormones and other substances. Erythritol is present in the placenta and male genital tract of cattle, sheep, goats, and pigs but not in humans [32]. Female usually abort only once, after which a degree of immunity develops and the animals remain infected and large number of Brucella be expelled in the fetal fluids at subsequent parturition [31]. Cattle susceptibility to B. abortus infection is influenced by age, sex, breed and reproductive status of the individual animal [33].
Agent risk factors
Naturally infected animals and those vaccinated as adults with strain 19 remain positive to the serum and other agglutination tests for long periods. The antibody response to Brucella consists of an early IgA and IgM is a type response, the timing of which depends on the route of exposure, the dose of bacteria and the health status of the animal. The IgM response is followed shortly by production of IgG1 antibody and later by IgG2 [34].
The total concentration of IgG2 increases with age. Most cross reacting antibody, resulting from exposure to microorganism other than Brucella spp., consist of IgM, making serological tests which measure IgM not specific as false positive results occur, leading to low assay specificity. In the case of Brucellainfection, the concentration of anti-Brucella total IgG2 increases with the level of antigen exposure, therefore the monitoring of IgG1 and IgG2 Brucella antibody levels is relevant for detection of Brucella-infected cattle [35].
Occupational risk factors
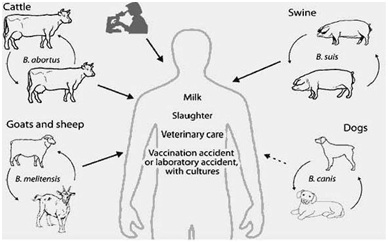 Figure 3: Transmission of Brucella to humans. Source: [37].
Figure 3: Transmission of Brucella to humans. Source: [37].Management risk factors
MOLECULAR EPIDEMIOLOGY OF BRUCELLOSIS
It is interesting to note that the second highest prevalence (71.42%) of brucellosis has been reported in mules from Egypt. Invariably, all domestic animals suffer from this disease. Brucellosis in buffaloes has been reported from Egypt (10.0%) and Pakistan (5.05%). Since cattle are found throughout the world, prevalence of brucellosis (0.85 to 23.3%) in cattle has been reported from a wide range of countries. In camels, brucellosis has been reported from Arabian and African countries (0.0-17.20%), where the disease also occurs in buffaloes, equines and swine. Variable prevalence of this disease has been reported in sheep and goats. Bio varieties of Brucella vary with respect to geographic region. B. melitensis biovar 1 from Libya, Oman and Israel and B. melitensis biovar 2 from Turkey and Saudi Arabia have been isolated. B. melitensis biovar 3 is the most commonly isolated species from animals in Egypt, Jordan, Israel, Tunisia and Turkey. B. abortus biovar 1 in Egypt, biovar 2 in Iran, biovar 3 in Iran and Turkey and biovar 6 in Sudan have been reported [40].
The countries with the highest incidence of human brucellosis include Saudi Arabia, Iran, Palestinian Authority, Syria, Jordan and Oman. Bahrain is reported to have no incidence [41]. The percent prevalence of bovine brucellosis has been reported to decrease in Ireland and Italy during the year 1999-2000 but there had been a trend towards a significant increase in Azores [42].
Characterization of the molecular epidemiology of B. abortus is an important component of efforts by APHIS and state animal health agencies to control the disease among wildlife and livestock. One of the initial protocols used for this purpose was the HOOF-Prints assay which exploited the presence of 8-base pare tandem repeat sequences at 8 loci in the B. abortus genome. This assay was used to differentiate clusters and groupings among a panel of 97 B. abortus reference strains and field isolates, representing three biovars, collected from different geographic locales in the United States [43].
Based on their agglutinating properties with specific antisera, B. melitensis can be differentiated into three biovars, biotypes 1, 2 and 3 of which biotype 1 is known to be present in Peru [8]. Recently, a highly discriminatory method for the genotyping of Brucella known as MLVA analysis has become available. This method makes use of various loci on the Brucella genome that are composed of repeats of short nucleotide sequences. These tandem-repeat units tend to occur in various numbers, and various alleles can be observed in different species and isolates. The recently published MLVA-16 assay, developed for the genotyping of Brucella, makes use of eight mini-satellite loci for species identification, supplemented with a selection of eight more polymorphic microsatellite loci for the further characterization and differentiation of isolates. Whereas the MLVA-16 assay can be used for the biovar classification of B. abortus and B. suis, no correlation between biovars and genotype has been observed for B. melitensis [44].
The MLVA-16 typing of animal and human Brucella isolates has revealed that clusters of individual genotypes within a species may show a distinct geographic distribution. For instance, human isolates of B. melitensis from Europe and North Africa can be divided according to their geographic origin into a west and an east Mediterranean cluster. Within the west Mediterranean cluster (which includes isolates from France, Switzerland, Tunisia, and Algeria), a clearly separate cluster originating from Italy can be identified. Genotypes are relatively stable, and isolates with identical MLVA patterns have been obtained from the same geographic area during a time span of almost three decades. A considerable number of distinct B. melitensis genotypes already have been identified [20]. MLVA typing additionally has some practical clinical applications, such as tracing sources of infections and discriminating relapse from re-infection [45].
High resolution phenotypic and molecular approaches have been developed for Brucella speciation, bio typing, and epidemiological trace-back. To date, advanced molecular technologies have not been widely used in low income countries where brucellosis is endemic in livestock and humans. Thus, information on the prevailing Brucella species, biovars, and genotypes/strains in such areas of endemicity may shed new light on the epidemiology of Brucella infection and the species and biovars circulating. Besides this generic scientific rationale for undertaking such investigations, increased understanding of the Brucellaepidemiology is critical for refining control of brucellosis in resource weak countries where the same measures as in high income countries cannot be applied [8]. Table 2 shows the origin of Brucella strains and their profiles.
| Strain | Biovar | Host | Source |
| B. abortus UK8/01 | 1 | Human | Eire |
| B. abortus I12 | 1 | Bovine | Northern Ireland |
| B. abortus F6/0404376 | 1 | Human | New Zealand |
| B. abortus R51/03 | 1 | Bovine | United Kingdom |
| B. abortus 5/93 | 3 | Bovine | United Kingdom |
| B. melitensis F3/02 | 2 | Human | Norway |
| B. melitensis 1BM1 | 1 | Not known | Portugal |
| B. melitensis 63/19 | 2 | Human | India |
| B. melitensis 66/59 | 3 | Ovine | India |
| B. melitensis 65/155 | 3 | Ovine | Mongolia |
| B. melitensis UK19/4 | 1 | Human | Ethiopia |
| B. melitensis R3-60 | 1 | Livestock | Tanzania |
| B. ovis 79/60 | 3 | Ovine | France |
| B. ovis 63/96 | 3 | Ovine | Argentina |
| B. ovis 81/2 | 3 | Ovine | Germany |
| B. suis 1330 | 1 | Porcine | Croatia |
| B. suis F7/03 BSI | 2 | Porcine | Germany |
| B. suis 01-5744 | 2 | Porcine | South Africa |
| B. canis 79/85 | 1 | Canine | South Africa |
| B. canis 79/92 | 3 | Canine | France |
| B. canis 79/139 | 2 | Canine | United Kingdom |
ECONOMIC AND PUBLIC HEALTH SIGNIFICANCE
Economic significance
The economic losses due to bovine brucellosis include: Losses of calves due to abortion, reduced milk yield, culling and condemnation of valuable cows because of breeding failure, endangering animal export trading of a nation, loss of man power, medical costs and government cost for research and eradication programs. Available information indicates that brucellosis is one of the most serious diseases of cattle in Latin America and other developing areas. Official estimates put annual losses from bovine brucellosis in Latin America at approximately US$ 600 million [5].
Brucellosis in sheep caused by B. ovis has been reported in Australia, New Zealand the United States, South Africa and Europe. The incidence has been very high in some areas, and there was much economic loss at one time. In California, 30-40% of rams were thought to be affected and annual loss of US $ 2 million was estimated. B. suis is a chronic disease of swine manifested by sterility and abortion in sows, heavy piglet mortality and orchitis in boars. The disease owes its economic importance to the fertility and reduction in numbers of pigs weaned per litter that occur in infected herds [10].
Public health significance
Each year half a million case of brucellosis occurs in humans around the world. The prevalence of infection in animal reservoir provides a key of its occurrence in humans [48]. Humans are infected by eating or drinking something that is contaminated with Brucella, breathing organisms (in halation or wind infection). The relative importance of etiological agent, mode of transmission and path way of penetration varies with the epidemiological area, animal reservoirs and occupational groups at risk. Conception of sheep and goat milk contain B. melitensis is an important source of humanbrucellosis worldwide and has caused several out breaks. For exam ple, in some countries including Italy 99% of human brucellosis is caused by B. melitensis. In countries where milk and dairy products are always pasteurized, brucellosis principally affects persons who are close contact with animals and animal products [20]. The following map shows incidence of human brucellosis in worldwide (Figure 4).
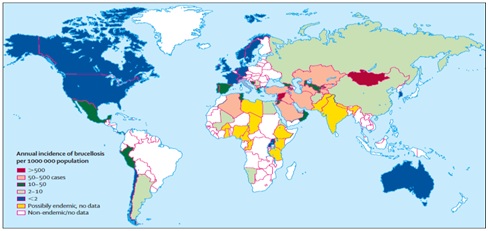 Figure 4: Worldwide incidence of human brucellosis. Source: [2].
Figure 4: Worldwide incidence of human brucellosis. Source: [2].Status of Brucellosis in Ethiopia
Animal brucellosis in Ethiopia
The evidences of Brucella infections in Ethiopian cattle have been serologically demonstrated by different authors. A relatively high seroprevalence of brucellosis (above 10%) has been reported from small holder dairy farms in central Ethiopia while most of the studies suggested a low seroprevalence (below 5%) in cattle under crop-livestock mixed farming. There is a scarcity of published literature on the status of cattle brucellosis in pastoral areas of the country where large population of cattle are reared. So far, a study carried out in east Showa zone of Ethiopia showed a relatively higher seroprevalence in pastoral than agropastoral system [50].
Most of the previous studies on cattle brucellosis have been carried out in central and northern Ethiopia, and do not provide an adequate epidemiological picture of the disease in different agro-ecological zones and livestock production systems of the country. In particular, there is no information on cattle brucellosis across various livestock production systems of southern and eastern part of the country, which gave impetus to the initiation of this study. The present study was therefore aimed at determining the prevalence of cattle brucellosis and associated risk factors across the two livestock production systems, pastoral and crop-livestock mixed systems, in Southern and Eastern Ethiopia [49]. There is summary on prevalence of brucellosis in Ethiopia by using RBPT and CFT in table 3.
| Locations | Breed | Number of Animals | Prevalence (%) |
| Tigray | Cross | 816 | 3.19 |
| Bahir Dar | Cross | 1135 | 0.26 |
| Sidama Zone | Cross | 811 | 2.5 |
| Local | 1627 | 1.7 | |
| Jimma Zone | Cross | 805 | 0.8 |
| Local | 1305 | 0.2 | |
| Northwest Ethiopia | Cross | 4243 | 22 |
Human brucellosis in Ethiopia
CONTROL AND PREVENTION
In animals
The decision about slaughter of test-positive animals is made after regulatory, economic and prevalence factors are considered. In most cases, test and slaughter of positive animals is only successful in reducing the incidence if the herd or flock prevalence is very low (e.g., 2%). Retention of positive animals is less hazardous if the remaining animals have been vaccinated but should only be considered as a last resort. The isolation of test-positive animals is essential, especially during and after parturition. The immediate slaughter of test-positive animals is expensive and requires animal owner cooperation. Compensation is usually necessary. Furthermore, the application of test and slaughter policies is unlikely to be successful with brucellosis of sheep and goats where the diagnostic tests are less reliable than in cattle [55].
The goal in the application of hygiene methods to the control of brucellosis is reduction of exposure of susceptible animals to those that are infected, or to their discharges and tissues. This is a classical procedure in disease control. Factors such as the methods of animal husbandry (e.g., commingling of herds or flocks), patterns of commerce, prevalence of clinical signs, type of facilities, and degree of dedication of the owners of animals, will also determine success. Owners are often poorly informed about disease transmission and recommendations, such as separation of parturient animals, can be difficult or impossible to implement [56].
Animals should be individually identified by brand, tattoo or ear tag. Unauthorized sale or movement of animals from an infected area to other areas should be forbidden. Similarly, importations into clean areas must be restricted to animals that originate from brucellosis-free areas, that have a herd/flock history of freedom from the disease and that have given negative reactions to recently performed diagnostic tests [54].
There is general agreement that the most successful method for prevention and control of brucellosis in animals is through vaccination. While the ideal vaccine does not exist, the attenuated strains of B. melitensis strain Rev.1 for sheep and goats and B.abortus strain 19 have proven to be superior to all others. The non-agglutinogen B. abortus strain RB51 has beesn used in the USA, Canada and some Latin American countries, South Africa and Egypt with encouraging results. The source and quality of the vaccines are critical. The dosages and methods of administration, especially with Rev.1, vary and these can affect the results [20].
It is often recommended that vaccination with strains 19 and Rev.1 should be limited to sexually immature female animals. This is to minimize stimulation of post vaccinal antibodies which may confuse the interpretation of diagnostic tests and also to prevent possible abortions induced by the vaccines. However, field and laboratory studies have demonstrated that conjunctival administration of these vaccines makes the vaccination of the herd or flock a practical and effective procedure. Rapid herd immunity is developed and application costs are minimized. The lowered dose results in lower antibody titers and serenade rapidly. Several diagnostic tests have been developed which are useful in differentiating antibody classes. Of these, the complement fixation test and ELISA are currently the most widely used [55].
In human
TREATMENT
Neither streptomycin nor doxycycline alone can prevent multiplication of intracellular Brucella. Although the DS regimen is considered as the gold standard treatment, it is less practical because the streptomycin must be administered parenterally for 3 weeks. A combination of doxycycline treatment (6 weeks duration) with parenterally administered gentamicin (5 mg/kg) for 7 days is considered an acceptable alternate regimen. Although DS combinations had been considered by the WHO to be the standard therapy against brucellosis for years, in 1986 the Joint FAO/WHO Expert Committee on Brucellosis changed their recommendations for treatment of adult acute brucellosis to rifampicin (600–900 mg/day orally) plus doxycycline (200 mg/day orally) DR for 6 weeks as the regimen of choice. However, the studies that compared the effectiveness of DR regimen with the traditional DS combination concluded that DR regimen is less effective than the DS regimen especially in patients with acute brucellosis [56].
CONCLUSION AND RECOMMENDATIONS
Based on the above conclusion the following recommendations are forwarded:
• Isolation and molecular characterization of species and biovars causing brucellosis in livestock and human should be identified for further control of brucellosis using the existing vaccines,
• High sensitive and specific diagnostic tests such as isolation combined with molecular based diagnostic techniques should be utilized for confirmatory diagnosis of brucellosis,
• In case of our country, even though molecular techniques of diagnosis are famous for their sensitivity and specificity, there is no any well-organized molecular diagnostic laboratory. Therefore the government should encourage the development of laboratories that conduct diagnosis at molecular level,
• Individuals at high risk of getting the infection should be well informed about the disease transmission route and proper safety materials and disinfection should be provided.
REFERENCES
- Boschiroli M, Foulongne V, O’Callaghan D (2001) Brucellosis: A worldwide zoonosis. Curr Opin Microbiol4: 58-64.
- Office International des Epizootic (OIE) (2008) Bovine brucellosis. In: Manual of diagnostic tests and vaccines for terrestrial animals. Office International des Epizooties, Paris, France.
- Pappas G, Akritidis N, Tsianos E (2005) Effective treatments in the management of brucellosis. Expert Opin Pharmacother 6: 201-209.
- Al Dahouk S, Sprague L, Neubauer H (2013) New developments in the diagnostic procedures for zoonotic brucellosis in humans. Rev Sci Tech 32: 177-188.
- WHO (2001) Zoonoses and communicable disease common to man, animals and wild life. Bacterioses and Mycoses, (3rd edn). WHO, Washington DC, USA.
- Al Dahouk S, Tomaso H, Prenger-BerninghoffE, Scholz H, Neubauer H (2005) Identification of Brucella species and biotypes using Polymerase Chain Reaction- Restriction Fragment Length Polymorphism (PCR-RFLP). Crit Rev Microbiol 3: 191-196.
- Scholz HC, Vergnaud G (2013) Molecular characterisation of Brucella species. Rev Sci Tech 32: 149-162.
- Lucero NE, Ayala SM, Escobar GI, Jacob NR (2008) Brucella isolated in humans and animals in Latin America from 1968 to 2006. Epidemiol Infect 136: 496-503.
- Dubie T, AdugnaM, Sisay T, Mukitar Y (2014) The economic and public health significance of brucellosis. Global Res J Public Health Epidemiol 1: 54-64.
- Radostits OM, Gay CC,Hinchcliff KW, Constable PD (2006) Veterinary medicine: The Diseaseof cattle, sheep, pigs, goats, and horses. Elsevier Health Sciences, USA.
- Carvalho Neta AV, Mol JP, Xavier MN, Paixão TA, Lage AP, et al. (2010) Pathogenesis of bovine brucellosis. Vet J 184: 146-155.
- Pizarro-Cerdá J, Moreno E, Gorvel J (2000) Invasion and intracellular trafficking of Brucella abortus in nonphagocytic cells. Microbes Infect 2: 829-835.
- Xavier MN, Costa EA, Paixão TA, Santos RL (2009) The genus Brucella and clinical manifestations of brucellosis. Ciência Rural 39: 2252-2260.
- Moreno E, Cloeckaert A, Moriyón I (2002) Brucella evolution and taxonomy. Vet Microbiol 90: 209-227.
- Lapaque N, Moriyon I, Moreno E, Gorvel JP (2005) Brucella lipopolysaccharide acts as a virulence factor. Curr Opin Microbiol 8: 60-66.
- López-Goñi I, Guzmán-Verri C, Manterola L, Moriyón I, Moreno E (2002) Regulation of Brucellavirulence by the two-component system. Int J Trop Med 9: 27-51.
- Briones G, Iñón de Iannino N, Roset M, Vigliocco A, Paulo P, et al. (2001) Brucella abortus cyclic beta-1, 2-glucan mutants have reduced virulence in mice and are defective in intracellular replication in HeLa cells. Infect Immun 69: 4528-4535.
- Robinson A (2003) Guidelines for coordinated human and animal brucellosis surveillance. FAO Animal Production and Health Paper. Pg no: 156.
- Hussein K, Awad S (2007) Trial to increase the sensitivity of Brucella antigens treated with binary ethylene amine as inactivated agent. Beni-Suef Vet Med J 17: 10-14.
- Al Dahouk S, Neubauer H, Hensel A, Schöneberg I, Nöckler K, et al. (2007) Changing epidemiology of human brucellosis, Germany, 1962-2005. Emerg Infect Dis 13: 1895-1900.
- Kauffman LK, Bjork JK, Gallup JM, Boggiatto PM, Bellaire BH, et al. (2014) Early detection of Brucella canis via quantitative polymerase chain reaction analysis. Zoonoses Public Health 61: 48-54.
- Scholz HC, Vergnaud G (2013) Molecular characterisation of Brucella species. Rev Sci Tech 32: 149-162.
- Rajashekara G, Eskra L, Mathison A, Petersen E, Yu Q, et al. (2006) Brucella: Functional genomics and host-pathogen interactions. Anim Health Res Rev 7: 1-11.
- Wang Y, Wang Z, Zhang Y, Bai L, Zhao Y, et al. (2014) Polymerase chain reaction-based assays for the diagnosis of human brucellosis. Ann Clin Microbiol Antimicrob 13: 31.
- García-Yoldi D, Marín C, de Miguel M, Vizmanos J, López-Goñi I (2006) Multiplex PCR assay for the identification and differentiation of all Brucella species. 210-345.
- Albert D, López-Goñi I, García-Yoldi D, Marín C, de Miguel M, et al. (2011) New Bruce-ladder multiplex PCR assay for the biovar typing of Brucella suis and the discrimination of Brucella suis and Brucella canis. Vet Microbiology 154: 152-155.
- Bricker BJ, Ewalt DR, Halling SM (2003) Brucella ‘HOOF-Prints’: Strain typing by multi-locus analysis of Variable Number Tandem Repeats (VNTRs). BMC Microbiol 3:15.
- Scholz HC, Vergnaud G (2013) Molecular characterisation of Brucella species. Rev Sci tech 32: 149-162.
- Wattiau P, Whatmore AM, Van Hessche M, Godfroid J, Fretin D (2011) Nucleotide polymorphism-based single-tube test for robust molecular identification of all currently described Brucella species. Appl Environ Microbiol 77: 6674-6679.
- Scholz HC, Nöckler K, Golner C, Bahn P, Vergnaud G, et al. (2010) Brucella inopinata sp. nov., isolated from a breast implant infection. Int J Syst Evol Microbiol 60: 801-808.
- Kassahun A (2004) Epidemiology of cattle and its sero-prevalence in animal health professionals in Sidama Zone Southern Ethiopia. Ethiop Vet J 21: 32-76.
- Quinn PJ, Markery BK, Carter GR (2002) Harcourt Publisher, Virginia, USA.
- Radostits OM, Gay CC, Blood CD, Hinchcliff KW, Arundel JH (2000) Veterinary medicine: A textbook of the disease of cattle, sheep, pigs, goats and horses, (9th edn). Saunders Company, New York, USA.
- Nielsen K (2002) Diagnosis of brucellosis by serology. Vet Microbiol 90: 447-459.
- Saegerman C, Vo TK, De-Waele L, Gilson D, Bastin A, et al. (1999) Diagnosis of bovine brucellosis by skin test: Conditions for the test and evaluation of its performance. Vet Rec 145: 214-218.
- Colibaliy ND, Yamego KR (2000) Prevalence and control of zoonotic disease: Collaboration between public health workers and veterinarians in Burkinafaso. ACTA Trop 76: 53-57.
- Minja KS (2002) Sero-epidemiological survey of Brucella antibodies in indigenous cattle and Human occupational groups in Babati and Hanang districts. MVM thesis, Sokoine University of Agriculture, Morogoro, Tanzania. Pg no: 124.
- Capasso L (2002) Bacteria in two-millennia-old cheese, and related epizoonoses in Roman populations. J Infect 45: 122-127.
- Godfroid J, Cloeckaert A, Liautard JP, Kohler S, Fretin D, et al. (2005) From the discovery of the malta fever’s agent to the discovery of a marine mammal reservoir, Brucellosis has continuously been a re-emerging zoonosis. Vet Res 36: 313-326.
- Refai M (2002) Incidence and control of brucellosis in the Near East region. Vet Microbiol 90: 81-110.
- Refai M (2003) Application of biotechnology in the diagnosis and control of brucellosis in the Near East Region. World J Microbiol Biotech 19: 443-449.
- Godfroid J, Käsbohrer A (2002) Brucellosis in the European Union and Norway at the turn of the twenty-first century. Vet Microbiol 90: 135-145.
- Higgins J, Stuber T, Linfield T, Rhyan J, Berte A, et al. (2012) Molecular epidemiology of Brucellaabortus isolates from cattle, elk, and bison in the United States, 1998 to 2011. Appl Environ Microbiol 78: 3674-3684.
- Le Flèche P, Jacques I, Grayon M, Guilloteau L, Vergnaud G, et al. (2006) Evaluation and selection of tandem repeat loci for a Brucella MLVA typing assay. BMC Microbiol 6: 9.
- Smits HL, Espinosa B, Castillo R, Hall E, Guillen A, et al. (2009) MLVA genotyping of human Brucellaisolates from Peru. Trans R Soc Trop Med Hyg 103: 399-402.
- Whatmore AM, Perrett LL, MacMillan AP (2007) Characterisation of the genetic diversity of Brucellaby multilocus sequencing. BMC Microbiol 7: 34.
- Chain PS, Comerci DJ, Tolmasky ME, Larimer FW, Malfatti SA, et al. (2005) Whole-genome analyses of speciation events in pathogenic Brucellae. Infect Immun 73: 8353-8361.
- Scholz HC, Nöckler K, Göllner C, Bahn P, Vergnaud G, Tomaso H, et al. (2010) Brucella inopinata sp. nov., isolated from a breast implant infection. Int J Syst Evol Microbiol 60: 801-808.
- Megersa B, Biffa D, Niguse F, Rufael T, Asmare K, et al. (2011) Cattle brucellosis in traditional livestock husbandry practice in Southern and Eastern Ethiopia, and its zoonotic implication. Acta Vet Scand 53: 24.
- Dinka H, Chala R (2009) Seroprevalence study of bovine brucellosis in pastoral and agro-pastoral areas of East Showa zone, Oromia Regional State, Ethiopia. American-Eurasian J Agri Environ Sci 6: 508-512.
- Tolosa T, Ragassa FG, Belihy K, Tizazu G (2007) Brucellosis among patients with fever of unknown origin in Jimma University Hospital South Western Ethiopia. Ethiopian Journal of Health Sciences 17: 59-63.
- Mussie H, Tesfu K, Mulugeta T, Kelay B, Yilkal A, et al. (2007) Seroprevalence of brucellosis in cattle and occupationally related human in selected sites of Ethiopia. Ethiop Vet J 11: 49-65
- Kassahun A, Shiv P, Yilkal A, Esayas G, Gelagaye A, et al. (2007) Seroprevalence of brucellosis in cattle and high risk professionals in Sidama Zone, Southern Ethiopia. Ethiop Vet J 11: 69-84.
- Mantur BG, Mangalgi SS (2004) Evaluation of conventional centrifugation blood culture techniques for diagnosis of human brucellosis. J Clin Microbiol 42: 4327-4328.
- Alp E, Koc RK, Durak AC, Yildiz O, Aygen B, et al. (2006) Doxycycline plus streptomycin versus ciprofloxacin plus rifampicin in spinal brucellosis. BMC Infectious Diseases 6: 72.
- Glynn MK, Lynn HK, TV (2008) Brucellosis. J Am Vet Med Assoc 233: 900-908.
- Acha N, Szyfres B (2003) Zoonoses and communicable diseases common to man and animals. Pan American Health Organization (PAHO), Washington, USA.
- Seleem M, Boyle S, Sriranganathan N (2008) Brucella: A pathogen without classic virulence genes. Vet Microbiol 129: 1-14.
Citation: Luelseged A, Zeleke E, Tessema F, Getaneh B, Enbiyale G (2018) Review on Molecular Epidemiology and Public Health Significance of Brucellosis. J Anim Res Vet Sci 2: 007.
Copyright: © 2018 Adonyas Luelseged, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.

