
Risk Assessment of Polycyclic Aromatic Hydrocarbons concentration in Cold Smoked Mullet Fish (Mugil Cephalus)
*Corresponding Author(s):
Mohamed HRFish Processing And Technology Laboratory, Fisheries Division, National Institute Of Oceanography And Fisheries, Cairo, Egypt
Tel:+20 33929900,
Email:hassanaboali66@yahoo.com
Abstract
The purpose of this work was to determine the concentration and risk assessment of Polycyclic Aromatic Hydrocarbons (PAHs) in cold-smoked mullet fish samples which were pre-frozen at -18°C for 6 months. Fish samples were obtained from two fish farms; A (Al-Batts drain) and B (El-Wadi drain), El-Fayoum governorate, Egypt. 16 components of PAHs concentration were determined by GC-MS. Results showed that the total concentration of PAHs recorded 16.2 and 7.4ppb sample of A and B-smoked mullet fish products, respectively. Also, levels of Benzo [a] Pyrene (B {a} P) equivalent were 0.0378 and 0.029ppb in A and B-products, respectively. Besides, content of Low Molecular Weight (LMW) components was higher in A-smoked mullet product than medium MW and vice versa in case B-smoked product however, high MW was not detected in products. In conclusion, PAHs concentration in smoked products processed from pre-frozen mullet samples for 6 months at -18°C are considered a minimally contaminated (16.2ppb) for A-smoked product and not contaminated (7.4ppb) for B-smoked product compared with recommended levels. In addition to the component of Benzo [a] Pyrene (B {a} P) was not detectable in all smoked fish products.
Keywords
INTRODUCTION
Smoking is one of the oldest methods used to process and preserve fish. It is a process of treating fish by exposing it to smoke from smoldering wood or plant materials. This process is usually characterized by an integrated combination of salting, drying, heating and smoking operations in a smoking chamber. The preservation properties of smoking treatment are mainly due to the partial drying and the precipitation of aliphatic and aromatic vapors on fish surface [1-6]. Food cooking and processing methods at high temperatures such as smoking, drying, roasting, baking or frying are recognized as a major source of food contamination by PAHs [7-9].
PAHs compounds are containing 2 or more fused aromatic rings. Polycyclic aromatic hydrocarbons containing 4 rings such as chrysene and benzo [a] anthracene consider weakly carcinogenic compounds, while PAHs which have 5 or more rings are a potentially carcinogenic and mutagenic for human such as Benzo [a] Pyrene (BaP), benzo [g,h,i] perylene, benzo [b] fluoranthene, indeno [1,2,3-c,d] pyrene and benzo [k] fluoranthene [10-12].
Wood smoke contains a hundreds (at least 100) of PAHs and their derivatives which have carcinogenic compounds such as Benzo [a] Pyrene (BaP). BaP consider a marker for carcinogenic PAHs in smoked fish and the maximum level is 2μg/kg. After metabolic activation in mammalian cells to diol-epoxides, PAHs bind covalently to cellular macromolecules, including DNA, thereby causing errors in DNA replication and mutations that initiate the carcinogenic process. This mechanism of activation, with some modifications, occurs with all carcinogenic PAHs [13]. The classification of the International Agency of Research on Cancer for Benzo [a] Pyrene (BaP) was changed from group 2A (probably carcinogenic to humans) to group 1 (carcinogenic to humans), chrysene was changed from group 3 (not classifiable for humans) to group 2B (possibly carcinogenic to humans), and benzo [a] anthracene was re-grouped from 2A to 2B [14,15].
Therefore, the main purpose of this work was to determine the concentration and risk assessment of Polycyclic Aromatic Hydrocarbons (PAHs) in cold-smoked mullet fish samples which were obtained from two fish farms, El-Fayoum governorate, Egypt and frozen storage at -18°C for 6 months.
MATERIALS AND METHODS
Fish samples
Smoking process
Analytical methods
B {a} P equivalent
Statistical analysis
RESULTS AND DISCUSSION
Polycyclic Aromatic Hydrocarbons (PAHs)
|
Compound |
Abbrev. |
Mw |
Rings |
Concentration ( ppb ) |
|
|
Farm (A) |
Farm (B) |
||||
|
Chrysene |
CHR |
228 |
4 |
ND |
ND |
|
Anthracene |
ANT |
178 |
3 |
2.4 |
2.4 |
|
Acenaphthene |
ACE |
153 |
3 |
ND |
ND |
|
Benzo(b)Fluoranthene |
BbF |
252 |
5 |
ND |
ND |
|
Benzo(k)fluoranthene |
BkF |
252 |
5 |
ND |
ND |
|
Dibenzo(a,h)Anthracene |
DahA |
278 |
5 |
ND |
ND |
|
Fluorene |
FLU |
166 |
3 |
3.8 |
?LOQ |
|
Naphthalene |
NA |
128 |
2 |
ND |
ND |
|
Benzo(a)pyrene |
BaP |
252 |
5 |
ND |
ND |
|
Benzo(g,h,i)perylene |
BghiP |
276 |
6 |
ND |
ND |
|
Indeno(1,2,3-c,d)pyrene |
IcdP |
276 |
6 |
ND |
ND |
|
Acenaphthylene |
ACY |
152 |
3 |
ND |
ND |
|
Fluoranthene |
FLA |
202 |
4 |
2.6 |
2.6 |
|
Pyrene |
PYR |
202 |
4 |
2.5 |
2.4 |
|
Benzo(a)anthracene |
BaA |
228 |
4 |
ND |
ND |
|
Phenanthrene |
PHE |
178 |
3 |
4.9 |
?LOQ |
|
Σ16PAHs |
16.2 |
7.4 |
|||
Table 1: Concentration of Polycyclic Aromatic Hydrocarbons (PAHs) in cold-smoked mullet fish samples pre-frozen for 6 months at -18°C.
Note: Farm (A): Al-Batts Drain. Farm (B): El-Wadi Drain. Mw: Molecular weight. LOQ: <2µg/kg.
ND: not detected.
Toxic Equivalent Factors (TEFs) and B {a} P equivalent of PAHs found in smoked mullet fish samples
The Toxic Equivalent Factors (TEFs) and B [a] P Equivalent of PAHs in smoked mullet fish are presented in table 2. The B [a] P Equivalent of Fluorene; Phenanthrene; Anthracene; Fluoranthene and Pyrene were 0.0038; 0.0049; 0.024; 0.0026 and 0.0025 respectively and the ∑(BaPeqi) was 0.0378 for farm A smoked samples (Figure 1). In the other farm samples (B) the B [a] P Equivalent of Anthracene; Fluoranthene and Pyrene were 0.024; 0.0026 and 0.0024 respectively and the sum of B [a] P Equivalent ∑(BaPeqi) were 0.029 (Figure 2).
|
Compound |
TEF |
Farm (A) |
Farm (B) |
||
|
Conc.(ppb) |
BaPeqi |
Conc. (ppb) |
BaPeqi |
||
|
Naphthalene |
0.001 |
ND |
- |
ND |
- |
|
Acenaphthylene |
0.001 |
ND |
- |
ND |
- |
|
Acenaphthene |
0.001 |
ND |
- |
ND |
- |
|
Fluorene |
0.001 |
3.8 |
0.0038 |
<LOQ |
- |
|
Phenanthrene |
0.001 |
4.9 |
0.0049 |
<LOQ |
|
|
Anthracene |
0.01 |
2.4 |
0.024 |
2.4 |
0.024 |
|
Fluoranthene |
0.001 |
2.6 |
0.0026 |
2.6 |
0.0026 |
|
Pyrene |
0.001 |
2.5 |
0.0025 |
2.4 |
0.0024 |
|
Benzo(a)anthracene |
0.1 |
ND |
- |
ND |
- |
|
Chrysene |
0.01 |
ND |
- |
ND |
- |
|
Benzo(b)fluoranthene |
0.1 |
ND |
- |
ND |
- |
|
Benzo(k)fluoranthene |
0.1 |
ND |
- |
ND |
- |
|
Benzo(a)pyrene |
1.0 |
ND |
- |
ND |
- |
|
Indeno(1,2,3,c)pyrene |
0.1 |
ND |
- |
ND |
- |
|
Dibenzo(a,h)anthracene |
1.0 |
ND |
- |
ND |
- |
|
Benzo(g,h,i)perylene |
0.01 |
ND |
- |
ND |
- |
|
∑(BaPeqi) |
0.0378 |
|
0.029 |
||
Table 2: Toxic Equivalent Factors (TEFs) and B [a] P Equivalent of PAHs found in pre-frozen cold smoked mullet fish for6 months at -18°C.
Note: TEF: Toxic Equivalent Factor; BaPeqi[a]: P equivalent.Farm (A): Al-Batts Drain; Farm (B): El-Wadi Drain.
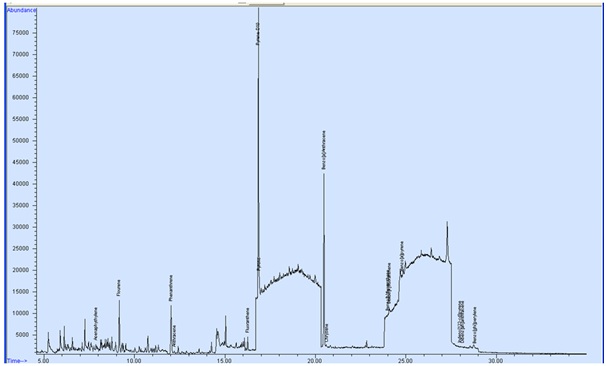 Figure 1: GC-MS chromatogram of smoked mullet fish (farm A).
Figure 1: GC-MS chromatogram of smoked mullet fish (farm A).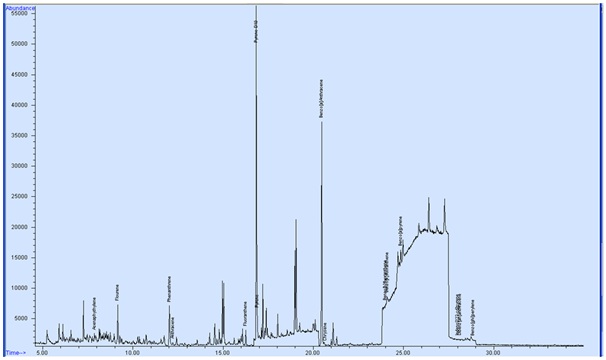 Figure 2: GC-MS chromatogram of smoked mullet fish (farm B).
Figure 2: GC-MS chromatogram of smoked mullet fish (farm B).Molecular weight of PAHs in smoked fish
|
Concentrations (ppb) of the PAHs for Farm A Samples |
Concentrations (ppb) of the PAHs for Farm B Samples |
||||
|
HMW |
MMW |
LMW |
HMW |
MMW |
LMW |
|
- |
5.1 |
11.1 |
- |
5 |
2.4 |
Table 3: Table 3: Total mean concentration (ppb) of PAHs in cold smoked fish, according to their molecular weights.
Note: HMW: High Molecular Weight; MMW: Medium Molecular Weight; LMW: Low Molecular Weight.
Farm (A): Al-Batts Drain; Farm (B): El-Wadi Drain: was not detectable.
Category of PAH concentration
Category of PAH concentration (ppb) in the studied smoked samples is shown in table 4. Concentrations of PAHs were 16.2 and 7.4ppb in smoked fish from farms (A) and (B), respectively. Based on these results, categories of concentration of PAH are considered a minimally contaminated (10-99ppb) for A-smoked product, may be due to pollutants presented in Al-Batsdrain and not contaminated (?10ppb) for B-smoked product compared with recommended levels as set by [28].
|
A-Smoked Mullet Product |
B-Smoked Mullet Product |
||
|
Category |
ΣPAHs (ppb) |
Category |
ΣPAHs (ppb) |
|
Minimally contaminated |
16.2 |
Not contaminated |
7.4 |
Table 4: Category of PAH concentration (ppb) in the studied cold smoked mullet samples.
Note: Farm (A): Al-Batts Drain. Farm (B): El-Wadi Drain.
CONCLUSION
It could be concluded PAHs concentration in smoked mullet products processed from pre-frozen mullet samples for 6 months at -18°C are considered a minimally contaminated (16.2ppb) for A-smoked mullet product and not contaminated (7.4ppb) for B-smoked product compared with recommended levels. In addition to the component of Benzo [a] Pyrene (B {a} P) was not detectable in all smoked fish products.
REFERENCES
- Swastawati F, Suzuki T, Dewi EN (2000) The effect of liquid smoke on the quality and omega-3 fatty acids content of tuna fish (Euthynnusaffinis). Journal of Coastal Development 3: 573-579.
- Simko P (2002) Determination of polycyclic aromatic hydrocarbons in smoked meat products and smoke flavouring food additives. J Chromatogr B Analyt Technol Biomed Life Sci 770: 3-18.
- Hultmanna L, BenczeRøra? AM, Steinslandc I, Rustad T, Ska?ra T (2004) Proteolytic activity and properties of proteins in smoked salmon. Food Chemistry 85: 377-378.
- Sto?yhwo A, Sikorski ZE (2005) Polycyclic aromatic hydrocarbons in smoked fish-A critical review. Food Chemistry 91: 303-311.
- Bilgin, ?, Ünlüsay?n M, ?zci L, Günlü A (2008) The determination of the shelf life and some nutritional components of gilthead seabream (Sparusaurata L., 1758) after cold and hot smoking. Turkish Journal of Veterinary and Animal Sciences (Turkey) 32: 49-56.
- Shalaby AR (2000) Relation between mackerel fish smoking and its chemical changes with emphasis on biogenic amines. J Agric Sci Mansoura Univ 25: 353-365.
- CCFAC (2005) Codex Committee on Food Additives and Contaminants. Discussion paper on polycyclic aromatic hydrocarbons contamination. 37th Session, The Hague, The Netherlands.
- Yurchenko S, Mölder U (2005) The determination of polycyclic aromatic hydrocarbons in smoked fish by gas chromatography mass spectrometry with positive-ion chemical ionization. J Food Comp Anal 18: 857-869.
- Scientific Committee on Foods of EC, SCF (2002) Opinion of the Scientific Committee on Food on the risks to human health of Polycyclic Aromatic Hydrocarbons in food. Scientific Committee on Foods of EC, SCF, Brussels.
- Martorell I, Perelló G, Martí-Cid R, Castell V, Llobet JM, et al. (2010) Polycyclic Aromatic Hydrocarbons (PAH) in foods and estimated PAH intake by the population of Catalonia, Spain: Temporal Trend. Environ Int 36: 424-432.
- Zhang H, Xue M, Dai Z (2010) Determination of polycyclic aromatic hydrocarbons in aquatic products by HPLC-fluorescence. J Food Compos Anal 23: 469-474.
- Alomirah H, Al-Zenki S, Al-Hooti S, Zaghloul S, Sawaya W, et al. (2011) Concentrations and dietary exposure to Polycyclic Aromatic Hydrocarbons (PAHs) from grilled and smoked foods. Food Control 22: 2028-2035.
- Falcó G, Domingo JL, Llobet JM, Teixidó A, Casas C, et al. (2003) Polycyclic aromatic hydrocarbons in foods: Human exposure through the diet in Catalonia, spain. J Food Protect 66: 2325-2331.
- IARC (1987) IARC Monographs on the Evaluation of Carcinogenic Risks to Humans. Overall Evaluations of Carcinogenicity. IARC Monographs. International Agency for Research on Cancer, Lyon, France.
- IARC (2010) IARC Monographs on the Evaluation of Carcinogenic Risks to Humans. Some Non-heterocyclic Polycyclic Aromatic Hydrocarbons and Some Related Exposures. International Agency for Research on Cancer, Lyon, France.
- Khorshid M, Souaya ER, Hamzawy AH, Mohammed MN (2015) QuEChERS Method followed by solid phase extraction method for gas chromatographic mass spectrometric determination of polycyclic aromatic hydrocarbons in fish. Int J Anal Chem.
- Nisbet IC, LaGoy PK (1992) Toxic Equivalency Factors (TEFs) for Polycyclic Aromatic Hydrocarbons (PAHs). Regul Toxicol Pharmacol 16: 290-300.
- Isioma T, Ozekeke O, Lawrence E (2017) Human health risk assessment of Polycyclic Aromatic Hydrocarbons (PAHs) in smoked fish species from markets in Southern Nigeria. Toxicol Rep 4: 55-61.
- AFSSA (2003) AFSSA opinion on a request for an opinion on the risk assessment of benzo [a] pyrene B [a] P and other polycyclic aromatic hydrocarbons (PAHs), present in various commodities or in certain vegetable oils, as well as PAH concentration levels in commodities beyond which health problems may arise. French Food Safety Agency.
- Varlet V, Serot T, Monteau F, Le Bizec B, Prost C (2007) Determination of PAH profiles by GC-MS/MS in salmon processed by four cold-smoking techniques. Food Addit Contam 24: 744-757.
- Perugini M, Visciano P, Giammarino A, Manera M, Di Nardo W, et al. (2007) Polycyclic aromatic hydrocarbons in marine organisms from the Adriatic Sea, Italy. Chemosphere 66: 1904-1910.
- EFSA (2002) European Food Safety Authority. Scientific Committee on Food. Opinion on the risks to human health of polycyclic aromatic hydrocarbons in food, Italy.
- Maga JA (1988) Smoke in Food Processing. CRC Press, Florida, USA.
- Bartle KD (1991) Analysis and occurrence of PAHs in food. In: Creaser CS, Purchase R (eds.). Food Contaminants: Sources and Surveillance. Royal Society of Chemistry, London, UK. Pg no: 41-60.
- Nakamura T, Kawamoto H, Saka S (2008) Pyrolysis behavior of Japanese cedar wood lignin studied with various model dimers. Journal of Analytical and Applied Pyrolysis 81: 173-182.
- Essumang DK, Dodoo DK, Adjei JK (2013) Effect of smoke generation sources and smoke curing duration on the levels of Polycyclic Aromatic Hydrocarbon (PAH) in different suites of fish. Food Chem Toxicol 58: 86-94.
- Chukwujindu MA, Francisca IB, Iwekumo A, Eferhire A, Grace OBI (2016) Concentrations and risks of polycyclic aromatic hydrocarbons in smoke-cured fish products in Nigeria. International Journal of Environmental Studies 73: 827-843.
- Seyedeh LMN, Wan RI, Mohamad PZ (2013) Residual concentration of PAHs in seafood from hormozgan province, Iran: Human health risk assessment for Urban population. International Journal of Environmental Science and Development 4: 393-397.
Citation: Hafez NE, Awad AM, Ibrahim SM, Mohamed HR, El-Lahamy AA (2019) Risk Assessment of Polycyclic Aromatic Hydrocarbons concentration in Cold Smoked Mullet Fish (Mugil Cephalus). J Toxicol Cur Res 3: 008.
Copyright: © 2019 Hafez NE, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.

