
Rpoe Gene Mutant Salmonella Enterica Serovar Typhimurium Protects From Wild Type Typhimurium Infection In Mice Model
*Corresponding Author(s):
Gopal NathDepartment Of Microbiology, Institute Of Medical Sciences, Banaras Hindu University, Varanasi, UP, India
Email:gopalnath@gmail.com
Abstract
Typhoid is a food and water-borne infectious disease transmitted through the faecal-oral route. The causative bacterium Salmonella Typhi has emerged resistant to several drugs and causes thousands of death worldwide. Ironically, the two available vaccines protect for 3 to 7 years only, with protection rates ranging between 50 and 70%. Therefore, the rpoE mutant of Salmonella species was screened for its vaccine potential against typhoid with the expectation of a long-lasting immune response. The immune potential of the ΔrpoE strain (ΔME) of Salmonella Typhimurium was evaluated in the BALB/c mice, a surrogate model. We took four groups with six mice in each. The LD50 (lethal dose) was decided for oral and intraperitoneal routes. The experimental groups were vaccinated with different doses of ΔrpoE strain, including the oral group. Multiple-dose of the mutant was given. Further, the mice with a lethal dose of wild type were challenged to see the protection against S. Typhimurium. In addition, we examined the faecal excretion of wild-type bacteria and humoral immune response. The booster dosage on the 7th and 14th days enhanced antibody titre significantly. In control groups, no mice survived after ten days of challenge, whereas in the vaccinated groups, more than 83% survived.
Keywords
Mutant; rpoE; Typhi; Typhoid; Vaccine
Introduction
Typhoid fever is caused by Salmonella enterica serovar Typhi (henceforth S. Typhi), a gram-negative bacteria belonging to Enterobacteriaceae family. It causes 22 million cases, with more than 200,000 deaths yearly [1]. This orofecally transmitted bacteria is highly prevalent in populations with poor sanitation and inadequate hygiene. It is difficult to diagnose enteric fever from other febrile illnesses clinically. Unfortunately, apart from prolonged illnesses, death in typhoid may occur due to intestinal perforation, hemorrhages, and other neurological manifestations if left untreated [2]. Although appropriate antibiotics reduce the duration of fever, bacterial shedding and mortality rate, in the recent past, indiscriminate use of antimicrobials has led to the emergence of multidrug resistance in enteric fever-causing serotypes. Intriguingly, humans are the only reservoir for these serovars. Despite developing the first typhoid vaccine (whole-cell killed), >100 years ago [3] we have only two anti-typhoid licensed vaccines internationally. Live attenuated Ty21a is given orally to a population of more than five years of age. The other vaccine is ViCPS, an injectable polysaccharide vaccine derived from Vi antigen of serovar Typhi that may be given to children >2 years of age. Currently available vaccines provide 65-70% protection for 3-7 years [4-6]. Live attenuated vaccine (LAV) is considered superior to subunit vaccine because LAV induces humoral and cell-mediated immunity. Different live attenuated candidate vaccines targeting mostly metabolic or virulence genes are under different phases of clinical trials. One such candidate vaccine is CVD909, a triple mutant of aroC, aroD and htrA and is under phase II trial constitutively expressing Vi antigen. The other candidate, Ty800 is a phoP-phoQ gene mutant developed by Avant Immunotherapeutics and under phase II trial. Several other mutants developed by targeting clpX, htrA, ssaV, aro operon, and cya/crp are under clinical trials. Other genes such as dam, wecA, cdt, recA, recB, relA, spoT, and rpoS mutant have also been checked for their vaccine potential. The search for an ideal vaccine against S. Typhi infection is still on (Plotkin and Cam, 1995). The lifestyle of this restricted human pathogen is a major hurdle in vaccine development. It survives extra- and intracellularly and tolerates our host defense system well. It has various mechanisms to evade and colonize the host organs and tissues.
Bacteria have a set of two types of genes; one is housekeeping and the luxury gene. A stress regulator is a luxury gene set induced in stress conditions. These stress regulator genes are shut off in normal situations and are induced exclusively to withstand harsh conditions. The rpoE is a stress regulator gene that is induced under periplasmic stress. The outer surface of bacteria is the primary site interacting with the host and targets of the defense system [3]. The SigmaE gene (rpoE in Salmonella) is an alternative sigma factor of Escherichia coli, which plays a role in maintaining cell envelope integrity in normal growth in stress conditions. It is required for cell viability and maintenance of membrane physiology during stress [7-9] . It is mainly induced either by heat stress or ethanol which disrupts the protein folding in the cell membrane. It may also be induced when overproduction of outer membrane porin or deactivation of periplasmic chaperones under normal conditions occurs for any reason [10-15]. The irony is that we do not have an exact animal model for S. Typhi infection. However, this is encouraging that S. Typhimurium, a murine pathogen mimics a similar infection process [16] It is also interesting to note that serotype Typhimurium encoded rpoE gene, controls its survival and proliferation inside macrophages, as is in the case of S. Typhi. It regulates Type-III secretion systems (T3SS) encoded by Salmonella Pathogenicity Island-1(SPI-1) and SPI-2, stress responses, and several global regulators. In addition, it controls SPI-2 by upregulation of ssrB and downregulation of the H-NS gene, which is required for intracellular survival [17] We hypothesize that if the rpoE (SigmaE) gene is deleted from S. Typhimurium, it will succumb to the stressful conditions inside the macrophage and be killed host in the macrophages. These macrophages may present the Salmonella antigen on their surface as antigen-presenting cells. This phenomenon may lead to induction of the better humoral and cell-mediated immune responses. Therefore, in the present study, we planned to delete the rpoE gene of S. Typhimurium and evaluate its efficacy in the mice model (BALB/c).
Results And Discussion
Deletion and characterization of rpoE mutant
The lambda red recombination system removed the rpoE from Salmonella enterica serovar Typhimurium. Mutants were confirmed by colony PCR using the internal sequences of rpoE gene (Figure. 1).
 Figure 1: Showing the plan for deletion of rpoE gene.
Figure 1: Showing the plan for deletion of rpoE gene.
The flanking genes of rpoE, left side genes are rseA, rseB, rseC and right side are nadB, yfiC, srmB. The sequence of rpoE gene is available in Pubmed data and shown just below the rpoE gene. The primers, H1 and H2 have been selected from the immediate neighbouring genes of rpoE, i.e. rseA on the left and nadB on the right side. The primers P1 and P2 show a priming site for extending the resistance gene (kanamycin) present on the pKD4 plasmid.
The growth curve was identical when the ΔME and WT were incubated at 37°C without acid exposure in the liquid medium (Figure. 2a); however, after giving a 10 min and 20 min exposure to pH 3.5, the WT could grow with a longer log phage of 7.5h at both the temperatures of 37°C and 42°C while ΔME could not enter the log phase despite the viability of the bacterial cells, which was evident by the recovery on inoculating the acid-exposed ΔME in bile broth with 9h of log phase (Figure 2a-c).
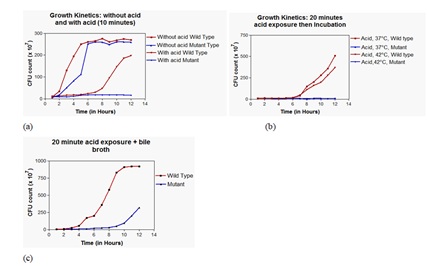 Figure 2: Growth kinetics (a) Without acid exposure and with 10 minutes acid exposure. (b) Acid exposure (20 minutes) and incubation at different temperatures. (c) 20 minutes acid exposure and bile broth enrichment.
Figure 2: Growth kinetics (a) Without acid exposure and with 10 minutes acid exposure. (b) Acid exposure (20 minutes) and incubation at different temperatures. (c) 20 minutes acid exposure and bile broth enrichment.
Evaluation of single-dose immunization through intraperitoneal route by ΔME
A single immunization via IP route with ΔME at 1.3 104 CFU/mouse gave 55.55%, and 1.3 106 CFU/mouse gave 61.6% protection (Table 1).
Evaluation of triple-dose immunization through intraperitoneal and oral routes by ΔME
The three booster doses of 1.3 104 CFU/mouse and 1.3 106 CFU/mouse given IP provided a protection rate of 61.1 and 83.3%, respectively, while oral doses of 1.3 104 CFU/mouse gave protection of 66.7%, and 1.3 106 CFU/mouse and 1.3 107 CFU/mouse protected 88.8% of the mouse (Table 1).
|
WT-STM (CFU/ mouse), IP |
Mortality (%) |
Total doses and route |
ΔME (CFU/ mouse), |
Mice survived (total number =18) |
Percentage of survival |
Antibody titre against the somatic antigen |
|
1.1 102 |
00 |
Single dose, IP |
1.3 104 |
10 |
55.5 |
<1:20 |
|
1.1 103 |
50 |
1.3 106 |
11 |
61.1 |
<1:20 |
|
|
1.1 104 |
70 |
Triple dose, IP |
1.3 104 |
11 |
61.1 |
>1:160 |
|
1.1 105 |
100 |
1.3 106 |
15 |
83.3 |
>1:640 |
|
|
|
|
Triple dose, O |
1.3 104 |
12 |
66.7 |
>1:160 |
|
|
|
1.3 106 |
16 |
88.8 |
>1:640 |
|
|
|
|
1.3 107 |
16 |
88.8 |
>1:320 |
Table 1: Evaluation of different doses through oral and intraperitoneal routes of ΔME for the protection from the IP challenge of WT of S. Typhimurium at the LD50 dose (1.1 103 CFU/mouse).
IP - Intraperitoneal route and O – oral
Safety of ΔME in mice model through intraperitoneal and oral routes
The table shows that the ΔME at doses 1.3 104, 1.3 105, 1.3 106, 1.3 107, 1.3 108 orally and IP up to the dose of 1.3 107 CFU/mouse did not cause mortality. All the groups could detect viable ΔME after 7 days of the last vaccination dose in their stool. However, the LD50 for WT was 1.1 103 CFU/mouse (Table 2).
|
WT-STM (CFU/ mouse), IP |
Mortality (%) |
ΔME (CFU/ mouse), IP |
Mortality (%) |
ΔME (CFU/ mouse), O |
Mortality (%) |
|
1.1 102 |
00 |
1.3 104 |
00 |
1.3 104 |
00 |
|
1.1 103 |
50 |
1.3 05 |
00 |
1.3 05 |
00 |
|
1.1 104 |
70 |
1.3 106 |
00 |
1.3 106 |
00 |
|
1.1 105 |
100 |
1.3 107 |
00 |
1.3 107 |
00 |
|
|
|
|
|
1.3 108 |
00 |
Table 2: Determination of lethal and safety of wild (WT) and mutant (ΔME) S. Typhimurium in BALB/c mice through different routes.
Even after 125 years of the first effective vaccine developed by Almroth Edward Wright, we do not have near-ideal vaccines against typhoid fever. These human-restricted human bacterial Salmonella serotypes can be eradicated from the globe if we could develop a satisfactory and acceptable vaccine. A live attenuated oral vaccine is the most desired of many vaccine-development designs since this bacterium may also go intracellularly, inducing a cell-mediated immune response. For the development of live oral vaccines, several approaches, e.g. chemical mutagenesis, genetically engineered vaccines with desired mutations in one or many genes, e.g. rpoS, phoPQ, ssaV, and htrA intended to block systemic infection have been tried [18]. They are either in the experimental or early stages of clinical trials. In a similar line, we planned to delete the rpoE gene. This gene is responsible for controlling 40% of the regulator genes of Salmonella (168 out of 411) [19]. for several functions, e.g. coordinates multiple stress response, intramacrophage survival and proliferation, maintenance of cell envelope integrity, regulates expression of both type 3 secretion system (T3SS) and all aspects of membrane physiology under stress environments. The intracytoplasmic pH is usually lower than 7.0, especially in Salmonella-containing vacuoles (SCV), where the S. Typhi resides. The lower pH in the SCV induces the expression of several proteins responsible for inhibition of fusion of SCV with lysosomal vesicles and intramacrophage survival. The genes regulated by this sigma factor (rpoE) can inhibit the killing of a bacterium by the host cell. Based on this principle, we deleted the rpoE gene expecting the bacterium to be killed in the cytoplasm since this deletion will result in the fusion of SCV with vesicles containing lysosomes. After killing, the phagosomes may present the Salmonella antigen for interaction with immune cells to induce a specific immune response. In the present study, we took S. Typhimurium, and its natural host, a rodent (mice), as a surrogate because enteric fever-causing serotypes can infect only human beings and, to some extent, a few higher primates (Chimpanzees). This is an accepted surrogate model for animal experimentation for S. Typhi and other serotypes. For creating the rpoE mutant, we used the one-step gene inactivation method [20]. After deciding on the lethal dose of the wild-type S. Typhimurium through oral and intraperitoneally routes in BALB/c mouse, single and multiple doses of the mutant were given through both routes mentioned above. The mice were observed for eight weeks after the challenge with the wild-type strain.
Interestingly, a 106 CFU/mouse IP dose protected more than 88% of the animals under experimentation. Interestingly higher doses (107 CFU/mouse) of mutant strain with multiple booster doses were required to achieve a similar level of protection if given orally. When antibody titres were estimated based on Widal's principle four weeks after the mutant strain's last dose, a significant rise in antibody titre could be seen in the mice given 106 CFU/mouse IP and 107 CFU/mouse orally.
It is worth mentioning that neither oral nor intraperitoneally administered mutant strain at a dose of 108 CFU/mouse result in fatality in any experimental mouse. The findings in the present study showed that rpoE gene mutant was very safe and able to induce a protective level of immunity in the BALB/c mouse model.
A live attenuated bacterial vaccine to eradicate typhoid fever is essential. The ideal candidate vaccine should induce both humoral and cell-mediated immunity systemically and at mucosal surfaces after a single oral administration. However, a significant rise in antibody titre against S. Typhimurium indicates the potential of the rpoE gene mutant to induce an immune response.
The rpoE mutant of S. Typhimurium generated in the present study survived at pH 3.5 for 20 min. This observation indicates that the mutant can pass through the stomach without compromising viability before reaching the small intestine. This is further supported by isolating mutant strains from faeces for 7 days after vaccination. Intriguingly, the faecal excretion was not associated with any visible morbidity.
It is worth mentioning here that despite being safe, the lacunae of the mutant were that a single dose was unable to induce a protective level of immunity. As the bacterium was excreted in the faeces for more than a week, it might have been able to boost the immune response. However, given the lack of data relevant to mechanisms involved in disease clearance, it may not be denied that rpoE mutant experimentally proven to be safe and highly immunogenic may nonetheless fail to offer satisfactory protection when administered orally/IP as a single dose. If a schedule of administration of the present mutant is standardized in animals providing satisfactory protection even with multiple doses will negate the need to re-engineer the mutant further to provide significant immunity.
Deleting stress response genes makes the bacteria susceptible to the hostile intracellular environment of reticuloendothelial cells leading to killing and its’ antigens being presented to immune responsive cells. We had ΔrpoE mutant of S. Typhimurium, which was observed to be sufficiently attenuated and gave adequate protection with booster doses.
Materials And Methods
The ethical clearance for this work was taken from the Institutional Animal Ethics Committee of the Institute of Medical Science, Banaras Hindu University, Varanasi, 221005 (no. Dean/2015-16/EC/1709 dated 19/03/2016).
Generation of the rpoE deletion mutant in S. Typhimurium
The ΔrpoE (ΔME) was constructed in the wild-type reference strain of S. Typhimurium ATCC 14028. The ΔrpoE (ΔME) was generated by a one-step gene inactivation strategy described by Wanner et. al., [20]. The ATCC 14028 strain of Typhimurium was transformed with pKD20 carrying lambda red recombinase system under the arabinose inducible promoter. The transformants carrying the helper plasmid pKD20 were grown in LB containing ampicillin (100 mg/ml) and 7 mM L-arabinose (Himedia) till the OD reached 0.5–0.6 at 600 nm. Electro-competent cells were prepared by washing the cells thrice with ice-cold Milli-Q water and 10% glycerol. PCR product containing the kanamycin resistance gene (amplified from pKD4) flanked by sequences upstream and downstream of the rpoE gene was amplified with primers (rpoE H1 and rpoE H2) were electroporated into the S. Typhimurium carrying pKD20 as per instructions given by the manufacturer's instructions (Bio-Rad, USA). Mutants were selected for their ability to grow on LB-containing kanamycin (75 mg/ml). The deletion was confirmed by specific primers targeting the inside sequences of the rpoE. In the ΔrpoE strain of S. Typhimurium, no amplification could be detected, while in the wild-type, 124 bp segment was amplified. Thus, the deletion mutant was designated as Δ rpoE (ΔME) in Typhimurium. The primers used in the study are shown in (Table 3).
|
|
Sequences |
Annealing temperature |
Amplicon Size |
|
Deletion primers |
|||
|
rpoE:H1 F |
5’GGTCCTGGTTGAACGGGTCCAGAAGGGAGATCAGAAAGCCGTGTAGGCTGGAGCTGCTTC3’ |
58°C |
1.5 kbp |
|
rpoE:H2 R |
5’GAACTTTATTATCAATAGCTTCCCGCGCCCGGAAGATACGCATATGAATATCCTCCTTAG3’ |
||
|
Confirmation primers for deletion |
|||
|
rpoE: F |
5'CATGATAGCCGCTATCTCTTC3' |
59°C |
124 bp |
|
rpoE:R |
5’GTTGTCAGAAGAACTGAGACAG3’ |
||
Table 3: In-house designed primers for deletion of rpoE and confirmation of mutant with a deletion in Salmonella Typhimurium.
The plan for mutant generation is shown in figure-1.
The growth curve at different pH
Ten microliters of overnight broth culture were inoculated in 50 ml LB in triplicate and incubated at 37°C, shaking at 150 rev/min know the in-vitro growth kinetics of ΔME and WT strain.
Animal experimentation
Six to eight-week-old BALB/c female mice were procured from Central Drug Research Institute, Lucknow and maintained in Central Animal Facility, Banaras Hindu University, Varanasi, India, under specific pathogen-free conditions. The plan of experimentation has been shown in (Tables 1 & 2).
Determinations of Lethal Dose (LD50) of WT and ΔME
Six to eight-week-old BALB/c female mice were inoculated to determine the LD50 value. First, four mice were injected with the wild and mutant serotype intraperitoneally (IP), and orally, as shown in (Table 2). Deaths of the animal were recorded for up to 2 weeks.
Fecal shedding of the Salmonella Typhimurium (WT) and ΔME
Three groups of mice were inoculated intraperitoneal routes with WT and ΔME through oral and IP at the dose of 106 CFU/mouse. Freshly recovered faecal pellets were collected, and the colony count of the bacterium per gram of stool was calculated.
Evaluation of vaccine potential of mutant strain (ΔME)
Single-dose immunization through oral and IP routes and multiple booster doses through oral and IP routes, as shown in Table 3, was evaluated after giving the challenge of WT S. Typhimurium at the LD50 dose of 1.1 103 CFU/mouse.
Estimation of humoral immune response
One millilitre of blood was collected and preserved for control. Antigen was prepared from overnight growth WT. It was washed thrice and fixed with formalin. The concentration of bacteria was brought to 1.5 108 CFU/ml. The agglutination was done with the pre and post-vaccination serum collected from the experimental mice following the tube method of agglutination.
Acknowledgements
This work was supported by the Council of Scientific and Industrial Research (CSIR) as a JRF to Shailendra Singh and by the Department of Biotechnology (DBT) under MINISTRY OF SCIENCE & TECHNOLOGY, Government of India as JRF to Virendra Bahadur Yadav. In addition, we gratefully acknowledge the lab facilities and equipment provided by Viral Research and Diagnostic Laboratory, Department of Microbiology, Institute of Medical Sciences, Banaras Hindu University, Varanasi.
Conflicts of interest
No conflict of interest declared.
Significance and impact of the study
The SigmaE gene (rpoE in Salmonella) is a luxury gene induced in stress conditions which play a role in maintaining cell envelope integrity in normal growth in stress conditions rpoE. Lower pH in the intracellular niche leads to the killing of the mutated bacteria and antigens are expressed on the surface of macrophages inducing a better immune response. In the present study, we have observed a significant rise in antibody titre against S. Typhimurium indicates the potential of the rpoE gene mutant to induce an immune response. Thus, rpoE mutants may be a potential candidate vaccine for typhoid.
References
- Nath G, Yadav, Bahadur V, Singh SK (2022) Biofilm and Chronic Typhoid Carriers with Special Reference to Bacteriophage Therapy. Journal of Gastrointestinal Infections 11: 36-41.
- Crump J. A, Mintz ED (2010) Global trends in typhoid and paratyphoid fever. Clinical Infectious Diseases 50: 241-246.
- Plotkin SA, Cam NBL (1995) A New Typhoid Vaccine Composed of the Vi Capsular Polysaccharide. Archives of Internal Medicine 155: 2293-2299.
- Allal R, Kastler B, Gangi A, Bensaid AH, Bouali O, et al. (1993) Splenic abscesses in typhoid fever: Us and ct studies. Journal of Computer Assisted Tomography 17: 90-93.
- Klugman KP, Koornhof HJ, Robbins JB, Le Cam NN (1996) Immunogenicity, efficacy and serological correlate of protection of Salmonella typhi Vi capsular polysaccharide vaccine three years after immunization. Vaccine 14: 435-438.
- Engels EA, Falagas ME, Lau J, Bennish ML (1998) Typhoid fever vaccines: A meta-analysis of studies on efficacy and toxicity. British Medical Journal 316: 110-116.
- De Las Penas A, Connolly L, Gross C A (1997) σ(E) is an essential sigma factor in Escherichia coli. Journal of Bacteriology 179: 21.
- Dartigalongue C, Missiakas D, Raina S (2001) Characterization of the Escherichia coli σE Regulon. Journal of Biological Chemistry 276: 20866-20875.
- Rezuchova B, Miticka H, Homerova D, Roberts M, Kormanec J (2003) New members of the Escherichia coli σE regulon identified by a two-plasmid system. FEMS Microbiology Letters 225: 1-7.
- Erickson JW, Gross CA (1989) Identification of the sigma E subunit of Escherichia coli RNA polymerase: a second alternate sigma factor involved in high-temperature gene expression. Genes & development 3: 1462-1471.
- Wang QP, Kaguni JM (1989) A novel sigma factor is involved in expression of the rpoH gene of Escherichia coli. Journal of bacteriology 171: 4248-4253.
- Mecsas J, Rouviere PE, Erickson JW, DonohueTJ, Gross CA (1993) The activity of σ(E), an Escherichia coli heat-inducible σ-factor, is modulated by expression of outer membrane proteins. Genes and Development 7: 2618-2628.
- Raina S, Missiakas D, Georgopoulos C (1995) The rpoE gene encoding the σ(E) (σ24) heat shock sigma factor of Escherichia coli. EMBO Journal 14: 1043-1055.
- Rouviere PE, Penas ADL, Mecsas J, Lu CZ, Rudd KE, et al. (1995) rpoE, the gene encoding the second heat-shock sigma factor, σ(E), in Escherichia coli. EMBO Journal 14: 1032-1042.
- Missiakas D, Betton JM, Raina S (1996) New components of protein folding in extracytoplasmic compartments of escherichia coli SurA, FkpA and Skp/OmpH. Molecular Microbiology 21: 871-884.
- Yadav VB, Nath G (2022) Bacteriophage therapy of human?restricted Salmonellaspecies-a study in a surrogate bacterial and animal model. Letters in Applied Microbiology 75: 422-430.
- Li J, Overall CC, Nakayasu ES, Afshan SK, Marcus BJ, et al. (2015) Analysis of the Salmonella regulatory network suggests involvement of SsrB and H-NS in σE-regulated SPI-2 gene expression. Frontiers in Microbiology 6: 27.
- Galen JE, Amanda DB, Sharon MT, Marcela FP (2016) Live Attenuated Human Salmonella Vaccine Candidates: Tracking the Pathogen in Natural Infection and Stimulation of Host Immunity. EcoSal Plus 7: 10.
- Li J, Overall CC, Johnson RC, Marcus BJ, Jason E M, et al. (2015) ChIP-seq analysis of the σe regulon of salmonella enterica serovar typhimurium reveals new genes implicated in heat shock and oxidative stress response. PLoS ONE 10: 138466.
- Datsenko K A, Wanner B L (2000) One-step inactivation of chromosomal genes in Escherichia coli K-12 using PCR products. Proceedings of the National Academy of Sciences of the United States of America 97: 6640-6645.
Citation: Nath G, Singh S, Yadav VB (2023) Rpoe Gene Mutant Salmonella Enterica Serovar Typhimurium Protects From Wild Type Typhimurium Infection In Mice Model. J Vaccines Res Vaccine 9: 23.
Copyright: © 2023 Gopal Nath, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.

