
Seroprevalence, Isolation and Associated Risk Factors of Contagious Bovine Pleuropneumonia at Bako Tibe and Ilu Galan Districts of West Shoa Zone, Western Ethiopia
*Corresponding Author(s):
Abebe Olani BultoNational Animal Health Diagnostic And Investigation Center, Sebeta, Ethiopia
Tel:+251 913176665,
Email:abebenaol@gmail.com
Abstract
Contagious bovine pleuropneumonia is a disease of cattle caused by Mycoplasma mycoides subspecies mycoides small colony and it is one of the most important threats to cattle health and production. Therefore, a cross-sectional study was conducted to assess the occurrence and risk factors of contagious bovine pleuropneumonia, using serological methods, and isolation of the causative agent in the selected districts of West Shoa Zone, Western Ethiopia from December 2019 to April 2020. A total of 106 households were purposively selected and interviewed with structured questionnaire, and from interviewed households a total of 604 cattle were randomly selected and serum samples were collected for c-ELISA test. The overall animal levels, herd level seroprevalence of contagious bovine pleuropneumonia and culture were 33.77% (95%C7I: 30.037.70), 55.7% (95%CI: 45.6865.31) and 6.67% (95%CI: 1.8416.19), respectively. Multivariate logistic regression analysis showed that risk factors such as district, Peasant associations (PAs), age, history of respiratory disorder, herd size, parity and management (p<0.05) associated with CBPP seroprevalence. Cattle that are found in Ilu Galan district were (OR=3.4, P=0.000) more affected by contagious bovine pleuropneumonia than those in Bako Tibe. Cattle from large herds (OR=4.6, P= 0.000) were more likely to be affected by Contagious bovine pleuropneumonia disease than from small herds, poor body condition animals (OR=1.8, P=0.015) were more likely to be seropositive compared to those with good body condition.The cultural result shows that isolation the causative agent from lung tissue. Contagious bovine pleuropneumonia was endemic in the study area and confirmed by serology and isolation of the causative agent Therefore, further study with large coverage using reliable tools like molecular technique is important, farmers should be aware of the disease, and use the combination of vaccination with therapeutic.
Keywords
Bako Tibe; CBPP; Ilu Galan; Isolation; Risk factors; Serology
INTRODUCTION
Ethiopia is one of the countries in Africa with huge livestock potential and heavily depending on agriculture sector which, the back bone of economy by employing about 78% of the work forces in the country. Among the agricultural sectors, livestock is the one which is ranked first in Africa, and tenth in the world [1-4].
Ethiopia has 60.39 million of cattle; 30.7 million of sheep, 30.2 million of goat population and this livestock sector has a significant role in socioeconomic activities of the country, and contributes to much the national economy [5,6]. Livestock provides a livelihood for 65% of the total population and 80% of the rural population of the countr, and contributes 15 17% of Gross Domestic Product (GDP) and 35 49% of agricultural GDP, and 37 87% of the household incomes [7].
Among the livestock, cattle are the most important to the GDP of the country [8] and they are used as source of draught power for the rural farming population, supply farm families with milk, meat and also as source of cash income, playing a significant role in the social and cultural values of the society [9-11].
Livestock contributes a major contribution for national economy particularly with regard to foreign currency earnings is through exportation of live animals, meat, skin, and hides. However, development of this sector is hampered by different constraints such as endemic diseases including viral, bacterial and parasitic infestation are the most important constraints widespread [4,12].
Infectious disease like Contagious Bovine Pleura Pneumonia (CBPP) is considered to be one of the most economically important, and major problem for Ethiopian livestock development. Lack of appropriate disease control policy, lack of appropriate veterinary services, and lack of attention from government are other important bottleneck for the development of this sector include [13-15] Contagious bovine pleura pneumonia (CBPP) is a highly contagious disease of cattle that is caused by Mycoplasma mycoides subspecies mycoides small colony (MmmSC) [16-19] and the disease is endemic in many African countries and the Sahara region is under constant threat due to the carrier status of its host. It is one of the most serious contagious animal diseases and Office International Des Epizooties (OIE) listed it in the group of notifiable animal diseases of high socio-economic impact and is regarded as one of the major transboundary animal diseases (TADs) [15,19-22]. It is both an epidemic, and endemic disease of cattle that affects production through mortality and reduction in productivity. It also retards genetic improvement, and limits working ability of cattle [4,23,24].
CBPP mainly transmitted from one animal to others through aerosol by close contact with infected animals within herds, and from herd to herd through direct contact, and repeated contact between sick, and healthy animals, and occasionally from latent carriers intermittently shedding Mycoplasma organisms from sequestrated lung lesions [3,25]. In addition, nomadic people which move freely across borders of certain countries such as the Fulani in West Africa, and the Maasai in east Africa which may have contributed to CBPP spread [26]. Wars, famine and inadequate financing of veterinary departments have resulted in CBPP running high in east and central Africa [25,27,28].
The diagnosis of CBPP is based on a history of contact with infected animals, clinical findings, immunodiagnostic tests, and necropsy findings, cultural examination and serological testing. The Complement Fixation Test (CFT) and competitive Enzyme Linked Immune Sorbent Assay (cELISA) have been prescribed by the World Organization for Animal Health as herd level serological diagnostic tests [3,29,30].
Control strategies of CBPP are based on early detection of outbreaks, control of animal movements, and slaughter policy. The main problems for control are frequency of subclinical infections persistence of chronic carriers after the clinical phase, lack of extended vaccination coverage. Other factors including the deterioration in the quality of products, lack of control programmes, lack of controlled cattle movement, lack of financial suppor, resulted in the gradual spread of the disease [31,15,18].
Therefore, the study was conducted with the objectives of isolate the causative agent of CBPP, estimate seroprevalence and to identify the associated risk factors of of CBPP in cattle of Bako Tibe and Ilu Galan districts of west shoa zone, western Ethiopia.
MATERIALS AND METHODS
Description of the study areas
The study was carried out in two selected districts of West Shoa Zone (BakoTibe and Ilu Galan) of Oromia Regional State, Western Ethiopia.
Bako Tibbe district is located in the western part of Ethiopia in West Shoa zone of Oromia Regional State (Figure 1). It is located at 251 kms to the west of Addis Ababa. The district is located at geographical coordinates 9° 8' 0" N and 37°3' 0" E. The total area of the district is about 64,469 hectares of land with animal population of 138,608 local and 480 exotic cattle, 12,627 sheep, 14,354 goats, 3721 horses, 8415 donkeys, 8499 mules, and 97709 poultry. The area is characterized by having an altitude ranging from 1300-2998 meter above sea level, and average rain fall of 886.5mmrelative humidity of 57.83%, and an average annual temperature of 21.2°c.The agro-ecology of the area is 52% low land, 37% mid land and 11 high lands. The main rainfall patterns are rainny season extending from June to September and the adry season being from December to April. According to the report of Bako Tibe livestock health agency, the major endemic livestock diseases in the district were trypanosomosis, babesiosis, blackleg, pasturellosis, lumpy skin disease, contagious bovine pleuropneumonia, African horse sickness, internal and external parasite [32].
 Figure 1: Map showing the location of study area.
Figure 1: Map showing the location of study area.
Ilu Galan district is found in the western part of Ethiopia in West Shoa Zone of Oromia Regional State (Figure 2). The district is located at about 200 km from West of Addis Ababa. The district is located at geographical coordinates 8°59'51"N and 37°19'49"E and annual temperature is 27.3%. The livestock populations of the district based on species were 147,874 cattle, 8,644 of sheep, 8,930 goats, 41,4853 poultry, 711 horses, 984 mules and 5,393 donkeys. According to the report of Ilu Galan livestock health agency, the major endemic livestock diseases in the district were pasturellosis, foot and mouth disease, contagious bovine pleuropneumonia, African horse sickness, trypanomosis, blackleg, epizootic lymphagitis, endo and ecto parasite [33].
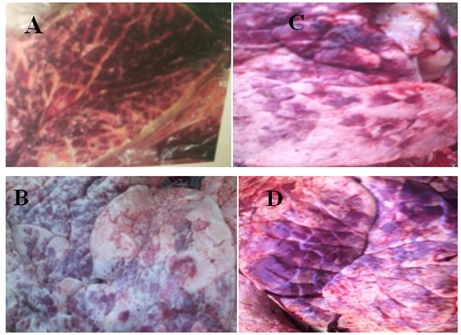 Figure 2: Severely inflamed lung tissue observed during postmortem examination(A) marbling appearance of lung tissue (C) CBPP of lung organ adhesion to the chest wall and thickening extensive fibrin, (B and D) are the lung of cattle affected by CBPP slaughtered .
Figure 2: Severely inflamed lung tissue observed during postmortem examination(A) marbling appearance of lung tissue (C) CBPP of lung organ adhesion to the chest wall and thickening extensive fibrin, (B and D) are the lung of cattle affected by CBPP slaughtered .
Study animals
The study animal includes Horro breed and crossbred cattle that were managed under extensive and semi-intensive production systems. For the serological study, cattle above the age of six months with no history of vaccinations to CBPP were included. Study animals related information on each tested cattle (such as, age, sex, body condition score, breed, herd size, parity, management and history of respiratory disease) were recorded at the time of sample collection. All lung tissue samples from the two districts were sampled from male animals purposively.
Study design and methods
Across-sectional study was conducted from December 2019 to April 2020 in selected villages of west shoa zone. Sample size was considered for the two districts based on the livestock populations and Peasant Associations (PAs) size of the district in the study area. On each study household, a questionnaire based survey was conducted by interviewing cattle owners regarding their perception towards the general respiratory disease and CBPP disease. Furthermore, animals’ blood sample and relevant data was collected from each interviewed household. For further confirmations of the presence of CBPP in the study area, isolationof the causative agent performed from clinically suspected cases (includes the cattle which were come into veterinary clinic) as well as slaughtered animals from municipality abattoirs.
Sample size determination
For this study, the required sample size was calculated based on [34] formula. From the previous reports of prevalence of CBPP disease in western part of the country such as 28.5% was reported by [26] and 19% was reported by [27] the average of the two was 23.75%. So that, in this study the sample size was based on 23.75% was considered to obtain the maximum sample size and a desired absolute precision (d) of 5% (0.05) was used to determine the sample size of this study.
So, using the [34] formula,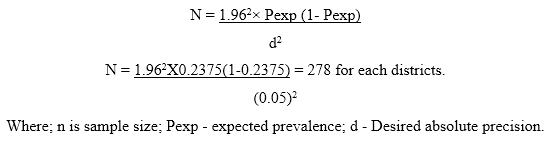 However, 281 sampled from Ilu Galan and 323 animals were sampled from Bako Tibe based on the district livestock populations and Peasant associations (Pas’) size of the districtsin order to increase the precision, which gives a total estimated sample size of 604 animals.
However, 281 sampled from Ilu Galan and 323 animals were sampled from Bako Tibe based on the district livestock populations and Peasant associations (Pas’) size of the districtsin order to increase the precision, which gives a total estimated sample size of 604 animals.
Therefore, the calculated sample size for the sero prevalence study was 604. Whereas an estimated sample size for households that participated in the questionnaire survey was calculated by dividing the total sample size (n=604) by the number of animals sampled within each herd (8) given an estimated of 76 households for inclusion in the questionnaire survey [35]. However, due to the inclusions of households that had less than eight animals, the total sample size of households was inflated to 106 [35]. Therefore, in this study a total of 106 households and 323 serum, 80 nasal swab, 40 lung tissue from Bako Tibe and 281 serum, 40 nasal swab, 20 lung tissues were collected from Ilugalan district.
Sampling techniques
In the first stage the two study districts were purposively selected based on based on accessibility, availability of infrastructure and number of cattle populations. In the second stage, with the help of animal health workers, development agencies, and peasant associations (PAs’), which means the smallest administrative unit of the districts were randomly selected from the two districts. Bako Tibe district has 32 PAs’ whereas Ilu Galan has 17 PAs’ and from Ilu Galan district three PAs’ and from Bako Tibe four PAs’ were randomly selected. Therefore, from Bako Tibe (Dambidima, Sadden kite, Bacaraoda gibe and Cekadimtu) and from Ilu Galan (Allewarailu, Sibabiche and Obora Benaso) were sampled.
Households and individual animal were selected using two stage sampling methods. The selected households were informed by animal health worker to provide their cattle for sampling purpose. The primary sampling unit was household that having at least one cattle in each selected PAs’ considered as herd and the secondary sampling unit was individual animals of households. From each list of herd the maximum sample size sampled was eight. From households those having greater than eight cattle, only eight animals were sampled using random sampling method. On the other hand, the households that have less than eight cattle, all animals were sampled [36].
Owners of the study area were recognizes each cattle owned by their name, thus, animals were randomly sampled using the name of animal as ID number and simple random sampling technique was used to select animal for serum sample collection.
Data collection
Blood sample collection: Animals were restrained by owners, and 7ml of blood sample was collected from the jugular vein using vacationer tubes under aseptic condition. The samples were kept at room temperature in a slant position for 24 hr and serum samples were separated after 24 hr through gently transferring to serum tubes (cryovial tube) and kept at -20°C until tested. Then serum samples were analyzed using competitive ELISA (c-ELISA) for the detection of CBPP antibodies according to [3]. During blood sample collection variable that considered as risk factors were; Sex (male and female); herd size (grouped in to three, since the interviewed had minimum and maximum number of herd sizes ranges 2-27 categorized as 2-7 called small herds, 8-15 called medium herds and greater than 16 large herds [37]. The body condition scores (BCS) were characterized according to [38] principles. History of respiratory disorder (yes/no); and parity categorized as heifer, single and multiple parity. Age were categorized into two, 6 months-3 years old called young and adult (3 years and above) according to [39].
Nasal swab and lung tissue sampling: The animals were restrained by the owner and 120 nasal swabs were collected from cattle showing clinical sign of CBPP and from seropositive animal by using swab into the tubes contain PPLO broth. A total of 60 lung tissue samples were collected from lung with lesion suspected of CBPP cases into tubes contains PPLO broth and immediately stored at -20OC at Bako veterinary clinic laboratory from two abattoirs and transported by cold chain to National Animal Health Diagnostic and Investigation Center (NAHDIC) for culture.
Diagnostic methods
Competitive ELISA (c-ELISA): The wells of the polystyrene micro-plate were coated with a Mycoplasma mycoides subspecies mycoides Small Colony (MmmSC) purified lysate. Test serum samples were premixed with a specific monoclonal antibody Mab 117/5 in a pre-plate. This mixture was then transferred into the MmmSC antigen coated micro-plate. Any antibody specific to MmmSCin the serum forms an immune complex with MmmSC antigen coated on the microplate by competing with Mab 117/5 for the specific epitopes. After washing away unbound material, an anti-mouse antibody enzyme conjugate was added. In presence of immune-complex between MmmSC antigen and antibody from the sample, Mab 117/5 cannot bind to its specific epitopes and the conjugate is blocked from binding to Mab 117/5. In the absence of MmmSC antibodies in the test sample, Mab 117/5 bind to its specific epitopes and the conjugate is free to bind to Mab 117/5. After washing unbound conjugate away, enzyme substrate (TMB) was added.
In the presence of the enzyme, the substrate is oxidized and develops a blue compound becoming yellow after blocking. The amount of antMmmSC Antibodies in the test sample is inversely proportional to color development. The result was expressed in “percentage of inhibition “by comparing the optical density in the test well with the optical densities in the Mab control wells [3].
Isolation and identification: Swab samples and lung tissues were incubated at 37oC under candle jar to generate (3-5% Co2) for seven days. Cultured samples were mixed by vortexing the mixtures and a loopful sample from the medium were plated on PPLO agar which was contained the Mycoplasma supplement selective G (Yeast extract, horse serum, thallous acetate and penicillin) and also growth factors (50% glucose, 0.2% DNA and Sodium pyruvate) and incubated at 37oC under candle jar to generate (3-5% Co2).
Data entry and analysis
Data were classified, filtered, coded using Microsoft Excel spread sheet, and was transferred and analyzed using STATA version14.0 software. Description statistics such as frequency, percentage, were used While Pearson’s chi- Square was used to evaluate the associations of different categorical variables with sero-prevalence of CBPP. Logistic regression analyses were performed to quantify effects of pre-specified risk factors on CBPP sero-reactivity. P-value less than 5% or p
RESULTS
Prevalence of CBPP by using c-ELISA
Over all prevalence: In the present study, a total of 604 cattle were examined and the overall animal level seroprevalence of CBPP in the study area were 33.77% (95% CI: 30.0-37.70). From a total of 106 sampled herds, 59 herds were infected and the overall herd level seroprevalence of contagious bovine pleuropneumonia (CBPP) was 55.7% (95%CI: 45.68-65.31).
Animal level seroprevalence of CBPP within the risk factors: In this study, different animal level seroprevalence was recorded and the higher seroprevalence (48.04%) was in Ilu Galan district, while the lowerseroprevalence (21.36%) was recorded in Bako Tibe district of west shoa Zone. There was a statistically significant significance (p < 0.05, χ2 = 47.82) in seroprevalence among the two districts (Table 1).
|
Districts |
Animals examined |
Positive |
Prevalence (%) |
95 % CI |
X2 |
P- value |
|
BakoTibe |
323 |
69 |
21.36 |
17.01-26.24 |
||
|
Ilu Galan |
281 |
135 |
48.04 |
42.07-54.05 |
47.82 |
0 |
|
Total |
604 |
204 |
33.77 |
30.0-37.70 |
Table 1: Seroprevalenceof CBPP based on individual animals at districts level.
Among the seven villages sampled, the prevalence was highest (58.59%) in Ale warailu of the Ilu Galan district while the lowest prevalence (14.85%) was recorded in Dambidima of Bako Tibe district. There was statistically significant variation (p < 0.05, χ2 = 72.27) in seroprevalence among the seven villages (Table 2).
|
PA (villages) |
Examined |
Positive |
Prevalence % |
95 % CI |
X2 |
P-value |
|
Dambidima |
101 |
15 |
14.85 |
8.55-23.30 |
||
|
Bacara Oda Gibe Saden Kite |
86 |
18 |
20.93 |
12.9-31.04 |
||
|
Cheka Dimtu |
78 |
18 |
23.08 |
14.28-33.99 |
||
|
Obora Benaso |
58 |
18 |
31.03 |
19.53-44.54 |
72.27 |
0 |
|
Siba Biche |
88 |
26 |
29.55 |
20.29-40.22 |
||
|
Alle WaraIllu |
94 |
51 |
54.26 |
43.65-64.57 |
||
|
Total |
99 |
58 |
58.59 |
48.24-68.39 |
||
|
604 |
204 |
33.77 |
30.0-37.70 |
Table 2: Seroprevalence of CBPP based on individual animals at sampled villages.
A chi-square analysis revealed that (age, body condition score, herd size, parity, history of respiratory disease and management) showed statistically significant variation (P< 0.05) with CBPP sero-positivity among the risk factors considered during the study. Variables (district, age, history of respiratory disease, body condition score, parity, management, herd size and villages) showing statically significant association (P<0.05) with CBPP were subjected to univariable logistic regression analysis (Table 3). Accordingly, the animal level seroprevalence was significantly higher in Ilu Galan district than in BakoTibe, this was cattle that found in Ilu Galan (OR=3.4, 95% CI: 2.38-4.85, P<0.001) were three times more affected by CBPP disease than Bako Tibe. Similarly, cattle that kept at SibaBiche (OR = 6.8, 95% CI: 3.43-13.45, P<0.001) were 6.8 times more likely to be CBPP seropositive than Dambidima, seropositivity with CBPP disease of adult cattle (OR=1.75, 95%CI:1.23-2.49, P<0.001) were 1.75 times more seropositive than young, animals which had history of respiratory disorders (OR=2.4, 95% CI:1.74-3.49, P<0.001) were two times more likely to be seropositive than those which had not, poor body condition animals (OR=1.8, 95% CI: 1.12-2.89, P=0.015) were 1.8 times more likely to be seropositive than good body condition, cattle that found in large herds (OR=4.6, 95% CI: 3.03-7.15, P< 0.001) were 4.6 times more likely to be affected by CBPP disease than small herds.
|
Risk Factors |
Category |
Examined |
Positive |
Prevalence (% ) |
95 % CI |
X2 |
P- value |
|
|
Age |
Young |
206 |
87 |
42.23 |
35.40-49.29 |
9.99 |
0.002* |
|
|
Adult |
398 |
117 |
24.4 |
24.96-34.14 |
||||
|
Sex |
Male |
295 |
108 |
36.61 |
31.10-42.38 |
2.07 |
0.15 |
|
|
Female |
309 |
96 |
31.07 |
25.94-36.55 |
||||
|
Breed |
Local |
572 |
192 |
33.57 |
29.70-37.60 |
0.209 |
0.647 |
|
|
Cross |
32 |
12 |
37.5 |
21.10-56.30 |
||||
|
Herd size |
Small |
263 |
63 |
23.95 |
18.92-29.58 |
|||
|
Medium |
188 |
50 |
26.6 |
20.42-33.51 |
60.85 |
0.000* |
||
|
Large |
153 |
91 |
59.48 |
51.25-67.32 |
||||
|
Parity |
Heifer |
90 |
21 |
23.33 |
15.06-33.42 |
|||
|
Single |
111 |
32 |
28.83 |
20.62-38.19 |
8.38 |
0.039* |
||
|
Multiple |
108 |
44 |
40.74 |
31.38-50.62 |
||||
|
BCS |
Good |
118 |
28 |
23.73 |
16.38-32.43 |
|||
|
Medium |
373 |
134 |
35.72 |
31.05-41.02 |
6.67 |
0.035 |
||
|
Poor |
113 |
42 |
37.17 |
28.26-46.76 |
||||
|
History of RD |
Yes |
287 |
126 |
44.06 |
38.07-49.85 |
28.22 |
0 |
|
|
No |
317 |
77 |
24.29 |
19.67-38.02 |
||||
|
Management |
Extensiv |
415 |
155 |
37.35 |
32.67-29.39 |
7.57 |
0.006* |
|
|
Sem-intensive |
189 |
49 |
25.93 |
19.83-32.78 |
||||
|
Total |
604 |
204 |
33.77 |
30.0-37.70 |
Table 3: Effects of risk factors on Seroprevalence of CBPP tested by X2.
RD=Respiratory disease; *=statistically significant; BSC= body condition score.
The cattle getting risk of infection with CBPP disease of multiple parity (OR=2.15; 95% CI: 1.16-4.00, P=0.015) were two times more than heifers and cattle that kept in extensive system (OR =1.7, 95% CI: 1.16-2.49, P =0.006) were 1.7 times more getting high risk of infection by CBPP than semi intensive system (Table 4).
|
Risk factors |
Category |
Odds ratio |
(95% CI) |
P-value |
|
Sex |
Female |
1 |
||
|
Male |
1.28 |
0.91-1.79 |
0.15 |
|
|
Age |
Young |
1 |
||
|
Adult |
1.75 |
1.23-2.49 |
0.002* |
|
|
Breed |
Cross |
1 |
||
|
Local |
0.84 |
0.40-1.75 |
0.647 |
|
|
History of RD |
No |
1 |
||
|
Yes |
2.47 |
1.74-3.49 |
0.000* |
|
|
BCS |
Good |
1 |
||
|
Poor |
1.8 |
1.12-2.89 |
0.015* |
|
|
Medium |
1.9 |
1.07-3.36 |
0.027* |
|
|
Herd size |
Small |
1 |
||
|
Medium |
1.15 |
0.74-1.76 |
0.523 |
|
|
Large |
4.6 |
3.03-7.15 |
0.000* |
|
|
Parity |
Heifer |
1 |
||
|
Single |
1.33 |
0.70-2.51 |
0.38 |
|
|
Multiple |
2.15 |
1.16-4.00 |
0.015* |
|
|
Management |
Semi intensive |
1 |
||
|
Extensive |
1.7 |
1.16-2.49 |
0.006* |
Table 4: Univariate logistic regression analysis of sero-prevalence between different risk factors.
RD= Respiratory disease; BSC= body condition score; PA= peasant association.
Initially, univariate logistic regression was used to screen all potential risk factors for statistical significance at (P<0.05). The risk factors statistically significant byunivariate logistic regression analysis, and variables that are colliniarity with each other that found statistically significant were subjected to multivariate logistic regression analysis with forward and backward variable selection approach based on the likelihood ratio statistic (p<0.05) analysis. The risk factors (district, age, history of respiratory disease, body condition score, parity, management, herd size and PA (villages) that statistically significant in univariable logistic regression analysis were included. Therefore, the final multivariable logistic regression analysis result showed that PA (kebele), age, history of respiratory disorder, herd size and management were statistically significant association with CBPP seroprevalence (P < 0.05) (Table 5).
|
Risk factors |
Category |
Odds ratio |
(95% CI) |
P-value |
|
Age |
Young |
1 |
||
|
Adult |
1.56 |
1.07-2.29 |
0.020* |
|
|
History of RD |
`No |
1 |
||
|
Yes |
2.4 |
1.68-3.65 |
0.000* |
|
|
Herd size |
Small |
1 |
||
|
Medium |
1.22 |
0.78-1.90 |
0.38 |
|
|
Large |
4.6 |
3.0-7.3 |
0.000* |
|
|
Management |
Semi intensive |
1 |
||
|
Extensive |
1.8 |
1.22-2.72 |
0.003* |
Table 5: Multivariate logistic regression analysis of seroprevalence between differentrisk factors.
RD=Respiratory disease; *= statistically significant; PA= peasant association
Herd level seroprevalence of CBPP and the risk factors: In the present study the risk factors that considered at herd level seroprevalence were district, age, herd size and history of respiratory health problems within the herd. The herd level CBPP seropositivity was higher in Ilu Galan (59.2%) than in Bako Tibe (31%). Univariate logistic regression analysis results showed that among herd level risk factors (district, age, history of respiratory problem and herd size) that considered, only district and herd size were statically significant effect on seropositivity (p<0.05). Such as cattle herds infection with CBPP disease in Ilu Galan district (OR =2.4, 95% CI: 1.38-5.34, p=0.0021) two times more than Bako Tbe. The chance of seropositivity with CBPP in larger herd is 9.1 (OR=9.1, 95%CI: 2.8-3.4 p=0.001) times higher than smaller herd (Table 6).
|
Risk factors |
Category |
Total herd |
%(95% CI) |
OR(95% CI) |
P-value |
|
|
Districts |
BakoTibe |
56 |
31(81.8-44.1) |
1 |
||
|
Illugalan |
50 |
59.2(45.18-73.6) |
2.4(1.38-5.34) |
0.0021* |
||
|
Age |
Young |
50 |
41(28.18-56.8) |
1 |
||
|
Adult |
56 |
62.7(48.5-75.08) |
2.3(4.53-7.44) |
0.791 |
||
|
Herd size |
Small |
29 |
81.9(64.2-94.15) |
1 |
||
|
Medium |
36 |
50.8(32.9-67.07) |
2.3(0.93-5.6) |
0.004** |
||
|
Large |
41 |
30.6(18.08-48.08) |
9.1(2.8-3.4) |
0.001** |
||
|
History of RD |
No |
56 |
47.9 (34.6-61.9) |
1 |
||
|
Yes |
50 |
53.9 (39.3-68.2) |
1.19 (0.41-1.74) |
0.463 |
Table 6: Multivariable logistic regression analysis of seroprevalence of CBPP at herd level.
Isolation and growth condition of Mmmsmall colony
The colonies of Mycoplasma showing typical fried eggs morphology were observed and classical colonies were recorded at seven days of incubation. The colonies were observed under inverted stereomicroscope (32x) with transmitted light. From the total of 60 lung lesions subjected for isolation, 4(6.67%) were found positive for MmmSC.
DISCUSSION
Contagious Bovine Pleuropneumonia (CBPP) has great potential for rapid spread and causes major impact on cattle production, still nationwide surveillance and control activities are often inadequate or unavailable in most African countries including Ethiopia [40].
In the present study, a total of 604 cattle were examined and the overall animal level seroprevalence of CBPP in the study area were 33.77% (95% CI: 30.0-37.70). From a total of 106 sampled herds, 59 herds were infected and the overall herd level seroprevalence of Contagious Bovine Pleuropneumonia (CBPP) was 55.7% (95%CI: 45.68-65.31). This is in agreement with previous reports in the country and other places.
Thus, [41,42] reported prevalence of 31.8% and 30.2% from Amaro district of Southern Nation, Nationalities and Peoples of Ethiopia and agro-pastoral areas of Nigeria, respectively.And also a total of 60 lung tissue samples were purposively tested from the two district and isolates Mycoplasma Mycoides Subspecies Mycoides Small Colony (MmmSC) 6.67% (CI=1.84-16.19) which, were 7.5% from BakoTibe and 5% from Ilu Galan districts. This finding is in line with that of [27] who reported statistically significant variation (p<0.05) in seroprevalence between districts.
On the other hand, the finding of this study was lower than the report of [28] with 40.3% in Gobbu Sayyo district of Eastern Wollega Zone, the report of Almaw et al. [43] with 71.8% in Bako research dairy farm, 39% reported by Gedlu [44] in Somali Regional State, the report of [45] with 47% in Kaduna State of Nigeria, the report of [46] with 55.05% in Derashe District, Southern Ethiopia.
However, higher than the previous reports of [26] with 28.5% in selected districts of western Oromia, [47] report with (25.3%) in districts of Sidama Zone, Southern Ethiopia, [48] in Bishoftu abattoir and Adama quarantine (6.85%), [49] in Borena pastoral of Oromia (0.4%), [50] in export quarantine of in and around Adama (4%), [24] in Dello Mena and Sawena districts of Bale zone (6.51%), [23] in Southern Zone of Tigray region of Ethiopia (12%), [51] in Nigeria, state of central Nigeria (14%), [52] in Khartoum State of Sudan (17.19%), [53] with 21.05% in Guinea, [35] with 8.1% in Gimbo District, Southwest Ethiopia. The rates of CBPP infection reported to vary from one region to another even within the region [3,26].
The highest was reported in Ilu Galan district (48.04%) while the lowest was reported in BakoTibe district (21.36%), this indicating that, there is a variation within the district. There was statistically significant difference (p<0.05) in the occurrence of the disease based on districts. This finding is in line with that of [27] who reported statistically significant variation (p<0.05) in seroprevalence between districts.
Seroprevalence of CBPP was highest in cattle with poor body condition (37.17%, 95%CI: 28.26-46.76) as compared to cattle with medium body condition (35.72%, 95%CI: 31.05-41.02) and good body condition (23.73%, 95%CI: 16.38-32.43). This finding is in agreement with the report of [41,42,54]. This could be due the weak protective immune response in poor body conditioned cattle. Loss of body condition is one of the indications for the presence of the infection in the animal. Mostly CBPP chronic carrier animals became emaciated because of the clinical characteristics of the disease [26].
Age was considerably associated with the sero positivity of CBPP with higher seroprevalence recorded in adult animals (42.23%) than young animals (24.4%) which is supported by the finding of [50]. The authors reported that who reported that high sero-prevalence was recorded in aged (9.5%) animals than young (3%) at export quarantine centers in and around Adama. Moreover [54,55] stated that adult cattle chronic carriers’ (7.2%) had relatively higher numerical value than the young (4.4%). In addition to this, relatively higher prevalence in adult as compared to young animals is supported by the finding of [41] who reported relatively higher seroprevalence in adults as compared to the young. The likelihood of seropositivity of adult cattle was 1.75 times (OR=1.75, 95% CI: 1.23-2.49, P=0.002) higher than young cattle.
In contrast, there are different studies that reported insignificant associations such as [40] in Amaro district of SNNP region, [43] in southern zone of Tigray region, [26] in selected districts of western Oromia and [42] in agro-pastoral areas of Nigeria, which may be explained by the fact that increasing age is a surrogate measure of repeated exposure. Moreover, the study also agreed with [56] who reported that factor like extremes of age may predispose to tissue invasion of CBPP disease.
There was no significant difference of CBPP seroprevalence among the sex which was (36.61%, 95%CI: 31.10-42.38) in male and (31.07%, 95%CI: 25.94-36.55) in female animal. This finding is agreed with the work done by [38] in Amaro district of SNNP region,[35]in selected districts of western Oromia, [23] in Southern zone of Tigray region of Ethiopia, [46] in Derashe District, Southern Ethiopia, [42] in agro-pastoral areas of Nigeria, and [51] in Niger state of north central Nigeria. Therefore, those reported sex has not been considered as the risk factors that affecting the susceptibility of cattle to CBPP disease infection. On the other hand, this finding is contradicted with the finding of [57] in south western Kenya and [54] in the Maasai ecosystem of south-western Kenya who reported statically significant difference among sex.
There was statistical significant between history of previous respiratory disorder and seroprevalence of CBPP disease (P=0.000). The prevalence was highest in respiratory disordered animals (44.06%, 95%CI: 38.07-49.85) compared to cattle that had not experienced respiratory problems (24.29%, 95%CI: 19.67-38.02). Respiratory disordered animals (OR=2.4, 95% CI: 1.68-3.65) were two times more seropositive of CBPP disease as compared to healthy animals which in line with the report of [54,58].
The prevalence was highest in cattle with large herd size (58.49%, 95%CI: 51.25-67.32) as compared to cattle with small herd size cattle (23.95%, 95%CI: 18.92-29.58). The large herd size groups (OR=4.6, 95% CI: 3.03-7.15, P=0.000) were four times more likely to have the CBPP infection compare to the small herd size cattle groups. The finding is in line with the report of [23] in Dello Mena and Sawena Districts of Bale Zone, South Eastern Ethiopia, [49] in Borena pastoral area of Southern Ethiopia, [42] in agro-pastoral areas of Nigeria who reported large herd size cattle groups significantly associated with CBPP seroprevalence.
The rate of effective contact between CBPP-infected and susceptible cattle is reported to be higher in larger herds, reason for which could be explained as the contagious nature of CBPP and its direct mode of transmission which might be increased by crowding and increased frequency of contacts as herd size increases. Furthermore, [59] reported that CBPP prevalence may even be higher in parts of predominated by pastoral systems such as nomadic and transhumant where frequently larger herds are kept.
In this study, the seroprevalence was found to be higher in multi parity cows (39.64%, 95%CI: 30.48-49.36) as compared to with single parity (28.83%, 95%CI: 20.62-38.19) and heifers (23.33%, 95%CI: 15.06-33.42). This finding was agreed with the suggestion of previous studies that is parallel with the parity susceptibility to CBPP disease by Mersha [35]. In recent result explained that the seropositivity of CBPP was increased with increased cattle parity with CBPP disease of multiple parity (OR= 2.5; 95% CI: 1.16-5.51, P=0.020) was 2.5 times more than heifer as well as the likelihood of seropositivity with CBPP disease of single parity cows (OR=2.15, 95% CI: 1.16-4.00, P=0.015) were two times more than heifers. The reason of higher prevalence in multi parity could be associated with age of animal because those multiple parity animals were older than single parity and heifers.
In recent result explained that the seropositivity of CBPP higher animals that kept in extensive management system than semi intensive management system (OR= 1.8; 95% CI:1.22-2.72, P=0.003) was 1.8 times more than semi intensive as well as the likelihood of seropositivity with CBPP disease. The variation of these findings might be due to the variation in temporal and spatial distribution of the disease, animal management difference and the sensitivity of the serological tests used.
The overall herd level seroprevalence of CBPP was (55.66%,95%CI: 2.8-3.4 p=0.001)which is closely in agreement with the finding of [42] with 54.7% in agro-pastoral areas of Nigeria. However, higher than the previous report of [60] with 30.4% in Somali regional state of Ethiopia [29] with 17% in north east states.On the other hand the finding was lower than the report of [54] with 85% in the Masai ecosystem of south-western Kenya.
The isolation of MmmSC from cattle slaughtered in Bako tibe and Ilu Galan districts of abattoirs. The positive CBPP pathology was taken as a definite mark of MmmSC infection in the lungs of the animals. The MmmSC isolates in this study were obtained from consolidated lungs that were suggestive of CBPP and the four isolates confirmed as Mycoplasma Mycoides Subspecies Mycoides small colony were from lungs tissue. This could be explained by the fact that the main predilection site for MmmSC is the lung where MmmSC causes pathological lesions accompanied with the production of pleural fluids thus, creating a more conducive environment for survival of the organism. The identification of the causative agents of CBPP from lung tissue in this finding is equally agreed with earlier report of [61] Maiduguri Borno State of Nigeria which isolates 6.67% from lung tissue. Moreover, this study higher than an abattoir based study [62] who reported a lesion based prevalence of 0.29% in States of Northern Nigeria.
From the current study it is evident that nasal samples were taken from seropositive animals. However, no isolate can be recovered from the samples. because of the sample might be taken from the chronic stage of the diseased animals, incubation period and the animals were maybe treated by antibiotics, due to the labile nature of organism.
CONCLUSION AND RECOMMENDATIONS
The output of this study has indicated that contagious bovine pleuropneumonia was endemic in the study area and confirmed by serology and isolation of the causative agent. This study also established a relatively high prevalence of CBPP in cattle in two selected districts, suggesting that the disease could be causing considerable economic losses\Among the potential risk factors district, age, history of respiratory disease, body condition score, herd sizes and parity and management system were significant associated with CBPP seropositivity. But, no MmmSC was isolated from nasal swab samples in both districts because of the sample might be taken from the chronic stage of the diseased animals.
Even though the disease is prevalent in the districts, there was knowledge and attitude gap among the farmers towards CBPP. Particularly, large proportion of owners didn’t mention important signs of the disease and they have limited awareness on the means of transmission. Besides, majority of farmers had practices with regard to the prevention and control poor animal management that create favourable condition for CBPP dissemination.
Therefore, based on the above conclusion the following recommendations were forwarded:
- • Further investigation in wide areas and increasing sample size by using molecular technique like PCR and biochemical tests are needed.
- • Modification of the protocol for bacterial isolation from nasal swab.
- • Use the combination of vaccination with therapeutics start in seropositive area.
- • Awareness creation for the farmers about the means of transmission, controlling techniques and economic impact of the disease via animal health extension.
Authors' Contributions: AF performed experiments and statistical analysis, interpreted the results and prepared the manuscript. AO and ML revised the manuscript and provided valuable advices. BT and ED participated on experimental design, TB participated on serological tests and MT participated on bacteria isolation. IT supervised the study and revised the study for important intellectual content. All authors read and approved the final manuscript.
CONFLICT OF INTEREST
The author has no conflict of interest to declare
ACKNOWLEDGEMENT
The authors are thankful to National Animal Health Diagnostic and Investigation Center (NAHDIC) and Ambo University for facilitation and assistance of this research work. We are also grateful to Bako Tibe and Ilu-Gela district Livestock and Fishery Resource Development Office for their continuous encouragement, guidance and kind correspondence up to completion of this work
REFERENCES
- Hailu S (2014) A broken Value chain: Why Ethiopia imports livestock products while it ranks first in Africa in resources? Topic Tweet, No.16.
- Martin R, Yue L, Mark R, Frederico GS (2014) Structural change in Ethiopia: an employment perspective, The World Bank, Washington, DC, USA.
- Office International Des Epizooties (2014) Manual of diagnostic tests and vaccines terrestial animals in (chapter 2.4.9) Contagious bovine pleuropneumonia. Pg No: 1-5.
- MamoY, Bitew M, TeklemariamT, Soma M, Debebe G, et al. (2018) Contagious Bovine Pleuropneumonia: Seroprevalence and Risk Factor s in Gimbo District, Southwest Ethiopia. Vet Med Int 2018: 5729296.
- Beyi AF (2016) Feed the future innovation lab for livestock systems Ethiopia: Animal Source Food Production and Marketing Brief. Feed the Future Innovation Lab for Livestock Systems, Florida, USA.
- CSA (2018) Federal Democratic Republic of Ethiopia. African Union. Addis Ababa, Ethiopia.
- Leta S, Mesele F (2014) Spatial analysis of cattle and shoat population in Ethiopia: growth trend, distribution and market access. SpringerPlus 3: 310.
- Metaferia F, Cherenet T, Gelan A, Abnet F, Tesfay A, et al. ( 2011) A Review to improve estimation of livestock contribution to the national GDP. CGIAR, Montpellier, France.
- Ulfina G, Zelalem B, Jemal D, Gemeda D, Chala M, et al. (2005) Survey of cattle production and marketing practices in DannoDistrict, Western Ethiopia, using PRA tools. PRA Report 98-107.
- Melaku T (2011) Oxenization Versus Tractorization: Options and Constraints for Ethiopian Framing System. Int J Sustai Agric 3: 11-20.
- Tonamo A (2016) A review on cattle husbandry practices in Ethiopia. Int J Livest Prod 7: 5-13.
- Abdela N, Teshome E, Hassan A, Deressa F (2017) Prevalence and associated risk factors of equine wound in and around Asella town, Ethiopia. J Vet Med Anim Health 9: 63-71.
- Swai E, Mwezimpya E, Ulicky A, Moshy W (2013) An abattoir survey of contagious bovine pleuropneumonia lesions in slaughtered cattle in selected districts in Northern Tanzania. Asian Pac J Trop Biomed 3: 303-306.
- Musa JA, LivinusTI, Fati AL, Enenche FE, Quagar JT (2015) Detection of Antibodies to Mycoplasma mycoides subspecies mycoides in Cattle using Competitive Enzyme-Linked Immunosorbent Assay. Int J Curr Microbiol Appl Sci 4: 770-777.
- Abdela N, Yune N (2017) Seroprevalence and Distribution of Contagious Bovine Pleuropneumonia in Ethiopia: Update and Critical Analysis of 20 Years (1996-2016) Reports. Front Vet Sci 4: 100-110.
- Yaya A, Manso Silvan L, Blancha A, Thiaucourt F (2008) Genotyping of Mycoplasma mycoides subsp. mycoides small colony by multilocus sequence analysis allows molecular epidemiology of contagious bovine pleuropneumonia. Vet Res 39: 14.
- Billy IL, Balami AG, Sackey KB, Tekdek LB, Sa'idu NA, et al. (2015) Awareness, knowledge and practices of pastoralists towards contagious bovine pleuropneumonia in Kaduna State, Nigeria. J Vet Med Anim Health 7: 296-301.
- Yansambou SM, Diallo AA, Gagara H, Malick AH, Haido AM, et al. (2018) Serological Prevalence of Contagious Bovine Pleuropneumonia in Niger in 2017. Frontir Vet Sci 5: 238.
- Weldearegay BY, Müller S, Hänske J, Kostka R, Brehm P, et al. (2019) Host pathogen Interactions of Mycoplasma mycoides of in Caprine and Bovine Precision Cut Lung Slices (PCLS) Models. Pathogens 8: 82.
- Food and Agriculture Organization of the United Nations (FAO) (2012) Recognizing contagious bovine pleuropneumonia. FAO Animal Manual Health Manual, Rome 13: 3-17.
- Wade A, Yaya A, El-Yuguda AD, Unger H, Nafarnda D W, et al. (2015) The Prevalence of Contagious Bovine Pleuropneumonia in Cameroon: A Case Study Garoua Central Abattoir, Cameroun. J Vet Med Res 2: 1029.
- Musa JA, Bale JO, Kazeem HM, Nwankpa ND, Provvido AD, et al. (2016) Molecular detection of Nigerian field isolates of Mycoplasma mycoides subsp. mycoides as causative agents of contagious bovine pleuropneumonia. Int J Vet Sci and Med 4: 46-53.
- TeklueT, Tesfay T, Nirayo T, Hailu B, Wayu S, et al. (2015) Epidemiological Status of Contagious Bovine Pleuro Pneumonia in Southern Zone of Tigray Regions, Northern Ethiopia, Animal and Veterinary Sciences 3: 32-36.
- Geresu MA, Kedir K, Birhanu D, Teshome A (2017) Sero-epidemiological investigation and risk factors for contagious bovine pleuro pneumonia infection of cattle in Dello Mena and Sawena Districts of Bale Zone, South Eastern Ethiopia. J Public Health Epidemiol 9: 122-132.
- Billy LI, Balami AG, Anthony KB, Baba LS, Shehu N, et al. (2017) Sero-Prevalence of Contagious Bovine Pleuropneumonia in Three Senatorial District of Kaduna State, Nigeria Using Latex Agglutination Test. World Vet J 7: 65-73.
- Mersha T (2016) Sero-prevalence of contagious bovine pleuropneumonia and its potential risk factors in selected sites of Western Oromia, Ethiopia. Ethiop Vet J 20: 31-41.
- Daniel G, Abdurahaman M, Tuli G, Deresa (2016) Contagious bovine pleuropneumonia: Seroprevalence and risk factors in Western Oromia, Ethiopia. Onderstepoort J Vet Res 83: 958.
- Wakgari M, Kitila G, Chali I, Merdasa D, Guta D, et al. (2018) Sero Prevalence of Contagious Bovine Pleuro Pneumonia (CBPP) and Its Associated Potential Risk Factors in Selected Districts of East Wollega Zone,Oromia Region, Ethiopia. Austin J Vet Sci & Anim Husb 5: 2.
- Schubert EK, Sachse J, Jores J, Heller M (2011) Serological testing of cattle experimentally infected with Mycoplasma mycoides subsp. Mycoides small colony using four different tests reveals a variety of seroconversion patterns. BMC Vet Res 7: 72.
- Zarina M, Zamri S, Latiffah H, Shahrom M, Norlida O (2016) Seroprevalence and Detection of Contagious Bovine Pleuropneumonia (CBPP) in Northeast States of Peninsular Malaysia. J Trop Agric Sci 39: 257-265.
- Food and agriculture organization of the United Nations (2016) Animal Production and Health Proceedings. Italy Iowa state university. Instiute forint Coop in Animal Biol, Rome, Italy.
- Bako Tibe district Livestock and Fishery Resource Development Office (2019).
- Ilu Gelan district Livestock and Fishery Resource Development Office (2019).
- Thrusfield MV (2007) Veterinary epidemiology. 3rd Ed. Can Vet J 11: 1117.
- Mersha T (2018) Epidemiology study on Contagious bovine pleuropnemonia and farmer’s knowledge, attitude and practice towards the disease in selected district of East Wollega and West Showa Zones,Western Ethiopia. Addis Ababa University, Bishoftu, Ethiopia.
- Gebremedhin EZ, Agonafir A, Tessema TS, Tilahun G, Medhin G, et al. (2013) Sero-epidemiological study of ovine toxoplasmosis in East and West Shewa Zones of Oromia Regional State, Central Ethiopia. BMC Vet Res 9: 117.
- Kemal J, Sibhat B, Abraham A, Terefe Y, Tulu KT, et al.(2019) Bovine tuberculosis in eastern Ethiopia: prevalence, risk factors and its public health importance. BMC Infect Dis 19: 69.
- Nicholson MJ, Butterworth M (1986) A guide to body condition scoring of zebu cattle. International Livestock Centre for Africa, Addis Ababa, Ethiopia.
- De-Lahunta A, Habel R (1986) Applied veterinary Anatomy. WB Sounders company, New Mexico, USA.
- FAO Animal Production And Health (2016) CBPP situation in Africa, Can contagious bovine pleuropneuma (CBPP) be eradicated? Proceeding of the FAO OIE AU/IBAR IAEA Consult ative group CBPP 5th meeting, Rome 14-16.
- Ebisa T, Hirpa E, Aklilu F (2015) Study on seroprevalence and risk factors of Contagious Bovine Pleuro pneumonia in Southern nation in southern Nation and Nationality and People of Ethiopia, Regional State in Amaro special district. Sci Technol Arts Res J 4: 106-112.
- Suleiman A, Bello M, Dzikwi A, Yaqub A, GeidamYA, et al. (2015) Serological prevalence of contagious bovine pleuropneumonia in agropastoral areas of Nigeria. Trop Anim Health Prod 47: 1033-1042.
- Almaw G, Duguma M, Wubetie A, Tuli G, Koran T (2016) A contagious bovine pleuropneumonia outbreak on a research farm in Ethiopia, and its dynamics over an eight-month period. Rev Sci Tech Off Int Epiz 35: 30-35.
- Mekonnen G (2004) Serological, Clinical and Participatory Epidemiological Survey of Contagious Bovine Pleuropneumonia in Somali Region, Ethiopia. Addis Ababa University, Debreziet, Ethiopia.
- Danbirni S, Okaiyeto SO, Pewan SB, Kudi AC (2010) Concurrent infection of contagious bovine pleuropneumonia and bovine tuberculosis in Bunaji nomadic cows. Sci J Anim Sci 4: 23-25.
- Aliy A, Lelisa K, Aklilu F, Damena D (2017) Contagious Bovine Pleuropneumonia: Serological Prevalence in Derashe District, Southern Ethiopia. Glob Vet 19: 616-621.
- Malicha G, Alemu S, Aklilu F, Abraha A (2017) Study of Seroprevalence and Associated Risk Factors of Contagious Bovine Pleuropneumonia in Sidama Zone, Southern Ethiopia. J Vet Sci Technol 8: 1-5.
- Atnafie B, Goba H, Sorri H, Kasaye S (2015) Sero-prevalence of contagious bovine pleuropneumonia in abattoirs at Bishoftu and export oriented feedlots around Adama. Glob Vet 15: 321-324.
- Alemayehu G, Leta S, Hailu B (2015) Sero-prevalence of Contagious Bovine Pleuropneumonia (CBPP) in bulls originated from Borena pastoral area of Southern Ethiopia. Trop Anim Health Pro 47: 983-987.
- Kassaye D, Molla W (2013) Seroprevalence of contagious bovine pleuropneumonia at export quarantine centers in and around Adama, Ethiopia. Trop Anim Health Prod 45: 275-279.
- Alhaji NB, Babalobi OO (2012) Molecular epidemiology of State, MSc Thesis University of Khartoum College of Veterinary Medicine, Sudan 53-67.
- Elhassan IH (2012) Prevalence and risk factors associated with Contagious Bovine pleuropneumonia it in Khartoum State, MSc Thesis University of Khartoum College of Veterinary Medicine, Sudan.
- Soromou L, Dabo W, Cissé M, Sidimé Y, Keyra M, et al. (2014) Seroprevalence of contagious bovine pleuropneumonia in the prefecture of Dabola, Upper Guinea. Afr J Microbiol Res 8: 1819-4214.
- Mtui Malamsha NJ (2009) Contagious Bovine Pleuropnemonia (CBPP) in the Maasai ecosystem of south western Kenya: Evaluation of seroprevalence, risk factor and vaccine safety and efficacy. University of Edinburgh 14: 97-207.
- Ikpa LT, Ankeli PI, Jambalang AR, Atuman YJ, Akalusi Y, et al. (2015) Detection of Contagious Bovine Pleuropnuemonia (CBPP) in mixed cattle contagious bovine Pleuropneumonia by detection, identification and differentiation of Mycoplas mamycoides subsp. mycoides in Niger State, Nigeria. Sokoto J Vet Sci 13: 1.
- Thomson G R (2005) Contagious bovine pleuropneumonia and poverty. A strategy for addressing the effects of the disease in sub- Saharan Africa, Research report, (DFID animal health programme, centre for Tropical Veterinary Medicine, University of Edinburgh, UK.
- Schnier C, Mtui-Malamsha NJ, Cleavel S, Kiara H, Grace D, et al. (2006) CBPP Seroprevalence and associated risk factors in the Maasai ecosystem of South-western Kenya. International Livestock Research Institute 5: 567-574.
- Amanfu W (2009) Contagious bovine pleuropneumonia (lung sickness) in Africa. Onderstepoort J Vet Res 76: 13-17.
- Majekodunmi AO, Fajinmi A, Dongkum C, Shaw AM, Welburn SC (2014) Pastoral livelihoods of the Fulani on the Jos Plateau of Nigeria. Pastoralism 4: 20.
- Mekonnen G (2004) Serological, Clinical and Participatory Epidemiological Survey of Contagious Bovine Pleuropneumonia in Somali Region, Ethiopia Addis Ababa University Faculty of veterinary Medicine. Debreziet, Ethiopia.
- Musa JA, Bale JOO, Kazeem HM, Nwankpa ND, Provvido AD, et al. (2016) Molecular detection of Nigerian field isolates of Mycoplasma mycoides subsp. mycoides as causative agents of contagious bovine pleuropneumonia. Inter J Vet Sciand Med 4: 46-53.
- Aliyu MM, Obi TU, Egwu GO (2000) Prevalence of contagious bovine pleuropneumonia (CBPP) in Northern Nigeria. Prev Vet Med 47: 263-269.
Citation: Fulasa A, Teshome I, Bulto AO, Lakew M, Tadesse B, et al. (2020) Seroprevalence, Isolation and Associated Risk Factors of Contagious Bovine Pleuropneumonia at Bako Tibe and Ilu Galan Districts of West Shoa Zone, Western Ethiopia. J Anim Res Vet Sci 4: 028.
Copyright: © 2020 Abdisa Fulasa, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.

