
Shoulder Injury Related to Vaccine Administration (SIRVA): A Systematic Literature Review
*Corresponding Author(s):
Vaidya BalaMedical Co-Director Public And Population Health, Senior Staff Specialist In Brain Injury Rehabilitation, The Wollongong Hospital, Australia
Email:vaidya.balasubramaniam@health.nsw.gov.au
Abstract
Shoulder Injury Related to Vaccine Administration (SIRVA) is a rare but increasingly recognized adverse event following intramuscular vaccination, particularly in the deltoid region. This review synthesizes current evidence on the pathophysiology, diagnostic imaging, treatment modalities, and demographic correlations of SIRVA, with a focus on cases following COVID-19 vaccination. MRI findings, patient characteristics, and vaccine types are analyzed to identify risk factors and guide clinical management.
Keywords
SIRVA; Shoulder Injury; Vaccine Administration; MRI; COVID-19; Bursitis; Capsulitis.
Introduction
SIRVA is defined as shoulder pain and dysfunction occurring within 48 hours of vaccine administration, typically due to improper injection technique leading to antigen deposition in synovial tissues [1,2]. Historically associated with influenza vaccines, the condition has gained increased attention during the COVID-19 pandemic due to mass vaccination efforts [1]. Common symptoms include subacromial bursitis, adhesive capsulitis, and rotator cuff injuries, which can significantly impair shoulder mobility and quality of life [3].v
Methods
A systematic review was conducted using PubMed, Europe PMC, and MDPI databases. The search included peer-reviewed articles published between 2019 and 2025, written in English, and focused on SIRVA diagnosis, imaging, treatment, and prevention. Exclusion criteria included non-peer-reviewed sources, articles not focused on SIRVA, and non-English publications. Study types included systematic reviews, retrospective cohort studies, case reports, and clinical guidelines (Table 1) [4].
|
Component |
Details |
|
Databases Searched |
PubMed, Europe PMC, MDPI |
|
Inclusion Criteria |
Peer-reviewed articles from 2019–2025, English language, focus on SIRVA diagnosis/treatment/imaging |
|
Exclusion Criteria |
Non-peer-reviewed sources, articles not focused on SIRVA, non-English publications |
|
Study Types Included |
Systematic reviews, retrospective cohort studies, case reports, clinical guidelines |
Table 1. Methodology Summary
Results
The review identified that SIRVA is more prevalent in women and individuals with higher BMI [1]. MRI findings commonly included subacromial bursitis (89.5%), tendonitis (47.4%), and adhesive capsulitis (36.8%). Specific vaccine associations were noted: AstraZeneca was linked to tendonitis, while Sinopharm was associated with adhesive capsulitis (Figure 1 & Table 2). Persistent symptoms were observed in 22.9% of cases, particularly among younger patients and those with right-sided injections [4].
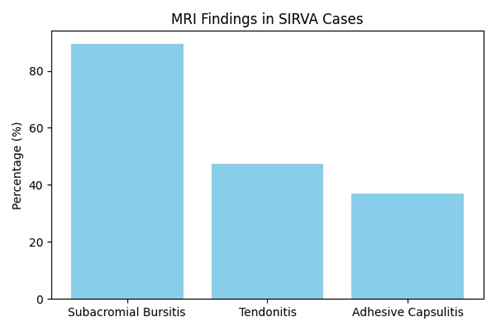 Figure 1. MRI Findings in SIRVA Cases
Figure 1. MRI Findings in SIRVA Cases
|
Vaccine |
Associated Condition |
|
AstraZeneca |
Tendonitis |
|
Sinopharm |
Adhesive Capsulitis |
Table 2. Vaccine Associations with SIRVA Conditions
Discussion
SIRVA is a preventable condition that underscores the importance of proper vaccination technique, including correct land marking and needle length selection [4]. MRI remains the gold standard for diagnosis, offering detailed visualization of soft tissue injuries [3]. Conservative treatment approaches such as NSAIDs, corticosteroid injections, and physical therapy are effective in most cases. However, misdiagnosis can occur, especially in patients with pre-existing shoulder conditions [2]. Increased awareness and training among healthcare providers are essential to reduce the incidence of SIRVA and improve patient outcomes [5,6].
Case Studies
- Sub coracoid Bursitis Post-HPV Vaccination
A 52-year-old woman developed anterior shoulder pain and limited ROM within 24 hours of receiving an HPV vaccine. Ultrasound and MRI confirmed subcoracoid bursitis. Treatment included corticosteroid injection and physiotherapy, resulting in significant improvement.
- Adhesive Capsulitis Following COVID-19 Vaccination
A 49-year-old female presented with shoulder stiffness and pain three days after her second Pfizer-BioNTech COVID-19 vaccine. MRI revealed adhesive capsulitis. Conservative treatment led to full recovery within three months.
- Rotator Cuff Tear Misdiagnosed as SIRVA
A 60-year-old male experienced persistent shoulder pain after influenza vaccination. Initial diagnosis was SIRVA, but MRI later confirmed a pre-existing rotator cuff tear. This highlights the importance of differential diagnosis.
Conclusion
This review highlights the clinical relevance of SIRVA and the need for vigilance in vaccine administration practices. With the global expansion of vaccination programs, early recognition and appropriate management of SIRVA are crucial to prevent long-term morbidity and maintain public confidence in immunization efforts.
References
- Obeidat N, Khasawneh R, Alrawashdeh A, Abdel Kareem AM, Al-Na'asan MK, et al. (2025) Shoulder injury related to vaccine administration (SIRVA) following COVID-19 vaccination: Correlating MRI findings with patient demographics. Tomography 11: 53.
- Jenkins PJ, Duckworth AD (2023) SIRVA: Shoulder injury related to vaccine administration. Bone & Joint Journal 8: 839-842.
- Quinn S (2021) Vaccine-related shoulder injury. Radsource MRI Web Clinic.
- Bancsi A, Houle SKD, Grindrod KA (2019) Shoulder injury related to vaccine administration and other injection site events. Canadian Family Physician 65: 40-42.
- Cagle PJ Jr (2021) Shoulder injury after vaccination: A systematic review. Europe PMC 56: 299-306.
- Macmahon A, Nayar SK, Srikumaran U (2023) What do we know about shoulder injury related to vaccine administration? Johns Hopkins University.
Citation: Bala V (2025) Shoulder Injury Related to Vaccine Administration (SIRVA): A Systematic Literature Review. J Orthop Res Physiother 11: 062
Copyright: © 2025 Vaidya Bala, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.

