
The Control of Neuroendocrine Mechanisms in Livestock to Facilitate Adaptation to Stress
*Corresponding Author(s):
Arushi KanwarDepartment Of Veterinary Physiology And Biochemistry, Lala Lajpat Rai University Of Veterinary And Animal Sciences, Hisar, Haryana, India
Email:arukanwar15@gmail.com
Abstract
This article discusses the concept of stress in animals, which is a complex set of physiological and behavioral responses to aversive stimuli. The article explores short-term vs long-term stress and the role of glucocorticoids in adaptation. It is crucial to regulate glucocorticoid levels for optimal physiological functioning and adaptation to stress. The article divides stress response into three stages: alarm, resistance, and exhaustion. The article also discusses the neuroendocrine axis affected by stress, including the ACTH axis, vasopressin axis, reproductive axis, growth hormone axis, thyroid axis, feed intake axis, immunity axis, and brain development axis. Overall, understanding the endocrine changes and their impact on the body can lead to better management of animals to avoid negative adaptations and to utilize positive ones.
Keywords
Animal ; Endocrine ; Physiology ; Stress
Introduction
The concept of "stress" refers to a complex set of physiological and behavioural responses that occur in response to aversive stimuli. In 1929, Cannon aptly described stress as the body's attempt to maintain homeostasis when faced with a range of stressors, activating the sympatho-adrenomedullary (SAM) system. Meanwhile, Selye coined stress as the "non-specific response of the body to any demand for change". The stress response in animals is a finely tuned, integrated phenomenon [1]. The World Health Organization has defined stress as the body's reaction to stimuli that require attention or action. Physiologically, "strain" refers to the internal displacement caused by stress, while "stress" refers to external factors that disturb the body's homeostasis. Animals are constantly exposed to environmental stressors that disrupt their state of balance, leading to the development of new adaptations that may or may not be beneficial to humans. The assessment of adaptability is crucial for effective cattle management, with the avoidance of negative adaptations and the utilization of positive ones being a key aspect of this process [2].
Short-Term Vs Long-Term Stress
Glucocorticoids play a vital role in supporting physiological and behavioral processes involved in adaptation. A brief surge in these hormones is actually protective and helps the animal respond more effectively to a stressful situation [3]. However, when glucocorticoid levels remain high or rise persistently, regulatory mechanisms can be adversely impacted. This can lead to a suppression of important physiological functions such as growth and reproduction.
The body's Sympathetic Nervous System (SNS) is also activated during times of stress, and the Paraventricular Nucleus (PVN) helps boost SNS activity to aid in the stress response. Oxytocin and epinephrine can promote the secretion of Adrenocorticotropic Hormone (ACTH), further supporting the body's response to stress. In acute stress, ACTH increases the concentration of glucocorticoids, which lead to decrease in growth hormone and Gonadotropin Releasing Hormone (GnRH). These hormones together decrease insulin levels in body, increase blood glucose, epinephrin and nor-epinephrin and thereby increases ability of body to undergo fight and flight reaction.
However, prolonged exposure to high levels of cortisol can have negative effects on the body, inhibiting the hypothalamic-pituitary-gonadal, thyroid, and growth hormone-insulin-like growth factor 1 axes. This can lead to an increase in visceral adiposity, a loss of lean mass, and an increased risk of diseases like osteoporosis and insulin resistance [4]. To better understand the complex endocrine changes and their impact on the body, we have included figures 1 and 2 for reference. Overall, it is important to carefully regulatglucocorticoid levels in order to ensure optimal physiological functioning and adaptation to stress.
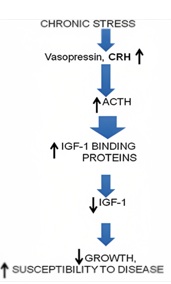 Figure 1: Effect of chronic stress on body
Figure 1: Effect of chronic stress on body
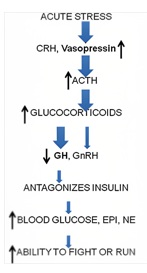 Figure 2: Effect of acute stress on body
Figure 2: Effect of acute stress on body
Stages Of Stress Response
Stress response is divided into three stages as shown in (Figure 3).
Stage 1: Alarm:
Upon encountering a stressor, body reacts with “fight-or-flight” response and sympathetic nervous system is activated. Hormones such as cortisol and adrenalin released into the bloodstream to meet the threat or danger. The body’s resources now mobilized.
Stage 2: Resistance:
Parasympathetic nervous system returns many physiological functions to normal levels while body focuses resources against the stressor. Blood glucose levels remain high, cortisol and adrenalin continue to circulate at elevated levels, but outward appearance of organism seems normal. Heart rate, blood pressure and breathing increases. Body remains on red alert.
Stage 3: Exhaustion:
If stressor continues beyond body’s capacity, organism exhausts resources and becomes susceptible to disease and death. [7].
Neuro-endocrine axis affected in stress
- ACHT Axis
- Vasopressin Axis
- Reproductive axis
- Growth hormone axis
- Thyroid axis
- Feed intake axis
- Immunity axis
- Brain development axis
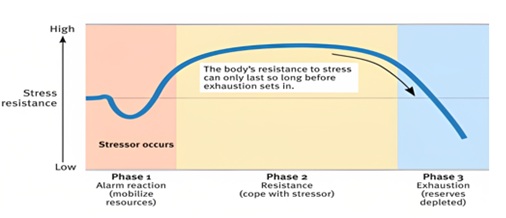 Figure 3: Phases of General Adaptation Syndrome.
Figure 3: Phases of General Adaptation Syndrome.
- ACTH axis
The Hypothalamic-Pituitary-Adrenal (HPA) axis mediates the main elements of the stress response. Corticotropin-Releasing Factor (CRF) is produced and released by nerve cells in the hypothalamus as a result of stress. The anterior pituitary gland receives CRF, which induces the creation of the Protein Proopiomelanocortin (POMC). Adrenocorticotropic hormone (ACTH), -Lipotropin (-LPH), and -endorphin are released by POMC in response to stress. When cortisol is released, it inhibits the release of ACTH and CRF. Physiological functions including growth and reproduction are momentarily suppressed when glucocorticoid levels are briefly raised. This helps the animal to utilize biological resources for reflex responses like flying more effectively [8]. When CRF binds to its receptor on pituitary corticotropes, it induces the release of Adrenocorticotropic Hormone (ACTH) into the systemic circulation. Circulating ACTH principally targets adrenal cortex, where glucocorticoid synthesis is stimulated and secreted from the zona fasciculata. Glucocorticoids are the downstream effectors of the HPA axis and regulate physiological changes through ubiquitously distributed intracellular receptors [9]. Endocrine pathway in ACTH is shown in (Figure 4).
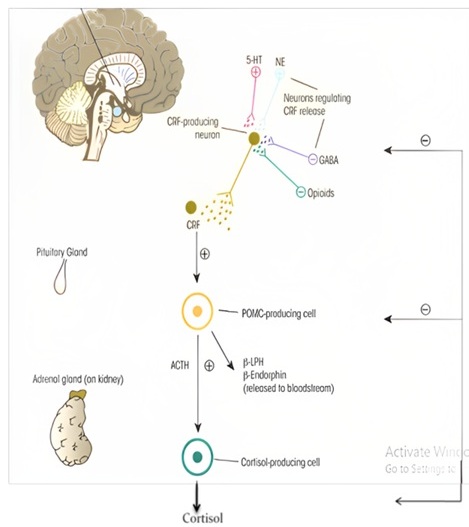 Figure 4: Effect of stress on ACTH axis.
Figure 4: Effect of stress on ACTH axis.
- Vasopressin Axis
Vasopressin-like peptides serve as hormones that preserve salt-water balance in animals. Vasopressin increases the blood volume by increasing the Na+ and reducing urine output. AVP together with CRH, stimulates the proopiomelanocortin-producing corticotrope cells of the anterior pituitary to secrete adrenocorticotropin (ACTH) [11]. ACTH elevation induces cortisol secretion in adrenal gland, thus stress response appears. AVP-induced stress hormone elevation is less sensitive to negative feedback than a CRH-induced one; suggesting this pathway play a critical role during chronic stress. AVP expression and receptor patterns is greatly influenced by the environment, playing a critical role in epigenetic programming in the fetus and during early childhood. AVP produced in the magnocellular region of the paraventricular nucleus (PVN) is transported through the posterior pituitary to the blood stream and is responsible for water reabsorption in the kidney [12]. AVP and corticotropin-releasing hormone (CRH) produced in the parvocellular population of PVN are released to the portal circulation of the pituitary, reaching the anterior lobe where they stimulate proopiomelanocortin-producing cells via acting on V1bR and CRHR1 receptors, thereby promoting adrenocorticotropic hormone (ACTH) synthesis and release. In the adrenal cortex, ACTH induces glucocorticoid production (e.g., cortisol), which triggers a response to stress [13]. Pathway and effect of stress on Vasopressin axis is shown in (Figure 5).
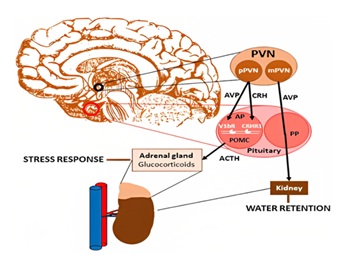 Figure 5: Pathway and effect of stress on Vasopressin axis.
Figure 5: Pathway and effect of stress on Vasopressin axis.
- Reproductive axis
It is comprised of the hypothalamus containing neurons that secrete GnRH in pulses. Pituitary releases (LH) and (FSH), and the gonads secrete the gonadal hormones estradiol and progesterone in females or testosterone in males. Stress impacts livestock reproduction by reduced feed intake, altered endocrine status, reduction in rumination and nutrient absorption. Increased maintenance requirements resulting in a net decrease in nutrient/energy availability for reproduction [15]. Stress (heat, nutritional, pH, immunological, physiological) prevents the basic cell function by causing improper folding of proteins. Stress could impair fertility by affecting GnRH–LH pulsatility, estradiol pro?les, LH surge timing and levels, follicular recruitment and development, and ovulation. Glucocorticoids, vasopressin, ACTH, CRH, opioids have an inhibitory effect on LH secretion [16]. Glucocorticoids can inhibit gonadal steroid secretion and alter the sensitivity of target tissues to these hormones. Effect of stress on reproductive functions is also severe. Stress inhibits reproductive functions. This is probably mediated through a reduction in norepinephrine (NE) levels in the hypothalamus, an increase in corticotrophin releasing hormone (CRH), or through an increase in cytokines that increases gamma amino butyric acid (GABA) to suppress NE levels. The net result is a reduction in gonadotropin releasing hormone (GnRH), luteinizing hormone (LH), and estradiol levels that functionally translates to loss of estrous cyclicity, reduced ovulation and conception rates and increasing intercalving intervals [17]. Effect of stress on male and female reproductive system is shown in (Figures 6 & 7) respectively.
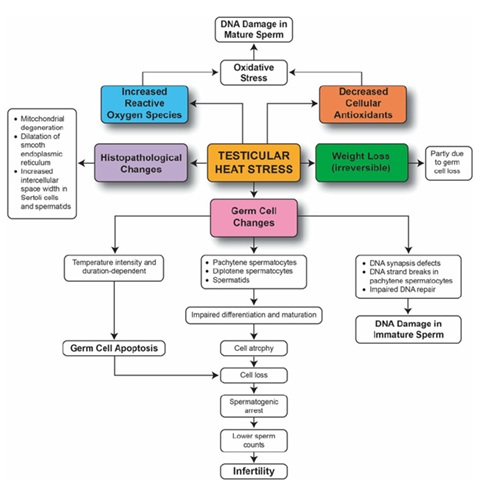 Figure 6: Effect of stress on testicular function
Figure 6: Effect of stress on testicular function
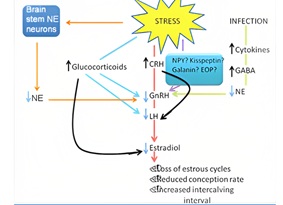 Figure 7: Effect of stress on female reproductive system.
Figure 7: Effect of stress on female reproductive system.
- Nutrition and its Effects on Reproduction
Undernutrition and malnutrition lead to an increase in CRH levels, which can lead to a decrease in the release of GnRH and LH [18-19]. Decrease in GnRH and LH release lead to a decrease in reproductive function, including a decrease in fertility and an increase in the risk of miscarriage [20]. Leptin produced primarily in adipose tissue, acts on the hypothalamus to regulate appetite and energy balance. Leptin has also been shown to play a critical role in the regulation of reproductive function. Leptin stimulates the release of GnRH, in turn stimulating the release of LH [21] and malnutrition can lead to a decrease in leptin levels, which can lead to a decrease in the release of GnRH and LH [22]. The mechanism has been discroibed in (Figure 8).
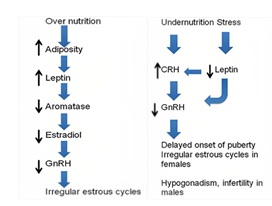 Figure 8: Effect of stress on nutrition.
Figure 8: Effect of stress on nutrition.
- Growth hormone axis
In livestock, growth hormone levels increase in stress, but IGF-1 levels decrease in response to stress. This might be due to an increase in GHRH levels through NE levels. Simultaneous activation of the HPA axis facilitates the increase in GHRH. Fasting increase growth hormone and decrease IGF-1 levels in livestock due to down regulation of growth receptors in liver [23]. Acute stressors decrease glucose utilization so that blood glucose levels increase and prepare the animal to ?ght or flight. Chronic stress decreases insulin-like growth factor (IGF)-1 production and inhibit growth. The reduction in IGF-1 levels result in reduced growth, and the conservation of energy and nutrients for survival. This is believed to be an important adaptive response, shifting energy resources from growth to survival [24]. Pathway and effect of stress on growth hormone axis is shown in (Figure 9).
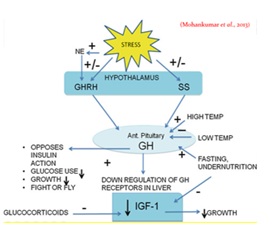 Figure 9: Effect of stress on growth hormone axis.
Figure 9: Effect of stress on growth hormone axis.
- Thyroid axis
Long-term exposures to cool temperatures stimulate the thyroid axis mainly by increasing TSH levels. The resulting increase in basal metabolic rate and increased body temperature can inhibit the thyroid axis [25]. In the context of survival, reduced thyroid activity would decrease metabolism and corresponding energy usage. This is especially useful when food is scarce. However, activation of the thyroid axis becomes bene?cial during cold stress. The resultant increase in endogenous opioids and glucocorticoid levels during stress can also cause suppression of the thyroid axis [26]. Effect of stress on thyroid axis has been shown in (Figure 10).
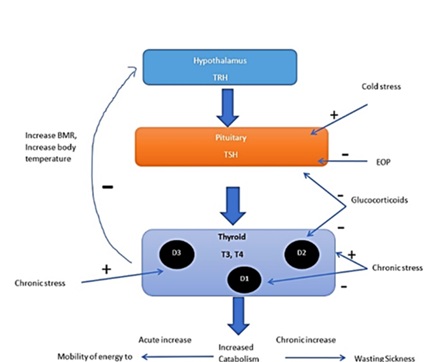 Figure 10: Effect of stress on Thyroid axis.
Figure 10: Effect of stress on Thyroid axis.
- Feed intake axis
Leptin, a hormone produced by fat cells, can suppress appetite by interacting with melanocortin (MC-4) receptors located in Proopiomelanocortin (POMC) neurons within the arcuate nucleus of the medulla oblongata [27]. When faced with stress, the body's release of CRH can also reduce appetite by activating the HPA axis. Pro-inflammatory cytokines can cause anorexia and stimulate the production of leptin, which can in turn decrease food intake by affecting the hypothalamus [28]. Effect of stress on feeding is described in (Figure 11).
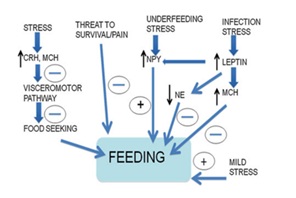 Figure 11: Effect of stress on feeding.
Figure 11: Effect of stress on feeding.
- Feeding Responses to Cold Stress
Under normal circumstances, leptin and NPY work together to regulate energy balance. However, when exposed to low temperatures, leptin levels decrease, leading to an increase in food intake [29]. Conversely, NPY levels in the hypothalamus increase, leading to an increase in appetite. The protein UCP1 plays a role in generating heat in brown adipose tissue (BAT) rather than energy, leading to thermogenesis. Animals exposed to cold for 21 days or more experience a decrease in leptin levels but no increase in NPY levels [30]. This leads to weight loss, likely due to increased thermogenesis in BAT. Animals lacking BAT tend to gain weight. These findings suggest that thermogenesis in BAT plays a crucial role in energy homeostasis, and that cold exposure can stimulate this process. Feeding response to acute and chronic cold stress is shown in (Figure 12).
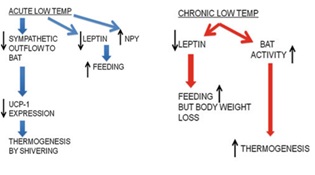 Figure 12: Feeding response to cold stress.
Figure 12: Feeding response to cold stress.
- Immunity axis
Chronic stress has been shown to increase levels of glucocorticoids [31]. These hormones are known to suppress the immune system, potentially leading to autoimmune disorders [32]. Glucocorticoids primarily affect the immune system by reducing levels of proinflammatory cytokines such as IL-1 and IL-6, while increasing levels of anti-inflammatory cytokines like IL-10 [33]. However, chronic suppression of the immune system by glucocorticoids is not recommended, as the immune system can rebound and regain its previous functionality when glucocorticoid levels decrease. Furthermore, additional stressors may prompt the body to produce more glucocorticoids, which could further weaken the immune system. As such, it is not advisable to repeatedly use glucocorticoids to depress the immune system.
- Stress Axis and Developmental Programming
Although animals possess an innate mechanism to counteract glucocorticoid exposure during fetal development, prolonged and elevated levels of glucocorticoids can still have adverse effects. The enzyme 11-hydroxysteroid dehydrogenase b (11-HSDb) plays a crucial role in converting active glucocorticoids to their inactive forms that are present in high concentrations in the placenta, thereby safeguarding the developing fetus from exposure to harmful glucocorticoids. Studies conducted on sheep have shown that prolonged stress and increased glucocorticoid levels can result in intrauterine growth retardation (IUGR), which is a disorder that could be observed in livestock affected by famine and drought [34]. This condition not only affects the physical growth of the offspring but also influences their neural circuits, which can have long-lasting effects on their behavior as adults [35].
Conclusion
The neuroendocrine responses to different stressors vary depending on the type, severity, and duration of the stressful experience. When animals are exposed to prolonged or severe acute stressors, their survival mechanisms take priority over growth, output, and reproduction. A stressor triggers a stress response that involves various homeostatic systems, including physiological, immunological, and behavioral adaptations. Neuroendocrine adaptations help to conserve energy, promote thermogenesis, and facilitate the efficient utilization of energy sources for long-term survival. The animal's ability to regulate its stress response has little impact on the productivity of the herd, but if chronic stressors exceed an animal's coping abilities, it can negatively affect its growth, productivity, reproduction, and survival. Therefore, it is crucial to implement effective management techniques and improve animal welfare to maintain optimal stress responses in livestock.
References
- Chrousos GP, Gold PW (1992) The concepts of stress and stress system disorders. Overview of physical and behavioral homeostasis. JAMA J Am Me Assoc 267: 1244-1252.
- Stott GH (1981) What is animal stress and how is it measured?. Journal of Animal Science52: 150-153.
- Salak-Johnson JL (2011) Adaptation and stress: neuroendocrine, physiological, and behavioral responses. Encyclopedia of animal science1: 1-5.
- Smith SM, Vale WW (2006) The role of the hypothalamic-pituitary-adrenal axis in neuroendocrine responses to stress. Dialogues in clinical neuroscience 8: 383-395.
- Mohan Kumar SM, Balasubramanian P, Dharmaraj M, Mohan Kumar PS (2012) Neuroendocrine regulation of adaptive mechanisms in livestock. Environmental stress and amelioration in livestock production 263-298.
- Szabo S, Tache Y, Somogyi A (2012) The legacy of Hans Selye and the origins of stress research: A retrospective 75 years after his landmark brief “Letter” to the Editor of Stress 15: 472- 478.
- Chu B, Marwaha K, Sanvictores T, Ayers D (2021) Physiology, stress reaction. In Stat Pearls Publishing.
- Rivier C, Vale W (1983) Modulation of stress-induced ACTH release by corticotropin-releasing factor, catecholamines and vasopressin. Nature 305: 325-327.
- Bamberger CM, Schulte HM, Chrousos GP (1996) Molecular determinants of glucocorticoid receptor function and tissue sensitivity to glucocorticoids. Endocrine Reviews17: 245-261.
- Stephens M, Wand G (2012) Stress and the HPA axis: role of glucocorticoids in alcohol dependence. Alcohol research: current reviews34: 468-483.
- Treschan TA, Peters J, Warltier DC (2006) The vasopressin system: physiology and clinical strategies. The J of the American Society of Anaesthesiologists105: 599-612.
- Angelousi A, Margioris AN, Tsatsanis C (2020) ACTH action on the adrenals. Endotext South Dartmouth MA.
- Aguilera G, Subburaju S, Young S, Chen J (2008) The parvocellular vasopressinergic system and responsiveness of the hypothalamic pituitary adrenal axis during chronic stress. Progress in Brain Research 170: 29-39.
- Török B, Fazekas CL, Szabó A, Zelena D (2021) Epigenetic modulation of vasopressin expression in health and disease. International Journal of Molecular Sciences22: 9415.
- Marques P, Skorupskaite K, Rozario KS, Anderson RA, George JT et al. (2022) Physiology of GNRH and gonadotropin secretion. Endotext.
- Boni R (2019) Heat stress, a serious threat to reproductive function in animals and humans. Molecular Reproduction and Development 86: 1307-1323.
- Sheng JA, Bales NJ, Myers SA, Bautista AI, Roueinfar M, et al. (2021) The hypothalamic-pituitary-adrenal axis: development, programming actions of hormones, and maternal-fetal interactions. Frontiers in Behavioural Neuroscience 14: 601939.
- Durairajanayagam D, Sharma RK, du Plessis SS, Agarwal A (2014) Testicular heat stress and sperm quality. Male infertility: a complete guide to lifestyle and environmental factors 105-125.
- Abbara A, Jayasena CN, Izzi-Engbeaya C, Comninos AN, Harvey RA et al. (2014) The effects of undernutrition and obesity on the gonadotropin-inhibiting hormone (GnIH) and kisspeptin systems in female rats. Journal of Endocrinology 223: 49-58.
- Gardner S, Stavrou E, Rischitor PE, Faccenda E, Pawson AJ (2010) Journal of molecular endocrinology 44: 195-201.
- Chehab FF, Qiu J, Ogus S (2004). The use of animal models to dissect the biology of leptin. Recent Progress in Hormone Research 59: 245-266.
- Soliman AT, Yasin M, Kassem A (2012) Leptin in pediatrics: A hormone from adipocyte that wheels several functions in children. Indian Journal of Endocrinology and Metabolism 16: S577-S587.
- Straus DS, Takemoto CD (1990) Effect of fasting on insulin-like growth factor-I (IGF-I) and growth hormone receptor mRNA levels and IGF-I gene transcription in rat liver. Journal of Molecular Endocrinology4: 91-100.
- Marik PE, Bellomo R (2013) Stress hyperglycemia: an essential survival response!. Crit Care 17: 305.
- Mullur R, Liu YY, Brent GA (2014) Thyroid hormone regulation of metabolism. Physiological Reviews94: 355-382.
- Cicatiello AG, Di Girolamo D, Dentice M (2018) Metabolic effects of the intracellular regulation of thyroid hormone: old players, new concepts. Front Endocrinol9: 474.
- Baldini G, Phelan KD (2019) The melanocortin pathway and control of appetite-progress and therapeutic implications. The Journal of endocrinology 241: R1-R33.
- Gautron L, Layé S (2010) Neurobiology of inflammation-associated anorexia. Frontiers in Neuroscience 3: 59.
- Heilig M (2004) The NPY system in stress, anxiety and depression. Neuropeptides38: 213-224.
- Giraudo SQ, Kotz CM, Grace MK, Levine AS, Billington CJ (1994) Rat hypothalamic NPY mRNA and brown fat uncoupling protein mRNA after high-carbohydrate or high-fat diets. American Journal of Physiology-Regulatory Integrative and Comparative Physiology 266: R1578-R1583.
- Whirledge S, Cidlowski JA (2010) Glucocorticoids, stress, and fertility. Minerva Endocrinol35: 109-125.
- Silver EM, Ochoa W (2018) Glucocorticoid-induced myopathy in a patient with systemic lupus erythematosus (SLE): A case report and review of the literature. The American Journal of Case Reports19: 277.
- Strehl C, Ehlers L, Gaber T, Buttgereit F (2019) Glucocorticoids-All-Rounders Tackling the Versatile Players of the Immune System. Frontiers in Immunology 10: 1744.
- Cottrell EC, Seckl J (2009) Prenatal stress, glucocorticoids and the programming of adult disease. Frontiers in behavioral neuroscience 3: 19.
- Shonkoff JP, Phillips DA (2000) National Research Council The developing brain. In From neurons to neighborhoods: The science of early childhood development. National Academies Press (US).
Citation: Kanwar A, Virmani M, Chaudhary K, Sharma R, Saini P (2023) The Control of Neuroendocrine Mechanisms in Livestock to Facilitate Adaptation to Stress J Anim Res Vet Sci 7: 0.46
Copyright: © 2023 Arushi Kanwar, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.

