
The Expanding Spectrum of COVID-19 Lung Imaging: Exploring Inputs from Radiologic-Pathologic Data for Disease Detection
*Corresponding Author(s):
Venkatraman BhatDepartment Of Radiology, Mazumdar Shaw Medical Center, Narayana Health City, Bangalore, India
Tel:91 9481027387,
Email:bvenkatraman@gmail.com
Abstract
Rationale and objectives
This report attempts to interpret chest imaging observations in COVID-19 patients in the context of its histopathologic alterations. Chest High Resolution Computed Tomography (HRCT) images from patients with COVID-19 are analyzed and are correlated with morphologic and histo-pathological observations that have been published in autopsy-based pathology literature.
Materials and methods
A literature review of COVID-19 lung pathology was performed, with a focus on gross and microscopic alterations reported in autopsy studies. HRCT chest images from 2000 COVID-19 patients at our tertiary center were analyzed, and the spectrum of radiological findings seen in this patient dataset was noted. The HRCT images from our patients were then correlated with COVID-19 pathology data reported in literature and a histo-pathologic basis for certain distinct imaging findings were explored.
Results
The spectrum of imaging changes seen in COVID-19 patients correlated well with histopathologic changes. Frequent vessel occlusion and perfusion abnormalities noted on pathology data call for additional scrutiny of COVID patients by sensitive imaging techniques like dual energy CT for detection of vascular changes in clinical practice.
Conclusion
Correlation of radiologic and pathologic data in COVID-19 patients facilitates a more complete understanding of the disease process. Frequent vessel occlusion and thrombotic phenomena noted on autopsy data call for additional scrutiny while investigating patients with the use of sensitive imaging techniques such as dual energy CT. Few unique HRCT observations like ‘vessel arborisation sign’ are identified and might possibly be explained by the underlying pathologic changes.
Keywords
COVID-19 lung changes; DAD; HRCT; Organising pneumonia; Vessel arborisation sign.
ABBREVIATIONS
HRCT: High Resolution Computed Tomography
ACE2: Angiotensin Converting Enzyme
RDS: Respiratory Distress Syndrome
DAD: Diffuse Alveolar Damage
IHC: Immunohistochemistry
AFOP: Acute Fibrinous and Organizing Pneumonia
GGO: Ground Glass Opacities
INTRODUCTION
The COVID-19 pandemic has changed the world in view of its substantial disease related morbidity and mortality [1-3]. The disease is caused by infection with Severe Acute Respiratory Syndrome Coronavirus 2 (SARS-CoV-2) [4,5] and prompt laboratory confirmation of viral presence is essential for establishing early diagnosis, initiating early isolation and patient management. The importance of early detection of viral infection is underscored by the high transmissibility of the virus [5-7]. At present, molecular detection of viral nucleic material is the gold standard for diagnosis [5] and laboratory methods such as Reverse-Transcriptase Polymerase Chain Reaction are being used widely for detection. Although several clinico-radiologic observations in COVID-19 are non-specific, there does appear to be an important role for imaging modalities such as HRCT in early diagnosis and in assessment of disease prognosis. Over the years, HRCT has established itself as an invaluable tool for assessment of pulmonary pathology with adequate support in the radiology literature attesting to its accuracy [8-13]. Due to its high sensitivity for detecting lung changes at alveolar/secondary lobule level, HRCT is used for diagnosis as well as management of COVID-19. Additionally, HRCT has the ability to display global lung changes and provide quantitative information about the extent of these changes [12-17]. While pulmonary imaging data is available from COVID-19 patients with mild/moderate as well as severe disease, published autopsy-based pathology data only reflects a subset of patients with a severe and eventually lethal form the disease. This fact may account for a degree of discrepancy between radiologic and autopsy-based histopathologic findings. Nevertheless, autopsy based pathology data provides invaluable information and insight into the anatomic aberrations observed in severe COVID-19 pneumonia. In this report, published literature on autopsy-based data in COVID-19 patients is reviewed, and an attempt is made to correlate this pathology data with HRCT imaging findings noted in our dataset of patients.
HISTOPATHOLOGIC FINDINGS IN COVID-19 AUTOPSIES
Similar to other Coronaviruses, SARS-CoV2 has been shown to enter the cell through the Angiotensin Converting Enzyme (ACE2) Receptor [5,18]. Pulmonary and bronchial epithelial cells show high expression of ACE2 receptors and serve as important points of viral entry and replication [18-20]. High viral loads have been isolated from lower respiratory samples [21,22]. In an autopsy series of 7 COVID-19 cases conducted by Ackermann et al. [23], immunohistochemical analysis of ACE2 expression found higher counts of ACE2-reactive alveolar epithelial cells and endothelial cells in COVID-19 lungs than in uninfected control lungs [23]. The lungs of COVID-19 patients also showed ACE2-positive lymphocytes in perivascular tissue and within alveoli.
Clinically, SARS-CoV2 infection can range in presentation from asymptomatic/pauci symptomatic disease to severe, life-threatening respiratory failure [1,5]. The rapid replication of the virus within the respiratory system can trigger a hyperinflammatory response, eventually cascading into a cytokine storm syndrome that results in Acute Respiratory Distress Syndrome (ARDS) and respiratory failure [5,24]. It is worth noting that a subset of COVID-19 ARDS patients have been noted to exhibit higher levels of respiratory compliance than would be expected in classical ARDS, even in the setting of severe hypoxemia [25,26]. Coagulation abnormalities, associated with high d-dimer levels, are another important element of the disease pathophysiology, and manifest as thrombotic disease [23,27]. Older age, compromised respiratory status on admission, and presence of pre-existing comorbidities has been consistently correlated with greater risk of developing ARDS, severe respiratory failure and negative patient outcomes [6,28,29]. On a histopathologic level, these above-mentioned clinical states manifest predominantly as patterns of diffuse alveolar damage, capillary congestion, and widespread thrombosis with microangiopathy, which are noted as consistent themes identified in autopsy based series.
MACROSCOPIC
The lungs of COVID patients tend to be bulky and heavier than normal, with diffuse congestion and edema [30-34]. Massive congestion can give the lungs a blue-red hue [35]. Gross examination of lungs can show heterogeneous findings ranging from patchy, firm, discolored foci to diffuse areas of parenchymal consolidation [30]. The upper airways can exhibit areas of mucosal ulceration and inflammation, seen grossly as focal white patches, often 2-3 mm in diameter. Thickened alveolar septae and signs of interstitial congestion can sometimes be appreciated on gross examination. Focal or diffuse areas of superimposed suppurative bronchopneumonia can be present [32]. Another important feature to look for is the presence of thrombi within the pulmonary vasculature. Thrombosis can involve vessels of any caliber, ranging from large-vessel central thrombi including saddle emboli [30] to smaller emboli within the peripheral vessels. Presence of emboli can be focal or diffuse, and adjacent areas of pulmonary infarction may be seen [35].
MICROSCOPIC
Sections from the trachea can show inflammation ranging from small lymphoid aggregates [34] to focal areas of mucosal ulceration with mixed inflammatory cell infiltration and fibrin (corresponding to the focal white patches seen grossly [30]. No significant association has been found linking the presence of tracheitis with intubation, invasive ventilation or acute bacterial/fungal pneumonia [30]. Transmural lymphomonocytic inflammation within bronchi and bronchioalveolar walls has also been described, along with other non-specific features such as squamous metaplasia and luminal dense mucoid secretions [31].
Histologic changes in COVID-19 autopsies show a progressive disease that begins in the upper air passages and extends distally into the alveoli. The predominant pattern of lung lesions in patients with COVID-19 is Diffuse Alveolar Damage (DAD), which shows temporal heterogeneity manifesting as exudative and organizing phase changes [34]. The acute or exudative phase changes seen are characterized by formation of hyaline membranes. These hyaline membranes are composed of condensed fibrin and protein, and can be identified in both short duration as well as long standing disease [30]. A 12 case autopsy study by Schaller et al. found DAD findings to be unevenly distributed and prominent in middle and lower lobes [34]. Viral particles have been identified by Immunohistochemistry (IHC) within these hyaline membranes. Other features of the exudative phase include capillary congestion, interstitial and intra-alveolar edema, dilated alveolar ducts, collapsed alveoli and pneumocyte loss [31]. Capillarostasis can be a prominent finding [32]. The lung injury pattern of Acute Fibrinous and Organizing Pneumonia (AFOP) has also been described by some studies [36,37]. In contrast to the hyaline membranes seen in DAD, this pattern is characterized by deposition of ‘fibrin balls’, which are aggregates of intra-alveolar fibrin, which is seen along with patterns of organizing pneumonia.
Proliferative or organizing phase changes include squamous metaplasia, interstitial fibroblastic proliferation and type 2 pneumocyte hyperplasia [30]. Histologic sections taken during this phase can give an appearance of interstitial thickening and collapsed alveoli [33]. Although features of organizing/subacute DAD are associated with a longer duration of illness [30,35], these changes have been reported in patients who died as early as two days after onset of symptoms [34]. A histologic spectrum showing exudative and proliferative DAD changes occurring alongside each other can be seen. Hyperplastic pneumocytes express TTF-1 and can display atypia, enlargement, multinucleation and syncytial formation [30,32]. Intracytoplasmic basophilic inclusions within pneumocytes have been described on light microscopy [30], which are presumed to represent virus particles. A few studies have reported features indicative of the fibrotic phase of diffuse alveolar damage (mural fibrosis and microcystic honeycombing). While Carsana et al. describe these changes only focally [31] (suggesting that none of the patients had progressed to the fibrotic phase), Schaller et al. describe a case with fully established fibrosis [33].
Other histologic patterns of lung injury such as organizing pneumonia and interstitial pneumonia have been described. The organizing pneumonia pattern shows loose connective tissue plugs composed of spindle-shaped fibroblasts and myofibroblasts within alveolar ducts. This pattern is associated with longer disease duration. Interstitial pneumonia pattern is seen as septal thickening with inter-alveolar infiltrates composed of polytypic B (CD20+) and T lymphocytes (CD3+) and/or macrophage/monocytes (CD68+) [31,38]. Prominent intra-alveolar macrophages may also be seen [31]. An angiocentric distribution and perivascular cuffing of lymphocytes has been described [33,38].
The pathology of COVID-19 is associated with marked abnormalities in the coagulation system, including elevated D-dimer levels, which predispose patients to thrombotic disease [23,35]. A study by Borczuk et al. found large vessel thrombosis (either focal or diffuse) in 42% of cases examined, and this finding was associated with patients demonstrating longer average disease duration [30]. Furthermore, platelet–fibrin microthrombi have also been identified within peripheral small arterial vessels ([23]. Combined capillary and venous thrombi in the absence of arterial thrombi have been described [30]. Microthrombi can be diffuse, and can be associated with mixed inflammation [30] and platelets. Compared to large-vessel thrombi, diffuse microthrombosis has been reported in association with shorter average disease duration [30]. Thrombi have been described in vessels within areas of DAD [31] as well as in areas not involved by acute lung injury. Adjacent lung parenchyma can display areas of hemorrhage and infarction. Studies have reported no significant association between the presence of large-vessel or microthrombi on autopsy and administration of anticoagulation therapy [30,35]. Findings such as saddle emboli and alveolar wall inflammation in confirmed COVID-19 cases have been reported in the absence of hyaline membranes [30].
Ultrastructurally, deformed pulmonary capillaries with significant angiogenesis are seen in COVID-19 patients, and these elongated vessels demonstrate sudden changes in caliber [23]. Bacterial and fungal abscesses have been described in COVID-19 cases, although this is believed to represent nosocomial and superimposed infection rather than a true manifestation of the disease process. The spectrum of histopathologic changes seen in COVID-19 pneumonia is shown in figure 1 [39].
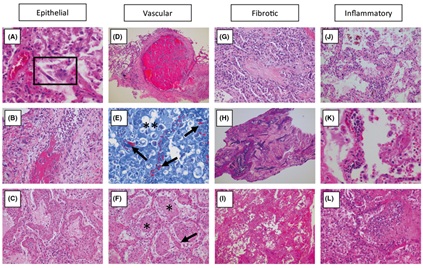
Figure 1: Spectrum of tissue response patterns in COVID-19 pneumonia. In the lung tissue of patients who died of respiratory failure due to COVID19 pneumonia, there can be evidence of epithelial infection with cytopathic effects of pneumocytes (A, box), Denudation of bronchiolar epithelium (B) and evidence of Diffuse Alveolar Damage (DAD) with hyaline membrane formation with organization (C). Observed vascular changes include extensive bilateral and diffuse (micro)vascular damage and its sequelae, with arterial thrombosis with organization (D), Microvascular fibrinoid change with hyaline thrombi (E, Fibrin-Lendrum (MSB) stain, arrows) and oedema (**), and extensive intra - alveolar fibrinous aggregates (F) With an Acute Fibrinous and Organizing Pneumonia (AFOP) pattern (F, *). Fibrotic changes vary in appearance and include organizing pneumonia with progression to fibrosis (G), Intra - alveolar fibroelastosis (Elastic - van Gieson, H) and fibrotic Nonspecific Interstitial Pneumonia (F - NSIP; I). There is often relatively sparse interstitial chronic inflammation with an Acute Interstitial Pneumonia pattern (AIP; J), Foci of lymphocytic vasculitis (K) and acute inflammation, especially in relation to areas of necrosis and possible secondary infection (L) [reproduced with permission [39]]
HRCT OBSERVATIONS DESCRIBED IN COVID-19 LUNG INVOLVEMENT
There has been extensive reporting of pulmonary imaging in COVID-19 patients subsequent to the initial descriptions of cases from Wuhan [1,2]. Computed Tomography (CT) has affirmed its role in the diagnosis and follow-up of COVID-19 patients [8-12,13]. Given the availability of molecular laboratory tests for confirmation of SARS-CoV2 infection, the optimal utilization of CT is now more clearly defined as a tool for monitoring disease progression and detection of disease-related complications. Lung changes manifest much earlier on CT than on plain radiography. These changes peak at approximately 10 days after symptom onset and subsequently begin to show resolution. The main elements that need to be evaluated on CT include morphology of the various types of lesions, distribution of these lesions in relation to different anatomical lung segments, quantification of lung changes for effective follow up, and development of disease-related complications.
Typical lesion patterns are Ground Glass Opacities (GGO), consolidation, and mixed pattern showing GGO and consolidation. Additional imaging findings such as crazy-paving pattern, interlobular septal thickening, vascular/perivascular thickening, bronchial wall thickening, air bronchograms, bronchiectasis and cavitation can also be seen. Vacuole sign and CT Halo sign have been described, defined as areas of radiolucency within consolidated lungs [3,12,13]. The location and distribution of lung lesions show considerable variation across different stages of the disease. Early lesions (1-5 days) are often bilateral, and are distributed in the middle or lower lobes. These lesions may be single or multiple, and are typically posteriorly distributed, with a predilection for peripheral or subpleural locations [3,16,17,13]. Lung aeration abnormalities and GGO are typically noted. In the later phases of the disease (6-14 days), lesions tend to be multiple, complex and can show evolution into denser consolidation, displaying a reticular and fibrotic pattern. Signs of organization, loss of volume, pleural thickening and/or retraction may be seen, possibly representing an organizing pneumonia [17].
Imaging studies demonstrate thrombi in large vessels, sluggish flow and occlusion of smaller vessels by platelet thrombi, leading to perfusion defects on pulmonary angiography [40]. A vascular tree-in-bud sign has been proposed, referring to the prominent vascular structures visible on CT studies. This observation has been suggested as an imaging marker for immunothrombosis and angiogenesis [40]. A study evaluating pulmonary angiopathy in severe COVID-19 found dilatation of smaller and larger vessels in varying numbers of cases (38-64%) and perfusion defects in all patients. Dual-energy CT is used for producing accurate iodine maps of the vascular tree undisturbed by parenchymal lung shadows. Extra pulmonary imaging manifestations that have been described include pleural thickening, pleural effusion, pneumothorax, mediastinal lymphadenopathy and pericardial effusion [13,17]. Statistically the data on the disease pattern and distribution appear nonspecific, not significantly contribute to the diagnosis. Limited statistical data is available on the vascular changes [40]. Pathology and Imaging correlations are presented in table 1.
|
COVID-19 Pathology and Imaging Correlations |
||
|
Pathology-Phase |
Histopathologic alteration |
HRCT Lung changes |
|
Normal appearance |
||
|
Exudative DAD Acute (days 1-5 days) |
- Hyaline membrane formation - Interstitial and intra-alveolar edema - Denudation/loss of pneumocytes and alveolar collapse |
Aeration abnormalites GGO Peripheral wedge shaped shadow |
|
Microthrombi (vascular pathology) |
Perfusion defect |
|
|
Proliferative DAD Intermediate (6-14 days) |
- Organisation of hyaline membranes - Interstitial thickening due to progressive fibrosis and inflammation - Pneumocyte hyperplasia |
GGO Peripheral wedge shaped shadow |
|
- Architectural distortion due to alveolar atelectasis and volume loss |
Reversible broncheictasis |
|
|
- Pleural inflammation |
Subpleural bands, Pleural shadows |
|
|
Late (15 days onward) |
- Organizing pneumonia and fibrosis |
Atelectasis/Fibrosis |
|
- Honeycombing and cavities |
bronchiectasis, distortion and cavities |
|
|
Vascular pathology (All phases) |
-Vascular and capillary stasis. Small vessel occlusion |
Perfusion abnormality |
|
- Large vessel occlusion - Pulmonary infarcts |
Pulmonary infarct Pleural retraction, Focal thickening |
|
|
- Angiogenesis |
||
Table 1: Display of Pathology and Imaging observations with the corresponding approximate time window.
RADIOLOGIC-PATHOLOGIC CORRELATION OF PULMONARY INVOLVEMENT IN COVID-19
The following imaging component of this report is based on an analysis of HRCT images from 2000 laboratory-confirmed cases of COVID-19 patients treated at our tertiary care hospital. The descriptive and statistical part of the study is under process. This report will describe the various morphological changes observed on imaging and will explore a pathological basis for them.
ALVEOLAR CHANGES
In the early phase HRCT appearances of the lungs may be normal [13]. HRCT images at the level of the alveoli manifest as GGO (Figure 2), crazy paving patterns (Figure 2) and classical complete consolidation with or without patent airways (Figure 3). These changes correspond to the exudative phase of DAD seen on microscopy and is caused by the presence of fibrin-inflammatory exudates within the alveoli and interstitium, in combination with the septal congestion and alveolar atelectasis due to inflammation or secondary to surfactant deficiency [8-12]. Certain features noted on our HRCT images are not typical for alveolar lesions of infective origin. For example, images from our COVID-19 cases rarely display bronchial tree-in-bud appearance, which is typically seen in consolidation due to the presence of airway exudates. On the contrary, our cases frequently showed distended peripheral airways and traction bronchiectasis (Figure 4). Additionally the volumes of affected lungs in COVID-19 patients appear decreased on imaging, probably a result of atelectasis secondary to alveolar damage and surfactant deficiency. Lack of significant exudation and a strong positive inspiratory force contribute to distended and clearly visualized airways. Consistent with reported literature, lesions identified on our HRCT images show a propensity for peripheral distribution, subpleural location, and bilateral involvement. Some lesions exhibit a band-like distribution along the lung periphery (Figure 5). These band-like densities are often separated from the chest wall by a lucent area. Lobulated and multifocal lesions are also seen (Figure 6).
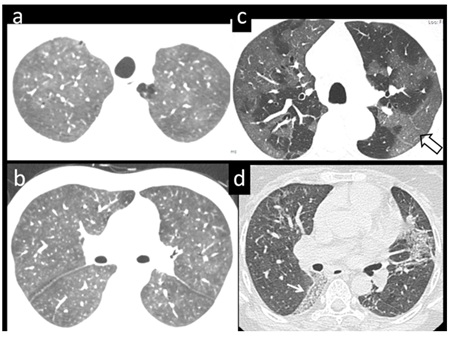 Figure 2: 39 yr-old, male patient (a,b) with respiratory difficulty, lab positive for COVID 19 pneumonia. Axial CT images show diffuse ground glass pattern of air-space disease, almost symmetrically involving both sides. (c) Predominant peripheral distribution of ground glass densities (open arrow) partially sparing the central parts in a 68 yr-male. (d) 58-yr-female, tested positive for COVID-19, show focal area of consolidation in the left lung with a clear demarcation from the surrounding lung (white arrow).
Figure 2: 39 yr-old, male patient (a,b) with respiratory difficulty, lab positive for COVID 19 pneumonia. Axial CT images show diffuse ground glass pattern of air-space disease, almost symmetrically involving both sides. (c) Predominant peripheral distribution of ground glass densities (open arrow) partially sparing the central parts in a 68 yr-male. (d) 58-yr-female, tested positive for COVID-19, show focal area of consolidation in the left lung with a clear demarcation from the surrounding lung (white arrow).
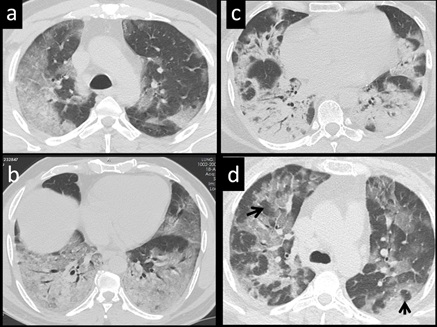 Figure 3: 54 yr-old male patient (a,b) with respiratory difficulty, lab positive for COVID 19 pneumonia show different imaging pattern of air-space disease: Axial CT images show (a) peripheral areas of consolidation (b) confluent areas of consolidation in both lower lobes revealing air bronchogram (c) 40 yr-old female with respiratory distress on an axial CT show multifocal areas of organized areas of pneumonia. (d) 35-yr-old female, lab proven for COVID 19, demonstrate on axial CT, Multifocal subsegmental pneumonic areas, showing a vacuole sign (black arrows).
Figure 3: 54 yr-old male patient (a,b) with respiratory difficulty, lab positive for COVID 19 pneumonia show different imaging pattern of air-space disease: Axial CT images show (a) peripheral areas of consolidation (b) confluent areas of consolidation in both lower lobes revealing air bronchogram (c) 40 yr-old female with respiratory distress on an axial CT show multifocal areas of organized areas of pneumonia. (d) 35-yr-old female, lab proven for COVID 19, demonstrate on axial CT, Multifocal subsegmental pneumonic areas, showing a vacuole sign (black arrows).
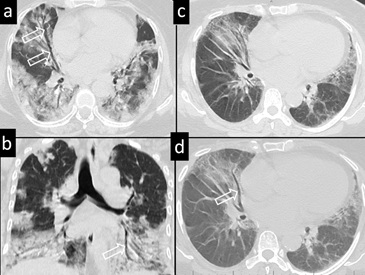
Figure 4: 62-yr-old female with breathing difficulty, hypoxia and tested positive for COVID 19, Axial and coronal CT images (a-c) dilated bronchus with the lack of normal tapering in areas of partial consolidation (open arrows). 47-yr, male with a lab proven COVID pneumonia show bronchial dilatation in the middle lobe on axial CT(c,d- Open arrow).
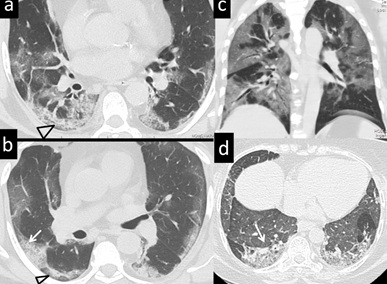
Figure 5: 55 yr-male with respiratory distress, positive for COVID 19. CT images in the axial plane (a,b) demonstrating band like peripheral distribution of lung lesions, located subpleurally (arrow) with air lucency at the periphery (Triangle) (c). Coronal CT image display combination of peripheral and central distribution of lesions. (d) 49-yr male, with breathing difficulty, proven positive for COVID 19 show bilateral lower lobe (arrow) band like opacities.

Figure 6: 45 yr-old, male with dry cough, fever, tested positive for COVID19. Axial CT images in different levels showing a lobulated, peripheral localised areas of consolidation. (arrows) (a-d) Lesions can be discrete either single or multiple.
INTERSTITIAL SEPTAL THICKENING
Interstitial changes seen on imaging represent the late exudative phase or proliferative phase of DAD, and are initially observed in the dependent parts of the lung and in subpleural locations (Figure 7). These changes, together with GGO, manifest as a pattern of crazy paving, linear shadows and subpleural bands. Lesions can be identified adjacent to fissures (Figure 7). Pleural bands and multiple linear shadowing are seen as additional peripheral densities. On a histopathological level, thickening of the interstitial tissue is brought about by septal congestion, edema, cellular infiltration, inflammation, hemorrhage and early fibrosis.
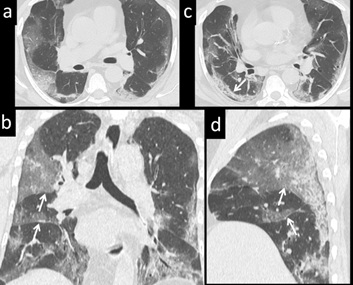 Figure 7: 56 yr-old male with respiratory distress, tested positive for COVID 19: Axial CT images (a,c) demonstrate band-like, subpleural location of consolidation. Coronal and Sagittal reconstruction (b and d) demonstrate the lesions to be located adjacent to and limited by fissures (white arrow).
Figure 7: 56 yr-old male with respiratory distress, tested positive for COVID 19: Axial CT images (a,c) demonstrate band-like, subpleural location of consolidation. Coronal and Sagittal reconstruction (b and d) demonstrate the lesions to be located adjacent to and limited by fissures (white arrow).
FIBROSING CHANGES
As the disease progresses, more proliferative phase changes emerge, with ensuing fibrosis and retraction resulting in foci of organization. Imaging signs seen during this phase of transition are traction bronchiectasis, bronchial distortion, focal pleural thickening and pleural retraction (Figure 8). Histologically, this corresponds to progressive interstitial myofibroblastic proliferation and septal collagen deposition associated with this phase. Organizing foci of DAD and AFOP can either show resolution or progress to permanent lung fibrosis with architectural distortion. In the late stages of the disease, pulmonary lesions on imaging tend to be more localized, retracted, dense, and destructive. Lung changes in this phase of the disease are seen as areas of dense consolidation, fibrosis, or even honeycombing and irregular cavitation. While such alterations have been described on autopsy histology in COVID-19 patients, an inquiry about history of pre-existing fibrosing lung diseases would be prudent in such cases. Imaging changes representing an organising pneumonia is seen in the late phase of the disease. Occasionally, complications such as pneumothorax are observed (figure 9). Vascular thrombosis, a well-established correlate in COVID-19 pneumonia, can play a contributing role in persistent lung density and perfusion defects on angiography.
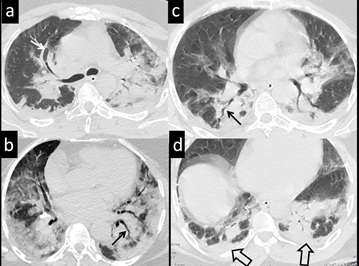 Figure 8: 59-yr-old female, presenting with breathing difficulty and positive RT-PCR for COVID-19. Axial CT image images demonstrate multifocal organized areas of pneumonia. Lesions are discrete, some show adjacent pleural thickening (a and d-open arrows) Associated bronchial dilatation (a-white arrow) and bronchial tortuosity-distortion (b,c-black arrows )
Figure 8: 59-yr-old female, presenting with breathing difficulty and positive RT-PCR for COVID-19. Axial CT image images demonstrate multifocal organized areas of pneumonia. Lesions are discrete, some show adjacent pleural thickening (a and d-open arrows) Associated bronchial dilatation (a-white arrow) and bronchial tortuosity-distortion (b,c-black arrows )
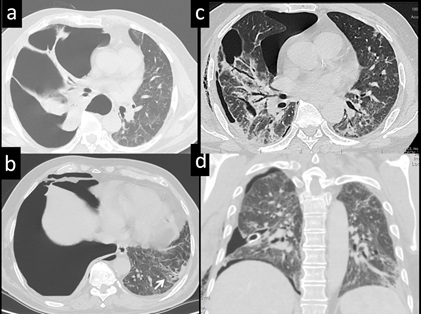 Figure 9: 38-year-old male with sudden onset of dysapnoea, tested positive for COVID 19, Axial CT images (a, b) demonstrate right pneumothorax. Left lung showed subpleural alveolar and the linear densities. Follow up axial and coronal CT images after chest tube drainage (c, d) demonstrate re-expansion of lung and residual lung changes.
Figure 9: 38-year-old male with sudden onset of dysapnoea, tested positive for COVID 19, Axial CT images (a, b) demonstrate right pneumothorax. Left lung showed subpleural alveolar and the linear densities. Follow up axial and coronal CT images after chest tube drainage (c, d) demonstrate re-expansion of lung and residual lung changes.
PROMINENT VASCULAR SHADOW
This observation is seen in several patients with COVID-19 pneumonia. A large, relatively dense vascular shadow is seen to enter an area of GGO or an area of mosaic crazy paving. This finding simulates the pattern of arborising vessels seen in the placenta (Figure 10); hence we suggest the name ‘vessel arborisation sign’. The name ‘vascular tree-in-bud sign’ has also been suggested, owing to a resemblance with dichotomously branching bronchi. This observation is also seen in smaller lesions, with the leading vessel reaching almost up to the subpleural region. The vessels can be single or multiple, and appear to be tributaries of the pulmonary veins in several cases (Figures 10 & 11). The arborising vessel sign represents congested veins with sluggish flow, with or without luminal thrombosis. This raises the possibility of a venous infarct contributing to the pulmonary shadow, a concept that has not been fully explored. Platelet microthrombi, capillarostasis and vascular stasis within both arteries and veins have been consistently described in autopsy pathology reports. The resulting sluggish vascular flow can contribute to increased density and thickness of the pulmonary vessels that we see on imaging. We have not studied lung perfusion extensively in this report, and our limited evaluation of CT pulmonary angiograms did not frequently demonstrate arterial thrombosis (Figure 11). Although a number of autopsy series have documented large vessel thrombosis, some reporting up to 42 % [30], this finding was not reflected in our cases.
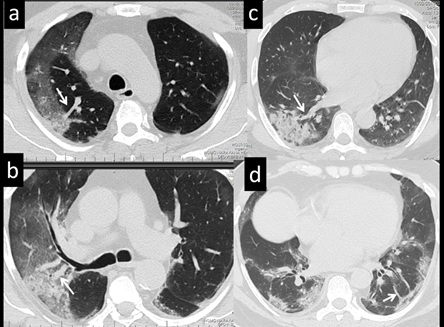 Figure 10: 69 yr-old male patient (a-d) with respiratory difficulty, proven positive for COVID pneumonia: Axial CT images show ‘Arborising vessel sign’. All images show a prominent vascular shadow in relation to an area of air space opacity (arrows). Leading vessels vary in size can be single (c) or multiple (a, b and d).
Figure 10: 69 yr-old male patient (a-d) with respiratory difficulty, proven positive for COVID pneumonia: Axial CT images show ‘Arborising vessel sign’. All images show a prominent vascular shadow in relation to an area of air space opacity (arrows). Leading vessels vary in size can be single (c) or multiple (a, b and d).
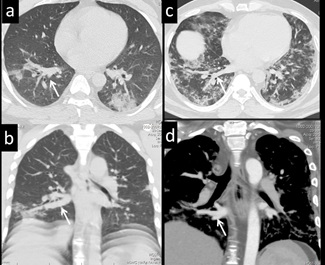 Figure 11: 40-yr-male, with breathing difficulty, hypoxia, lab positive for COVID-19. CT axial (a,c) and coronal plain (b) and contrast enhanced image (d) demonstrate ‘Arborising vessel sign’. Dilated prominent vessels represent right inferior pulmonary vein, as shown in the CT angiogram (arrow) (d).
Figure 11: 40-yr-male, with breathing difficulty, hypoxia, lab positive for COVID-19. CT axial (a,c) and coronal plain (b) and contrast enhanced image (d) demonstrate ‘Arborising vessel sign’. Dilated prominent vessels represent right inferior pulmonary vein, as shown in the CT angiogram (arrow) (d).
CONCLUSION
Although an extensive number of publications regarding COVID-19 have emerged recently, the radiologic and histopathologic features are rarely described in concert. This report provides a morphological perspective for lung changes and provide deeper understanding of the pathophysiology of COVID-19 pulmonary changes. On histology a spectrum of acute lung injury and reparative changes noted alongside frequent thrombotic disease. HRCT imaging with a well proven basis, provides extensive quantitative and qualitative information regarding early and late disease. On CT imaging, we note typical features of COVID-19 pneumonia such as bilateral, peripheral, subpleural distribution of disease. Pattern-wise hypoareation, GGO, consolidation, septal lines, pleural lines and focal pleural thickening are frequently noted. Interstitial changes are identified to a lesser extent. Often leading vessels are noted in relation to a lobulated and circumscribed pulmonary opacity, indicating an underlying vascular structural alteration. Extensive vascular occlusion, stasis, destructive lung lesions and organizing pneumonia are reported in patients with longer duration of disease. Vascular lesions have been increasingly described in autopsy studies and hence further exploration is merited in this direction.
ACKNOWLEDGMENTS
Authors would like to acknowledge and thank the Chairman of the hospital, Dr Devi Prasad Shetty for the generous administrative support; Dr Paul Salins, Mazumdar Shaw Medical foundation for academic support and for the team members of imaging services for their contribution in case assessment.
FINANCIAL SUPPORT
Nil
REFERENCES
- Huang C, Wang Y, Li X, Ren L, Zhao J, et al. (2019) Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. The Lancet 395 (10223).
- World Health Organization (2020) Coronavirus disease (COVID-19) pandemic. World Health Organization, Geneva, Switzerland.
- Bhandari S, Rankawat G, Bagarhatta M, Singh A, Singh A, et al. (2020) Clinico-radiological evaluation and correlation of CT chest images with progress of disease in COVID-19 patients. J Assoc Physicians India 68: 34-42.
- Zhu N, Zhang D, Wang W, Li X, Yang B, et al. (2020) China Novel Coronavirus Investigating and Research Team. A novel coronavirus from patients with pneumonia in China, 2019. N Engl J Med 382: 727-733.
- Hu B, Guo H, Zhou P, Shi ZL (2020) Characteristics of SARS-CoV-2 and COVID-19. Nat Rev Microbiol 6: 1-14.
- Guan W-J, Ni Z-Y, Hu Y, Liang WH, Ou CQ, et al. (2020) Clinical characteristics of coronavirus disease 2019 in China. N Engl J Med 382: 170-1720.
- Petersen E, Koopmans M, Go U, Hamer DH, Petrosillo N, et al. (2020) Comparing SARS-CoV-2 with SARS-CoV and influenza pandemics. Lancet Infect Dis 20: 238-244.
- Murata K, Itoh H, Todo G, Kanaoka M, Noma S, et al. (1986) Centrilobular lesions of the lung: Demonstration by high-resolution CT and pathologic correlation. Radiology 161: 641-645.
- Meziane MA, Hruban RH, Zerhouni EA, Wheeler PS, Khouri NF, et al. (1988) High resolution CT of the lung parenchyma with pathologic correlation. Radio Graphics 8: 27-54.
- Webb WR (1989) High-resolution CT of the lung parenchyma. Radiol Clin North Am 27: 1085-1097.
- Elicker B, Pereira CA de C, Webb R, Leslie KO (2008) High-resolution computed tomography patterns of diffuse interstitial lung disease with clinical and pathological correlation. J Bras Pneumol 34: 715-744.
- Zhang G, Liu Q, Zhao Z, Lin H, Wu C, et al. (2017) Correlation between imaging features of high-resolution computed tomography and histopathology of connective tissue diseases associated interstitial lung disease in chinese population: Avoid lung biopsy in those patients? Int J Radiol Med Imag 3: 118.
- Çinkooglu A, Hepdurgun C, Bayraktaroglu S, Ceylan N, Savas R (2020) CT imaging features of COVID-19 pneumonia: Initial experience from Turkey. DiagnInterv Radiol 26: 308-314.
- Chung M, Bernheim A, Mei X, Zhang N, Huang M, et al. (2020) CT imaging features of 2019 novel coronavirus (2019-nCoV). Radiology 295: 202-207.
- Fang Y, Zhang H, Xie J, Lin M, Ying L, et al. (2020) Sensitivity of chest CT for COVID-19: Comparison to RT-PCR. Radiology 296: 115-117.
- Zhou S, Wang Y, Zhu T, Xia L (2020) CT features of coronavirus disease 2019 (COVID-19) Pneumonia in 62 Patients in Wuhan, China. AJR Am J Roentgenol 214: 1287-1294.
- Salehi S, Abedi A, Balakrishnan S, Gholamrezanezhad A (2020) Coronavirus disease 2019 (COVID-19): A systematic review of imaging findings in 919 patients. AJR Am J Roentgenol 215: 87-93.
- Hoffmann M, Kleine-Weber H, Schroeder S, Krüger N, Herrler T, et al. (2020) SARS-CoV-2 cell entry depends on ACE2 and TMPRSS2 and is blocked by a clinically proven protease inhibitor. Cell 181: 271-280.
- Wölfel R, Corman VM, Guggemos W, Seilmaier M, Zange S, et al. (2019) Virological assessment of hospitalized patients with COVID-2019. Nature 581: 465-469.
- Hamming I, Timens W, Bulthuis MLC, Lely AT, Navis GJ, et al. (2004) Tissue distribution of ACE2 protein, the functional receptor for SARS coronavirus. A first step in understanding SARS pathogenesis. The Journal of Pathology 203: 631-637.
- Yu F, Yan L, Wang N, Yang S, Wang L, (2020) Quantitative detection and viral load analysis of SARS-CoV-2 in infected patients. Clin Infect Dis 71: 793-798.
- Pan Y, Zhang D, Yang P, Poon LLM, Wang Q (2020) Viral load of SARS-CoV-2 in clinical samples. Lancet Infect Dis 20: 411-412.
- Ackermann M, Verleden SE, Kuehnel M, Haverich A, Welte T, et al. (2020) Pulmonary vascular endothelialitis, thrombosis, and angiogenesis in Covid-19. N Engl J Med 383: 120-128.
- Jin Y, Yang H, Ji W, Wu W, Chen S, et al. (2020) Virology, epidemiology, pathogenesis, and control of COVID-19. Viruses 12: 372-374.
- Gattinoni L, Coppola S, Cressoni M, Busana M, Rossi S, et al. (2020) COVID-19 Does not lead to a “typical” acute respiratory distress syndrome. American Journal of Respiratory and Critical Care Medicine 201: 1299-1300.
- Gattinoni L, Chiumello D, Rossi S (2020) COVID-19 pneumonia: ARDS or not? Crit Care 24: 154-154.
- Bikdeli B, Madhavan MV, Jimenez D, Chuich T, Dreyfus I, et al. (2020) COVID-19 and thrombotic or thromboembolic disease: Implications for prevention, antithrombotic therapy, and follow-up: JACC state-of-the-art review. J Am Coll Cardiol 75: 2950-2973.
- Zhou F, Yu T, Du R, Fan G, Liu Y, et al. (2020) Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: A retrospective cohort study. Lancet 395: 1054-1062.
- Wu C, Chen X, Cai Y, Xia J, Zhou X, et al. (2020) Risk factors associated with acute respiratory distress syndrome and death inpatients with coronavirus disease 2019 pneumonia in Wuhan, China. JAMA Intern Med 180: 934-943.
- Borczuk AC, Salvatore SP, Seshan SV, Patel SS, Bussel JB, et al. (2020) COVID-19 pulmonary pathology: A multi-institutional autopsy cohort from Italy and New York City. Mod Pathol 33: 2156-2168.
- Carsana L, Sonzogni A, Nasr A, Rossi RS, Pellegrinelli A, et al. (2020) Pulmonary post-mortem findings in a series of COVID-19 cases from northern Italy: A two-centre descriptive study. The Lancet Infectious Diseases 20: 1135-1140.
- Menter T, Haslbauer JD, Nienhold R, Savic S, Hopfer H, et al. (2020) Postmortem examination of COVID-19 patients reveals diffuse alveolar damage with severe capillary congestion and variegated findings in lungs and other organs suggesting vascular dysfunction. Histopathology77: 198-209.
- Schaller T, Hirschbühl K, Burkhardt K, Braun G, Trepel M, et al. (2020) Postmortem examination of patients with COVID-19. JAMA 323: 2518-2520.
- Bradley BT, Maioli H, Johnston R, Chaudhry I, Fink SL, et al. (2020) Histopathology and ultrastructural findings of fatal COVID-19 infections in Washington State: A case series. Lancet 396: 320-332.
- Lax SF, Skok K, Zechner P, Kessler HH, Kaufmann N, et al. (2020) Pulmonary arterial thrombosis in COVID-19 with fatal outcome: Results from a prospective, single-center, clinicopathologic case series. Ann Intern Med 173: 350-361.
- Copin MC, Parmentier E, Duburcq T, Poissy J, Mathieu D, et al. (2020) Time to consider histologic pattern of lung injury to treat critically ill patients with COVID-19 infection. Intensive Care Med 46: 1124-1126.
- Kory P, Kanne JP (2020) SARS-CoV-2 organising pneumonia: Has there been a widespread failure to identify and treat this prevalent condition in COVID-19? BMJ Open Respir Res 7: 000724.
- Hanley B, Naresh KN, Roufosse C, Nicholson AG, Weir J, et al. (2020) Histopathological findings and viral tropism in UK patients with severe fatal COVID-19: A post-mortem study. Lancet Microbe 1: 245-253.
- von der Thüsen J, van der Eerden M (2020) Histopathology and genetic susceptibility in COVID-19 pneumonia. Eur J Clin Invest 50: 13259.
- Patel BV, Arachchillage DJ, Ridge CA, Bianchi P, Doyle JF, et al. (2020) Pulmonary angiopathy in severe COVID-19: Physiologic, imaging, and hematologic observations. Am J Respir Crit Care Med 202: 690-699.
Citation: Bhat V, Bhat V, Gadabanahalli K, Ramanjaneya R (2021) The Expanding Spectrum of COVID-19 Lung Imaging: Exploring Inputs from Radiologic-Pathologic Data for Disease Detection. J Pulm Med Respir Res 6: 053.
Copyright: © 2021 Varun Bhat, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.

