The Role of Mutations on Genes RPS14, MIR145, MIR146A, in Myelodysplastic Syndrome
*Corresponding Author(s):
Shahin AsadiMedical Genetics Harvard University, Director Of The Division Of Medical Genetics And Molecular Optogenetic Research, Boston Children's Hospital, United States
Tel:+1-857-600-7921,
Email:shahin.asadi1985@gmail.com
Abstract
Myelodysplastic syndrome (MDS) refers to a heterogeneous group of closely related clonal hematopoietic disorders commonly found in the aging population. All are characterized by one or more peripheral blood cytopenias. Bone marrow is usually hypercellular, but rarely, a hypocellular marrow mimicking aplastic anemia may be seen. Bone marrow cells display aberrant morphology and maturation (dysmyelopoiesis), resulting in ineffective blood cell production.
MDS affects hematopoiesis at the stem cell level, as indicated by cytogenetic abnormalities, molecular mutations, and morphologic and physiologic abnormalities in maturation and differentiation of one or more of the hematopoietic cell lines.
Keywords
Genetics Mutations, MIR146A genes, MIR145, Myelodysplastic syndrome (MDS), RPS14
OVERVIEW OF MYELODYSPLASTIC SYNDROME
Myelodysplastic syndrome, also known as minus 5q (-5q) syndrome, is a genetic disorder that affects the bone marrow. In myelodysplastic syndrome, the development of red blood cells is affected and results in the reduction of these cells (anemia) [1].
CLINICAL SIGNS AND SYMPTOMS OF MYELODYSPLASTIC SYNDROME
People with myelodysplastic syndrome are associated with decreased red blood cells (erythrocytes) and abnormal size of these cells (macrocytes). Most people with myelodysplastic syndrome have no symptoms of anemia, but some sufferers develop severe fatigue, weakness, and facial tiredness [1] (Figure 1).
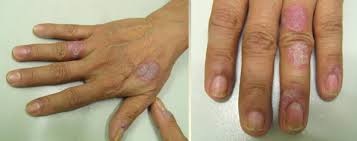 Figure 1: Image of human hands with myelodysplastic syndrome with skin malformations.(1)
Figure 1: Image of human hands with myelodysplastic syndrome with skin malformations.(1)
People with myelodysplastic syndrome also suffer from abnormal bone marrow cells called megakaryocytes that produce platelets. In addition, some people with myelodysplastic syndrome have excessive platelets. Patients with myelodysplastic syndrome are also at risk for leukemia. This cancer can progress very quickly to acute myeloid leukemia (AML). It is noteworthy that AML progression is less common in people with myelodysplastic syndrome [1,2] (Figure 2).
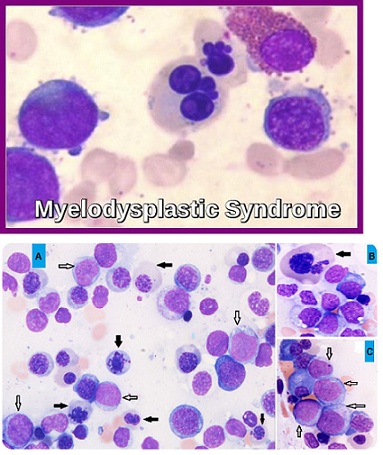 Figure 2: Microscopic image of peripheral blood flow associated with myelodysplastic syndrome.(2)
Figure 2: Microscopic image of peripheral blood flow associated with myelodysplastic syndrome.(2)
ETIOLOGY OF MYELODYSPLASTIC SYNDROME
Myelodysplastic syndrome is caused by the deletion of a DNA region from the long arm (q) of chromosome 5. In this knockout mutation, about 1.5 million bp (1.5Mb) is removed from the building blocks of the DNA molecule. The magnitude of the knockout mutation in this syndrome varies among affected individuals. This deletion mutation occurs in the immature blood cells of patients with myelodysplastic syndrome and affects one of two copies of chromosome 5 in each cell. The deletion region contains 40 genes, many of which play a critical role in the development of normal blood cells. Research shows that deleting multiple genes in the long arm of chromosome 5 contributes to the characteristics of myelodysplastic syndrome. Deletion of the RPS14 gene located on chromosome 5 arm 5q33.1 leads to impaired red blood cell development and deletion of the MIR145 gene located on chromosome 5 arm 5q32 or the MIR146A gene in the arm. The long chromosome 5 is located at 5q33.3, causing megakaryocyte and platelet abnormalities and may increase the immature cell growth. Researchers are trying to determine how deletion of numerous other genes from the long arm of chromosome 5 could be involved in the characterization of myelodysplastic syndrome. Myelodysplastic syndrome occurs as a result of new mutations in somatic cells and does not follow any inherited pattern [1,3 ] (Figure 3).
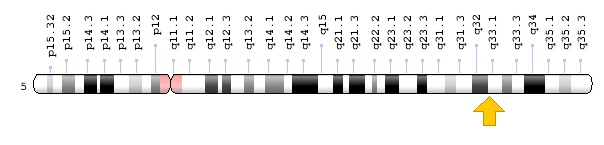 Figure 3: Schematic overview of chromosome 5 where the RPS14 gene is located in the long arm of chromosome 5q33.1. (3)
Figure 3: Schematic overview of chromosome 5 where the RPS14 gene is located in the long arm of chromosome 5q33.1. (3)
FREQUENCY OF MYELODYSPLASTIC SYNDROME
Myelodysplastic syndrome is a chromosomal disorder with a prevalence of 1 in 20,000 in the United States. Myelodysplastic syndrome is more common in men than in women [1,4] (Figures 4-6).
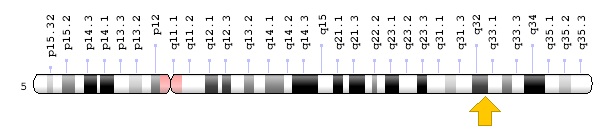 Figure 4: Schematic overview of chromosome 5 where the MIR145 gene is located on the long arm of chromosome 5q32.(4)
Figure 4: Schematic overview of chromosome 5 where the MIR145 gene is located on the long arm of chromosome 5q32.(4)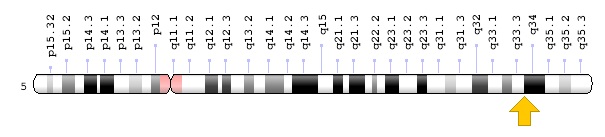 Figure 5: Schematic overview of chromosome 5 where the MIR146A gene is located in the long arm of chromosome 5q33.3. (5)
Figure 5: Schematic overview of chromosome 5 where the MIR146A gene is located in the long arm of chromosome 5q33.3. (5) 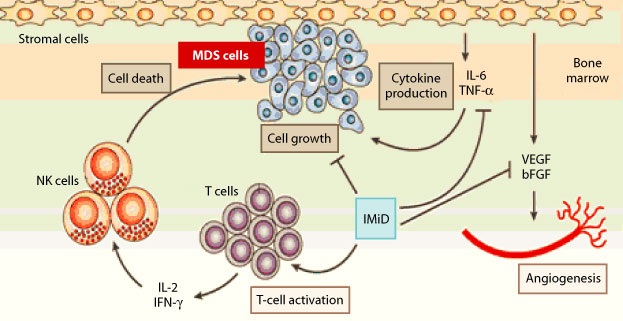 Figure 6: Schematic of the molecular pathway of myelodysplastic syndrome. (6)
Figure 6: Schematic of the molecular pathway of myelodysplastic syndrome. (6)
DIAGNOSIS OF MYELODYSPLASTIC SYNDROME
Myelodysplastic syndrome is diagnosed based on the clinical and physical findings of the patients and some pathological tests. The best way to diagnose this syndrome is by molecular cytogenetic testing, such as in situ fluorescence hybridization (FISH) technique, to investigate the presence of a deletion mutation in chromosome number 5 arm. Prenatal diagnosis is also possible using PGD and amniocentric fluid or sampling of fetal placenta chorionic villi [1,5] (Figure 7& 8). 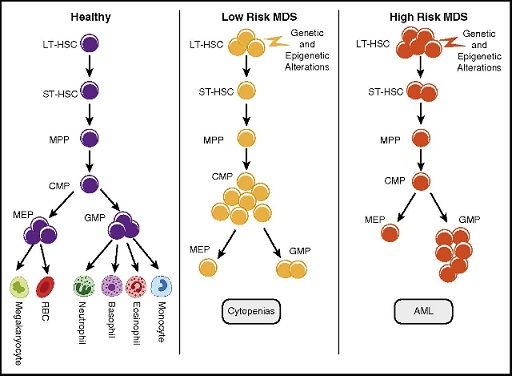 Figure 7: Schematic of normal blood cells (left) versus low-risk myelodysplastic syndrome (middle) blood cells and high-risk myelodysplastic syndrome blood cells that have acute myeloid leukemia or AML (the right side). (7)
Figure 7: Schematic of normal blood cells (left) versus low-risk myelodysplastic syndrome (middle) blood cells and high-risk myelodysplastic syndrome blood cells that have acute myeloid leukemia or AML (the right side). (7) 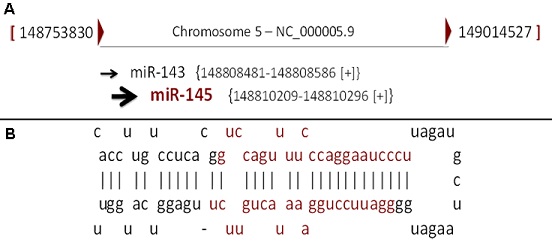 Figure 8: Schematic of the genetic codes for the miR-145 gene. (8)
Figure 8: Schematic of the genetic codes for the miR-145 gene. (8)
THERAPEUTIC PATHWAYS OF MYELODYSPLASTIC SYNDROME
The strategy of treatment and management of myelodysplastic syndrome is symptomatic and supportive. Treatment may be done with the efforts of a team of specialists including pediatricians, hematologists, oncologists and other health care professionals. There is little effective treatment for this syndrome and all clinical measures are to reduce the suffering of patients. Genetic counseling is also important for all parents who want a healthy baby [1,6] (Figure 9). 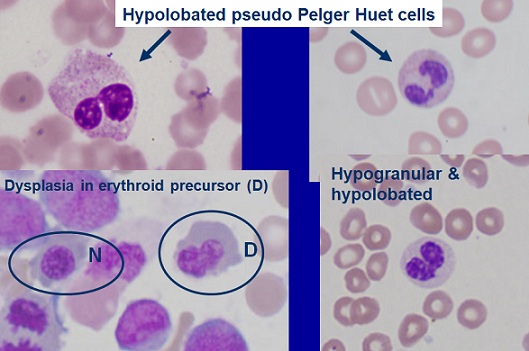 Figure 9: Microscopic overview of myelodysplastic syndrome blood cells. (9)
Figure 9: Microscopic overview of myelodysplastic syndrome blood cells. (9)
DISCUSSION AND CONCLUSION
In a patient with a myelodysplastic syndrome, the blood stem cells (immature cells) do not become mature red blood cells, white blood cells, or platelets in the bone marrow. These immature blood cells, called blasts, do not work the way they should and either die in the bone marrow or soon after they go into the blood. This leaves less room for healthy white blood cells, red blood cells, and platelets to form in the bone marrow. When there are fewer healthy blood cells, infection, anemia, or easy bleeding may occur. Different types of treatment are available for patients with myelodysplastic syndromes. Some treatments are standard (the currently used treatment), and some are being tested in clinical trials. A treatment clinical trial is a research study meant to help improve current treatments or obtain information on new treatments for patients with cancer. When clinical trials show that a new treatment is better than the standard treatment, the new treatment may become the standard treatment. Patients may want to think about taking part in a clinical trial. Some clinical trials are open only to patients who have not started treatment. Patients with a myelodysplastic syndrome who have symptoms caused by low blood counts are given supportive care to relieve symptoms and improve quality of life. Drug therapy may be used to slow progression of the disease. Certain patients can be cured with aggressive treatment with chemotherapy followed by stem cell transplant using stem cells from a donor [1,7]. Research shows that deleting multiple genes in the long arm of chromosome 5 contributes to the characteristics of myelodysplastic syndrome. Deletion of the RPS14 gene located on chromosome 5 arm 5q33.1 leads to impaired red blood cell development and deletion of the MIR145 gene located on chromosome 5 arm 5q32 or the MIR146A gene in the arm. The long chromosome 5 is located at 5q33.3, causing megakaryocyte and platelet abnormalities and may increase the immature cell growth. Researchers are trying to determine how deletion of numerous other genes from the long arm of chromosome 5 could be involved in the characterization of myelodysplastic syndrome. Based on the findings of this study, it can be concluded that mutations in each of these three genes lead to separate abnormalities, all of which are associated with blood cells [1,7].
REFERENCES
- Asadi S (2018) Pathology in Medical Genetics Book. Amidi Publications. Vol 6.
- Ebert BL, Pretz J, Bosco J, Chang CY, Tamayo P, et al. (2008) Identification of RPS14 as a 5q- syndrome gene by RNA interference screen. Nature 451(7176): 335-9.
- Gaballa MR, Besa EC (2014) Myelodysplastic syndromes with 5q deletion: pathophysiology and role of lenalidomide. Ann Hematol 93(5): 723-33.
- Giagounidis A, Mufti GJ, Fenaux P, Germing U, List A, et al. (2014) Lenalidomide as a disease-modifying agent in patients with del(5q) myelodysplastic syndromes: linking mechanism of action to clinical outcomes. Ann Hematol 93(1): 1-11.
- Komrokji RS, Padron E, Ebert BL, List AF (2013) Deletion 5q MDS: molecular and therapeutic implications. Best Pract Res Clin Haematol 26(4): 365-75.
- Kumar MS, Narla A, Nonami A, Mullally A, Dimitrova N, et al. (2011) Coordinate loss of a microRNA and protein-coding gene cooperate in the pathogenesis of 5q- syndrome. Blood 118(17): 4666-73.
- Starczynowski DT, Kuchenbauer F, Argiropoulos B, Sung S, Morin R, et al. (2010) Identification of miR-145 and miR-146a as mediators of the 5q- syndrome phenotype. Nat Med 16(1): 49-58.
Citation: Shahin Asadi (2020), The Role of Mutations on Genes RPS14, MIR145, MIR146A, in Myelodysplastic Syndrome. J Protein Res Bioinform 2: 004.
Copyright: © 2020 Shahin Asadi, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.