
The Therapeutic Use of Adipose Tissue-Derived Stromal Cells (ASCs) in Scar Tissue and its Potential in Scar Remodeling
*Corresponding Author(s):
L VriendDepartment Of Plastic And Reconstructive Surgery, Department Of Pathology And Medical Biology, University Of Groningen And University Medical Center Groningen, Hanzeplein 1, 9713 GZ Groningen, Netherlands
Tel:+31 620097606,
Email:lindavriend@gmail.nl
Abstract
Lipofilling, i.e., transplantation of adipose tissue, is a widely adopted technique in aesthetic and reconstructive plastic surgery as well as in regenerative medicine. Lipofilling is used to improve dermal scarring and to enhance wound healing. Its beneficial effect is ascribed to the regenerative potential of Adipose Tissue-Derived Stromal Cells (ASCs). Both ASCs as well as the paracrine factors ASCs secrete are believed to influence wound healing processes by enhancing angiogenesis, modulating inflammation and remodelling extracellular matrix. The Stromal Vascular Fraction (SVF), which consists mainly of ASCs, can be isolated from adipose tissue by means of either a mechanical procedure or an enzymatic procedure. This review aims to provide an overview of these SVF isolation procedures and discuss their known current and possible future role in treatment of scar tissue and scar tissue remodelling.
INTRODUCTION
Since the nineties, lipofilling i.e., the transplantation of autologous adipose tissue has become increasingly popular for both tissue augmentation and regenerative purposes [1]. The augmentative role of lipofilling used for restoration of soft tissue volume defects has become indispensable in clinical practice of both reconstructive and aesthetic plastic surgery. In the course of its significant clinical usage since the nineties, it has become apparent that lipofilling can be effectively used for regenerative purposes, such as improving scar appearance and wound healing.
The beneficial effects of lipofilling for regenerative purposes are mainly ascribed to the Stromal Vascular Fraction (SVF), which contains Adipose Derived Stromal Cells (ASCs). SVF consists of all non-adipocyte like cell types e.g. ASCs, endothelial cells, immune cells and fibroblasts and can be obtained by both mechanical and enzymatical isolation. ASCs reside in the SVF around vessels as precursor cell types e.g. periadventitial cells and pericytes [2,3]. Controversy remains about the phenotype of these precursor cell types [2-5]. ASCs are believed to be one of the key cell types in adipose tissue because of their ability to differentiate into the ectodermal [6], endodermal [7] and mesenchymal cell lineages [8]. Moreover, ASCs secrete an excess of pro-regenerative growth factors, cytokines, proteins and exosomes [9-11]. These factors can stimulate angiogenesis and decrease fibrosis, both important mechanisms contributing to improved wound repair as well as scar remodeling.
Scar tissue differs structurally and functionally from normal skin. It is defined by both an increased dermal and epidermal thickness combined with loss of hair follicles and sebaceous glands [12,13]. Dermal thickening is caused by excessive deposition of extracellular matrix leading to an increase of collagen (mostly collagen I) that consists of thick and disorganized bundles [14]. This collagen deposition leads to the formation of firm scars that cause functional limitations and usually are associated with unappealing appearance, limiting patients’ quality of life. Clinical studies and experimental studies have shown that lipofilling improves scar tissue appearance and quality [15]; after lipofilling skin structure improves significantly and collagen bundles start to remodel, thereby changing scar tissue to more closely resemble normal skin. It has been frequently suggested that presence of ASCs in lipografts is responsible for this phenomenon. The regenerative power of ASCs may even prevent scar formation due to intervention of pro-regenerative growth factors early during the wound healing process. The use of ASCs in treatment of scar tissue therefore seems to be promising. SVF (containing ASCs) used to improve wound healing and scar remodelling is currently obtained through one of two procedures: enzymatic isolation or mechanical isolation. Unfortunately, thus far, no clinical trials have been published demonstrating therapeutical application differences between these two types of SVF isolation procedures and as a result it is unclear which type of SVF isolation procedure is most suitable for clinical use. In this review, we aim to provide a brief overview of these two SVF isolation procedures and discuss their current and future role in the treatment of scar tissue and scar remodelling.
MECHANICAL AND ENZYMATIC ISOLATION OF ASCS: CELLULAR SVF VS. TISSUE SVF
Thus far, two types of methods have been developed to isolate SVF from adipose tissue [16]: enzymatic isolation procedures and mechanical isolation procedures. An enzymatic isolation procedure uses enzymes and animal derived products to disrupt all cell-cell connections, including extracellular matrix and adipocytes. An enzymatic isolation procedure therefore yields a suspension of cells only (cellular SVF or cSVF). A mechanical isolation procedure, however, uses shear stress to disrupt adipocytes, while preserving cell-cell connections and interactions with the supportive extracellular matrix. A mechanical isolation procedure also uses autologous materials only. Mechanical isolation yields a tissue like suspension of SVF cells (tissue SVF or tSVF). In both types of procedures, the most vulnerable and largest cell population by volume are being disrupted i.e., adipocytes, which make up approximately 85-90% of the adipose tissue.
Due to their vulnerability and size, adipocytes are susceptible to shear stress, especially when lipoaspirate is derived from infiltration fluid. Infiltration fluid functions as a pad around adipocytes to prevent adipocytes from falling apart due to shear stress [17]. Hence, optimal mechanical isolation of SVF is obtained when centrifuged lipoaspirate is used.
The difference in composition between both types of products i.e., cSVF vs. tSVF might have a different clinical effect as is their behaviour after injection: a single cell suspension tends to migrate shortly after injection in the tissues, whereas cells in their natural habit i.e., extracellular matrix might be preserved on site of injection.
Moreover, the extracellular matrix present in tSVF might have an important function in tissue regeneration as well; it directs cell proliferation, differentiation and survival of retained cells. Additionally, extracellular matrix binds regenerative trophic factors secreted by ASCs and can store and release these factors slowly to surrounding tissue. These regenerative trophic factors increase angiogenesis, stimulate matrix remodelling and downregulate inflammation [18-20]. All these processes support remodulation and regeneration of scar tissue. Furthermore, in tSVF, microvasculature is still embedded in extracellular matrix in fragmented form and may therefore induce angiogenesis [21-24]. cSVF is depleted of extracellular matrix by enzyme digestion and as a result the regenerative capacity of tSVF is almost certainly significantly larger as compared to cSVF.
Besides a potential clinical difference between cSVF and tSVF, substantial differences in procedural characteristics exists, such as time, costs, use of non-autologous materials, training of personnel and regulatory barriers. Firstly, (non)-intraoperative enzymatic isolation procedures are more time-consuming and require more complex facilities in comparison with mechanical procedures. Enzymatic procedures for example, can take up to 133 minutes (e.g. Cyotori), whereas mechanical isolation procedures require up to 20 minutes [16,25-27]. Enzymatic procedures also require multi-step lab-based collagenase digestion in good manufacturing practice facilities (cGMP) [28]. Secondly, the implementation of enzymatic isolation procedures in clinical practice requires necessary adjustments e.g. extensive training of personnel and (re)tooling of laboratories with expensive machinery. Thirdly, the aforementioned characteristics of enzymatic isolation result in higher costs as compared to mechanical isolation procedures [15]. Finally, the opportunities to use enzymatic isolation procedures clinically also depend on applicable regulations; in many countries, enzymatic digestion of adipose tissue is considered to be a significant manipulation of autologous tissue and thus legally prohibited for use in clinics. In contrast, mechanical isolation is not prohibited for use in clinics. It is considered to be a minimal manipulation of autologous tissue since mainly adipocytes are disrupted and native cell-cell connections remain intact.
As a result, in our opinion the feasibility of regular use of enzymatic isolation procedures in clinical practice is rather low for the near future, except when ASCs are used for infusion therapy (intravascular). Hence, studies evaluating ASCs for regenerative purposes in tissues should focus on optimizing mechanical isolation procedures to yield tSVF for clinical use and try to elucidate its role in tissue regeneration, such as wound healing or scar remodelling.
ASCS’ PARACRINE FACTORS AND PRECLINICAL RESEARCH IN DERMAL SCARRING
Preclinical studies have investigated the mechanisms through which ASCs may improve wound healing; paracrine factors secreted by ASCs and its extracellular matrix (which together comprise tSVF) are hypothesized to play the main role.
ASCs, when cultured, secrete anti-apoptotic, angiogenic and anti-fibrotic paracrine factors (Figure 1) that can be collected in medium, then called ASC conditioned medium (ASCcme). In vitro research has shown that ASCcme consists of paracrine factors like Vascular Endothelial Growth Factors (VEGF), Hepatocyte Growth Factor (HGF), Transforming Growth Factor Beta (TGF-β) and Matrix Metalloproteinases (MMPs), among others [11].These factors combined can augment wound healing through different processes: VEGF proteins stimulate angiogenesis; HGF cytokines play an important role in all stages of wound healing, for example in epidermal remodelling and in suppressing fibrogenesis after injury; TGF-β induces fibroblast to myofibroblast conversion, leading to expression of α-Smooth Muscle Actin (α-SMA), which enables wound closure through its contractile properties and MMP1 can suppress excess synthesis of collagen and accelerates the turnover of the extracellular matrix.
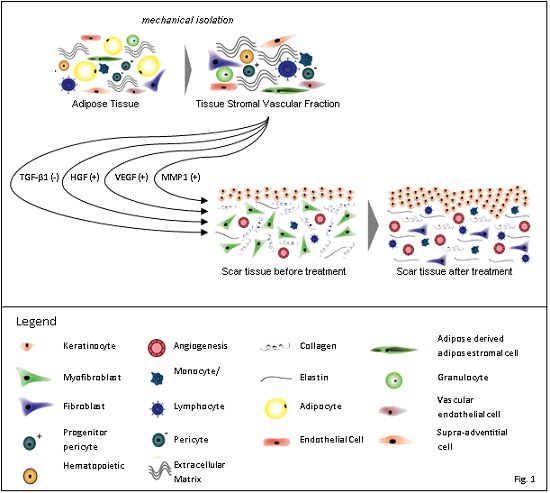
Figure 1: Schematic overview of a mechanical isolation procedure yielding tissue Stromal Vascular Fraction (tSVF) containing ASCs and its treatment application in a dermal scar on tissue level.
ASCs’ in vitro and in vivo characteristics differ from each other, so that one could argue that the outcomes in in vitro models cannot be directly translated to in vivo models e.g., in vitro cultured ASCs were shown to be highly susceptible to environmental changes. Induced hypoxia for example selectively upregulates the release of VEGF [11]. Likely, ASCs’ release of paracrine factors is also impacted by environmental conditions, such as biological fluctuation sand mechanotransduction caused by the 3D structure in which they are embedded in animal and human bodies. Furthermore, demonstrating the direct paracrine effects of ASCs in in vivo experiments is difficult compared to in vitro experiments since monitoring is challenging. Nonetheless, it is considered highly likely that in vivo effects are similar to in vitro effects. In fact, preclinical studies in animals show that ASCs do have regenerative potential in treatment of scar tissue.
For example, two relevant animal studies where scars were treated with cSVF and/or ASCcme, showed that scar formation reduced after treatment [29,30]. Both studies found that excessive extracellular matrix synthesis decreased, and more collagen bundles were aligned after ASCs treatment. This resulted in smaller and less elevated scars, with a colour more similar to normal skin.
These preclinical trials demonstrate the beneficial effects of ASCs on fibrotic matrix remodeling, which leads to scar reduction. An overview of clinical research on application of ASCsin scar tissue and scar remodelling is discussed hereafter.
CLINICAL APPLICATION OF ADIPOSE DERIVED STROMAL CELLS: CELLULAR PREVENTION OR TREATMENT OF DERMAL SCARRING
Treatment for scarring is one of the most obvious and promising clinical use cases for ASCs. Scarring of the skin is a process following wound healing due to e.g. traumatic events or systemic diseases such as diabetes mellitus or following fibroproliferative diseases of the skin. Dermal wound healing is initiated by a damaged epidermal layer causing an inflammatory reaction followed by proliferation and finally remodelling. During remodelling, fibroblasts differentiate into myofibroblasts and deposit extracellular matrix. Excessive deposition of extracellular matrix results in a fibrotic scar. Fibrotic tissue consists of a disorganised network of cross-linked thick collagen bundles and its properties are dissimilar from normal skin. This scar tissue restores the continuity of the skin, thereby protecting the body from environmental challenges and securing homeostasis. However, the formation of this scar tissue is often accompanied by loss of aesthetic function of the skin impairing patients’ quality of life.
Scars are functionally characterized by lack of strength, elasticity, sweating capability and oil secretion as compared to healthy skin. On a subcutaneous level, scar tissue can form adhesions with other structures, leading to impaired mobility, pain and a sensation of firmness. Aesthetically, scar tissue lacks normal skin colour due to a decrease of melanisation and vascularity as well a lack of hair follicles.
To date, lipofilling has proven to be an effective method in treating dermal scarring. In an extensive review, Spiekman M et al., [15] have shown that lipofilling is effective as anti-fibrotic treatment for scars of different origins such as those caused by burns, radiation or post-operative procedures (cleft lip, tracheotomy) and trauma. The authors concluded that lipofilling and application of ASCs improved scar appearance and reduced scar-related pain. In general, lipofilling and ASCs seemed to reduce the severity of scar formation as well as improve characteristics of existing scars.
The regenerative capacity of adipose tissue is initiated primarily by SVF containing ASCs. However, SVF makes up only about 10-vol% of the adipose tissue used in lipofilling. Adipose tissue consists of loose connective tissue of which 85-90-vol% is composed of adipocytes. Adipocytes have, among many others, a supporting function and can restore volume function when transplanted to other body parts but their functionality in tissue regeneration is rather limited. When performing lipofilling to improve scar tissue, only a small volume of adipose tissue, and thus an even smaller volume of SVF containing ASCs, can be injected before saturation is reached; tissue envelopes (i.e., space) underneath scars are relatively limited due to decreased elasticity of the skin, absence of the dermis or scar adhesions that cause contractions. As a result, multiple lipofilling treatments may be required to achieve satisfying results. As such, in cases where the objective is to regenerate damaged tissue in low volume areas, only the regenerative part of adipose tissue i.e., SVF or ASCs should be injected or lipofilling should be enriched by SVF or ASCs to increase the ratio regenerative cells/per ml of lipoaspirate.
To date, the efficacy of ASCs to improve scar appearance has been investigated in three clinical trials [31-33] and a clinical case report [34].
A study by Gentile P et al., investigated the effect of cSVF-enhanced fat grafts in 10 patients with burns sequela (n=6) and post-traumatic scars (n=4) and the effect of Platelet-Rich-Plasma (PRP)-enriched fat grafts in another 10 patients with burn sequelae (n=5) and post-traumatic scars (n=5) [31]. The control group existed of 10 patients who underwent fat grafting (Coleman technique) alone. Outcomes were evaluated based on team evaluation, MRI, ultrasound and patient self-evaluation. Patients treated with cSVF- enhanced fat grafts and PRP enhanced fat grafts showed 63% and 69% maintenance of contour restoring and 3D volume after 1 year respectively, compared to 39% of the control group. All patients were satisfied with the results on skin texture, softness and contour. However, no standardised evaluation tools were used to evaluate observer or patient satisfaction. As a result, these results are unreliable.
Lee JW et al., conducted a retrospective case-control study consisting of two studies investigating the effect of cSVF in scarring. In the first study, 17 patients with scars located on different parts of the body received cSVF injections, either alone or combined with scar reduction surgeries (specified as: scar revision with composite graft or dermofat graft, tissue expansion, dermabrasion and split-thickness skin graft). The second study comprised 15 patients with linear scars located in the face, of which 7 patients received cSVF injections combined with scar reduction surgery and 8 patients received scar reduction surgery only (control group) [33]. In both studies the validated Observer portion of the Patient and Observer Scar Assessment Scale (POSAS), the Stony Brook Scar Evaluation Scale (SBSES), the Vancouver Scar Scale (VSS) and a 10 cm Visual Analog Scale were used. For study 1, all scar scale scores showed significant improvements between pre-operative and post-operative measurements. The median values changed as follows: OSAS, -5 points, SBSES, +1 point, VSS, -3 points, VAS, +2 points. However, as in study 1 cSVF injections were combined with surgery, the impact of cSVF on these changes cannot be determined. In study 2, the scoring on the OSAS, VSS and VAS showed statistically significant favourable changes for treatment with cSVF when the control and cSVF groups were compared in pre-operative and post-operative measurements.
In contrast to the study of Gentile P et al., the study of Lee JW et al., used validated outcome measurements to study the anti-scarring effect of cSVF. Unfortunately, in this study only the observer portion of the POSAS questionnaire was measured. Measurements were performed in a blinded manner by a single examiner who did not participate in the surgeries. Hence, objective patient satisfaction rates remain unavailable. Because both studies used different outcome measurements, results are difficult to compare. Another difficulty in comparing the aforementioned studies is the lack of standardization in the isolation method, the number of cSVF cells used per cm2, as well as the different origins and locations of the scars.
In the study of Gentile et al., it is unclear what amount of cSVF cells were injected per cm2 of scar tissue. In respect of concentration, the study refers to having obtained 50,000 (+/-) 6,956 nucleated cells per ml of fat tissue through automatic extraction, using the Celution System. 5ml of this suspension was subsequently mixed with an unknown quantity of washed fat graft and applied to scars, but the surface area of the scars is not recorded. Gentile et al., included patients with scars in the face caused by trauma or burns. Lee JW et al., used cSVF in a concentration of 4.9X10^7 cells per ml and documented the maximum amount of cSVF injected (~5ml). The injected volume of cSVF per cm2 scar was not documented, although scars varied substantially in size. Lee JW et al., included patients with scars located on all body parts in the first study and only in the face in the second study.
Furthermore, neither study mentions the composition of different cell populations in cSVF based on CD marker expression. It is well known that composition of adipose tissue differs highly between patients. Although, the aforementioned clinical studies showed significant improvement after cSVF-enriched lipofilling as anti-scarring treatment, the results are difficult to interpret.
In another study, Eitta A et al., treated 10 patients with post-acne scars; one side of the face received an intradermal injection of cSVF underneath atrophic scars. The other side received three sessions of fractional carbon dioxide laser resurfacing with three-month intervals [33]. Scars were evaluated photographically with Goodman’s and Baron qualitative global acne grading system by two independent and blinded dermatologists.
Both procedures showed significant improvement in reduction of scar severity. Though, there was no difference between the two treatments. Patients also rated their satisfaction for the procedure as a whole and their satisfaction towards skin texture and homogeneity. The study showed that both treatments improved patient satisfaction on all three metrics, though treatment with a single cSVF injectable was as effective as three sessions of carbon dioxide laser therapy. However, the grading system on the three metrics on patient satisfaction is not a validated instrument and therefore difficult to interpret.
Mahajan PV et al., investigated the effect of a combined injection of bone marrow derived stem cells, ASCs and PRP on acne scars after three sessions of injection [34]. After the third injection, scars were classified with the Global Acne Scarring Classification and transitioned from grade 3 (moderate) to grade 1 (mild). Photographically, fading of hyper pigmentation was found. Again, no validated instrument was used supporting this finding.
In both studies, cSVF injection was combined with other therapeutic interventions i.e., bone marrow, PRP or carbon dioxide laser therapy without using a control group. In this manner, the therapeutic benefit of the addition of cSVF to other therapeutic interventions remains unclear.
In conclusion, all studies reported beneficial effects of cSVF or ASCs on the outcome of scar appearance. However, the clinical studies discussed all have limitations. To distinguish effectiveness of treatment and elucidate the exact role and mechanisms of ASCs and SVF as an anti-scarring treatment, prospective randomized controlled trials are needed. No complications occurred in any of the studies suggesting that injection of ASCs or SVF subcutaneously is a safe intervention.
FUTURE PERSPECTIVES
In conclusion, lipofilling and the application of adipose derived stromal cells have gained significant ground in aesthetic and reconstructive surgery. All cellular components in lipografts, i.e., adipocytes, SVF and ASCs, have different therapeutical effects. Adipocytes have shown to be effective in augmenting tissue, restoring volume and contouring. SVF and ASCs have regenerative capabilities and are small in volume, making them suitable for enrichment strategies and treatment of smaller damaged areas, such as scars.
Lipo harvesting, required for all aforementioned treatments, is an operative procedure that can be performed under local anaesthetics. Both lipo harvesting and mechanical isolation of tSVF are relatively simple procedures and can be performed with a limited number of inexpensive tools by a wide variety of care professionals and in a wide variety of settings including in developing countries. Using autologous tissue as a source of regenerative cells also means no premium need be paid to pharmaceutical manufacturers and simple distribution networks can be used for transportation as spoilage is not an issue for medical devices. The use of an autologous source of regenerative cells e.g. adipose tissue will also minimize a potential immune response. Having stated these benefits, some remarks should be made in respect of lipo harvesting. Lipo harvesting requires invasive suction of lipoaspirate with (low) risk of infection or necrosis, especially in patients with co-morbidities e.g. impaired diabetic wound healing or fibroproliferative diseases of the skin. Patients with multiple co-morbidities are often not eligible to undergo anaesthesia. Additionally, the regenerative function of adipose tissue might be impaired due to systemic diseases like diabetes mellitus.
In such cases where lipo harvesting is not feasible or desirable or where systemic diseases impair the regenerative potential of ASCs or SVF, allogenic transplantation of ASCs or SVF would be ideal. However, it is well known that allogenic transplantation of ASCs or SVF will result in an immune response. As such, transplantation of the regenerative components of adipose tissue i.e. extracellular matrix and ASCs secretome into a delivery medium that does not elicit an immune response is a promising avenue for further clinical development.
New developments in biotechnology may provide a solution. Biotechnology aims to develop an allogenic “of the shelf” product that delivers regenerative factors, instead of using an autologous transplant. For example, a hydrogel may be created consisting of extracellular matrix and the paracrine regenerative factors of SVF and ASCs; the extracellular matrix once implanted may then function as a slow release platform and secrete factors from ASCs to the surrounding damaged tissue.
Next to ASCs secretome, PRP containing numerous growth factors can be used to load the extracellular matrix-based hydrogels as well. For PRP harvesting only collection and centrifugation of whole blood is needed. These factors induce angiogenesis, downregulate inflammation and inhibit necrosis, while enhancing matrix remodelling. This may offer a cost-effective and controlled product available for patients for whom lipo harvesting is not feasible or otherwise undesirable. Depending on the indication or place of implantation, one can argue that there is a need for several products, consisting of extracellular matrix derived from fat but also other decellularized tissues such as skin or bone.
Currently, the efficacy and safety of ECM-hydrogels with conditioned medium derived from ASCs are being investigated in animal experiments for following purposes: wound healing, regeneration of skin and as anti-scarring treatment after radiotherapy. Additional future research should also focus on the following: composition of the hydrogel, dosage of ASCs or ASC derived growth factors, preservation of the product, safety and legal concerns.
CONCLUSION
Direct application of ASCs and SVF in treatment of scar tissue seems to be a promising method, as indicated by preliminary clinical studies and case reports. For use of ASCs in tissue regeneration, mechanical isolation procedures isolating tSVF outweigh the enzymatic isolation procedures resulting in cSVF in respect of costs, time and regenerative potential of ASCs and mechanical isolation is therefore preferable. Future research therefore should focus on the further study in good clinical trials of tSVF probably also in combination with the development of an of the shelf product, combining the extracellular matrix and paracrine factors of fat tissue or PRP to augment wound healing and improve scar treatment.
REFERENCES
- Coleman SR (2006) Structural fat grafting: More than a permanent filler. Plast Reconstr Surg 118: 108-120.
- Corselli M, Chen CW, Sun B, Yap S, Rubin JP, et al. (2012) The tunica adventitia of human arteries and veins as a source of mesenchymal stem cells. Stem Cells Dev 21: 1299-308.
- Lin G, Garcia M, Ning H, Banie L, Guo YL, et al. (2008) Defining stem and progenitor cells within adipose tissue. Stem Cells Dev 17: 1053-1063.
- Traktuev DO, Merfeld-Clauss S, Li J, Kolonin M, Arap W, et al. (2008) A population of multipotent CD34-positive adipose stromal cells share pericyte and mesenchymal surface markers, reside in a periendothelial location, and stabilize endothelial networks. Circ Res 102: 77-85.
- Zimmerlin L, Donnenberg VS, Pfeifer ME, Meyer EM, Péault B et al. (2010) Stromal vascular progenitors in adult human adipose tissue. Cytometry A 77: 22-30.
- Ferroni L, Gardin C, Tocco I, Epis R, Casadei A, et al. (2013) Potential for neural differentiation of mesenchymal stem cells. Adv Biochem Eng Biotechnol 129: 89-115.
- Baer PC (2011) Adipose-derived stem cells and their potential to differentiate into the epithelial lineage. Stem Cells Dev 20: 1805-1816.
- Zuk PA, Zhu M, Ashjian P, De Ugarte DA, Huang JI, et al. (2002) Human adipose tissue is a source of multipotent stem cells. Mol Biol Cell 13: 4279-4295.
- Zuk PA, Zhu M, Mizuno H, Huang J, Futrell JW et al. (2001) Multilineage Cells from Human Adipose Tissue: Implications for Cell-Based Therapies. Tissue Engineering 7: 211-228.
- Pawitan JA (2014) Prospect of Stem Cell Conditioned Medium in Regenerative Medicine. Bio Med Research International 2014: 1-14.
- Rehman J, Traktuev D, Li J, Merfeld-Clauss S, Temm-Grove CJ, et al. (2004) Secretion of angiogenic and antiapoptotic factors by human adipose stromal cells. Circulation 109: 1292-1298.
- Beausang E, Floyd H, Dunn KW, Orton CI, Ferguson MW (1998) A new quantitative scale for clinical scar assessment. Plast Reconstr Surg 102: 1954-1961.
- Ehrlich HP, Desmouliere A, Diegelmann RF, Cohen IK, Compton CC, et al. (1994) Morphological and immunochemical differences between keloid and hypertrophic scar. Am J Pathol 145: 105-113.
- van der Veer WM, Bloemen MC, Ulrich MM, Molema G, van Zuijlen PP, et al. (2009) Potential cellular and molecular causes of hypertrophic scar formation. Burns 35: 15-29.
- Spiekman M, van Dongen JA, Willemsen JC, Hoppe DL, van der Lei B, et al. (2017) The power of fat and its adipose-derived stromal cells: Emerging concepts for fibrotic scar treatment. J Tissue Eng Regen Med 11: 3220-3235.
- van Dongen JA, Tuin AJ, Spiekman M, Jansma J, van der Lei B et al. (2017) Comparison of intraoperative procedures for isolation of clinical grade stromal vascular fraction for regenerative purposes: a systematic review. J Tissue Eng Regen Med 12: 261-274.
- van Dongen JA, Tuin AJ, Harmsen MC, van der Lei B, Stevens HP, et al. (2020) The difference between Stromal Vascular Fraction isolation and fat emulsification: A crucial role for centriguation. Plast Reconstruct Surg 145: 232-233.
- van Dongen JA, Harmsen M, van der Lei B, Stevens HP (2018) Augmentation of Dermal Wound Healing by Adipose Tissue-Derived Stromal Cells (ASC). Bioengineering (Basel) 5: 91.
- van Dongen JA, Stevens HP, Parvizi M, van der Lei B, Harmsen MC (2019) The fractionation of adipose tissue procedure to obtain stromal vascular fractions for regenerative purposes. Wound Repair Regen 24: 994-1003.
- van Dongen JA, Harmsen MC, Stevens HP (2019) Isolation of Stromal Vascular Fraction by Fractionation of Adipose Tissue. Methods Mol Biol 1993: 91-103.
- Bosman FT, Stamenkovic I (2003) Functional structure and composition of the extracellular matrix. J Pathol 200: 423-428.
- SemonJA, Zhang X, Pandey AC, Alandete SM, Maness C, et al. (2013) Administration of murine stromal vascular fraction ameliorates chronic experimental autoimmune encephalomyelitis. Stem Cells Transl Med 2: 789-796.
- Atalay S, Coruh A, Deniz K (2014) Stromal vascular fraction improves deep partial thickness burn wound healing. Burns 40: 1375-1383.
- Premaratne GU, Ma LP, Fujita M, Lin X, Bollano E, et al. (2011) Stromal vascular fraction transplantation as an alternative therapy for ischemic heart failure: anti-inflammatory role. J Cardiothorac Surg 6: 43.
- SundarRaj S, Deshmukh A, Priya N, Krishnan VS, Cherat M, et al. (2015) Development of a system and method for automated isolation of stromal vascular fraction from adipose tissue lipoaspirate. Stem Cells Int 2015: 109353.
- Osinga R, Menzi NR, Tchang LA, Caviezel D, Kalbermatten DF, et al. (2015) Effects of intersyringe processing on adipose tissue and its cellular components: implications in autologous fat grafting. Plast Reconstr Surg 135: 1618-1628.
- Bianchi F, Maioli M, Leonardi E, Olivi E, Pasquinelli G, et al. (2013) A new nonenzymatic method and device to obtain a fat tissue derivative highly enriched in pericyte-like elements by mild mechanical forces from human lipoaspirates. Cell Transplant 22: 2063-2077.
- Zuk PA, Zhu M, Mizuno H, Huang J, Futrell JW, et al. (2001) Multilineage cells from human adipose tissue: implications for cell-based therapies. Tissue Eng 7: 211-228.
- Yun IS, Jeon YR, Lee WJ, Lee JW, Rah DK, et al. (2012) Effect of Human Adipose Derived Stem Cells on Scar Formation and Remodeling in a Pig Model: A Pilot Study. Dermatol Surg 38: 1678-1688.
- Zhang Q, Liu L-N, Yong Q, Deng J-C, Cao W-G (2015) Intralesional injection of adipose-derived stem cells reduces hypertrophic scarring in a rabbit ear model. Stem Cell Res Ther 6: 145.
- Gentile P, De Angelis B, Pasin M, Cervelli G, Curcio CB, et al. (2014) Adipose-Derived Stromal Vascular Fraction Cells and Platelet-Rich Plasma. J Craniofac Surg 25: 267-272.
- Lee JW, Park SH, Lee SJ, Kim SH, Suh IS. et al. (2018) Clinical Impact of Highly Condensed Stromal Vascular Fraction Injection in Surgical Management of Depressed and Contracted Scars. Aesthetic Plast Surg 42: 1689-1698.
- Abou Eitta RS, Ismail AA, Abdelmaksoud RA, Ghezlan NA, Mehanna RA (2019) Evaluation of autologous adipose-derived stem cells vs. fractional carbon dioxide laser in the treatment of post acne scars: a split-face study. Int J Dermatol 58: 1212-1222.
- Mahajan PV, Abbasi J, Subramanian S, Parab SC, Danke M (2017) Regenerative medicine using platelet rich plasma and stem cells in atrophicacne scars: a case report. J Cosmo Trichol 3: 2.
Citation: Vriend L, van Dongen JA, van der Lei B, Stevens HPJD (2020) The Therapeutic Use of Adipose-Tissue-Derived Stromal Cells (ASCs) in Scar Tissue and its Potential in Scar-Remodeling. J Stem Cell Res Dev Ther 6: 036.
Copyright: © 2020 L Vriend, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.

