
TotiCyte, a Paradigm Shift in Stem Cell Isolation and Storage from Umbilical Cord Blood
*Corresponding Author(s):
Lesley-Ann MartinCytetech Ltd, Units 2 And 3 Oak House, Woodlands Office Park, Albert Drive, Burgess Hill, RH15 9TN, United Kingdom
Email:Lesley-ann@cytetech.com
Abstract
Objectives: Storage of cord blood for therapeutic applications using volume reduction technologies is well established, but significant loss of the white cell fraction during processing and freezing remains problematic. Here we describe the validation of TotiCyte, a novel reagent which removes >99% of the erythrocyte content without centrifugation and improves post-thaw recovery.
Materials and Methods: Cord blood was processed using TotiCyte, Hydroxyethyl Starch (HES), AXP, Sepax 2 or MacoPress Smart to allow comparison. Viability was assessed using flow cytometry for CD45+ and CD34+. Colony Forming Unit Assays (CFU), were performed to establish post-thaw recovery.
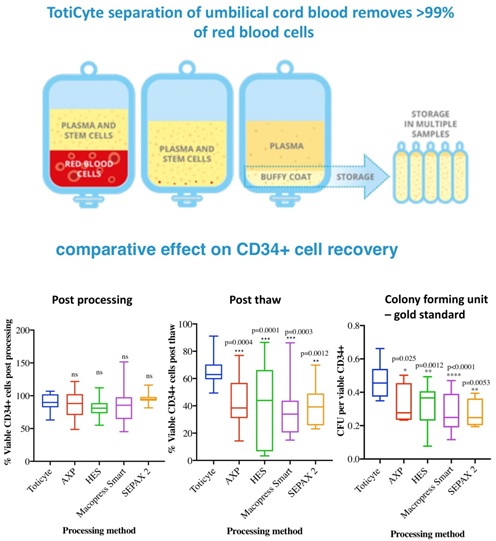
Results: TotiCyte depleted >99% of the erythrocyte fraction while maintaining good recovery of CD45+ and CD34+ cells. No significant difference in viable CD34+ post-processing was evident between the systems tested. However, post-thaw viable recovery of CD34+ cells using TotiCyte was significantly greater (66.24% SD ± 9.9) compared to AXP (42.42% SD ± 17.1), HES (41.05% SD ± 27.4), MacoPress Smart (37.92% SD ± 20.9) and Sepax 2 (40.02% SD ± 14.0). CFU assays showed a 1.4- to 1.7-fold increase of viable CD34+ cells using TotiCyte versus the other technologies. Overall, TotiCyte provided a 2.2 to 3.0-fold improvement in CD34+ cell recovery at the point of use.
Conclusion: We provide evidence to support the use of TotiCyte as a novel volume reduction technology capable of significantly improving CD34+ stem cell recovery.
Graphical Abstract
Introduction
Stem cell transplantation has been used to great effect to treat several disease states. There are several sources of stem cells for transplantation, such as adipose, bone marrow and apheresis treated peripheral blood. However, Umbilical Cord Blood (UCB) is regarded as the best source of stem cells due to its associated benefits, including lower incidence of acute Graft-versus-Host Disease (GvHD), and tolerance of a partial HLA match between donor and recipient [1-3].
However, the application of UCB transplants in larger individuals has been constrained, by cell-dose limitations, which is regarded as the single biggest disadvantage of using UCB [1,4-5]. To overcome this limitation, various strategies have been suggested, including the use of double UCB unit grafts [6-9] combining unrelated UCB with haploidentical peripheral blood stem cells [10,11], and performing direct infusion omitting washing steps. Some of these studies have demonstrated positive results [12,13].
With the introduction of delayed cord clamping, which has been shown to be beneficial for the development of both term and preterm infants, and is now supported by the World Health Organization, The American Academy of Paediatrics and The Royal College of Obstetricians and Gynaecologists; UCB volumes are becoming smaller. This is likely to exacerbate cell dose constraints.
There have been several attempts to develop methods to enable more efficient cell recoveries and improve the cryogenic storage of UCB; however, each has drawbacks [12-21]. These methods include whole blood storage and volume reduction techniques, that remove either most of the erythrocyte content to isolate the buffy coat selectively (volume reduction for erythrocyte removal - VRE), or to reduce plasma whilst retaining the erythrocyte fraction (volume reduced by plasma removal -VRP). Whilst VRP results in minimal loss of nucleated cells, its use has been associated with transfusion related adverse effects [22] whilst other volume reduction methodologies document nucleated cell losses as high as 40% [14]. In addition, all volume reduction methodologies result in significant numbers of erythrocytes being harvested along with the white cell fraction. In some instances, the erythrocyte content can exceed 50% of the resulting product.
In order to address these deficiencies, we developed a novel reagent, TotiCyte, and compared its efficacy against other methodologies used by both private and public UCB storage banks. Parameters assessed included recoveries of various cell types (CD34+ cells, CD45+), colony forming ability by assessment of (CFU)-Granulocyte–Monocytes (CFU-GM), CFU-Erythrocytes (CFU-E), CFU-Granulocyte–Erythrocyte–Monocyte–Megakaryocytes (CFU-GEMM), total CFU, and haematocrit.
Overall TotiCyte appeared superior, eliminating >99% of the erythrocyte fraction and significantly improving the recovery of viable CD34+ stem cells post thaw. This latter point is potentially the most important improvement seen when using TotiCyte and is reflected in colony forming unit assays (CFU) widely accepted as the “gold standard” signifying clinical utility [23].
Materials and Methods
Fresh umbilical cord blood units combined with Citrate Phosphate Dextrose Adenine (CPDA) (n=76) were purchased from Cells4Life LLP and stored at 4°C upon receipt and processed in <48 hours. Sample volumes processed were optimal for each comparator system. Prior to processing a 500 µl aliquot of whole blood was taken for analyses and acted as the baseline for cell loss calculations. Further samples were taken post-processing and post-thaw for comparisons of the various technologies.
Processing Technologies and cryogenic storage
TotiCyte
TotiCyte is a CE marked medical device which consists of dextran 500 (2.5% (w/v)), Dimethyl Sulfoxide (DMSO) (2.5% (v/v)) combined with phosphate buffered saline. An equal volume of TotiCyte was added to the cord blood/CPDA mix and a second receiving bag attached to the first. All ports were locked. The sample was mixed by gentle inversion and the blood bag suspended from a clamp stand for 30 minutes, allowing formation of rouleaux. The blood bag was then pressed using a Mikromatic press under manual setting. The appropriate locks were opened, and pressure applied to the cord blood bag. The white cell rich plasma was expelled into the secondary bag. Upon entry of the erythrocytes into the tubing, the connecting locks were sealed. The secondary bag containing the white blood cell fraction was centrifuged at 750 x g for 20 minutes to sediment the cellular fraction. The plasma was expressed off leaving between 20-25 ml in which the cell pellet was resuspend. A 500 µl aliquot was taken by syringe and labelled post-processing. The volume of cells was adjusted to contain 7.5% DMSO (CryoSure-DEX40 - 55% w/v Dimethyl Sulfoxide USP Grade, 5% w/v Dextran 40 USP Grade. WAK-Chemie Medical GmbH). After cryoprotectant addition, the cord blood samples were subjected to controlled rate freezing at –1°C/minute. After 24 hours the samples were placed in liquid nitrogen for long term storage.
HES
Hydroxyethyl starch (6% solution (Baxter, Deerfield, IL)) was added to the cord blood/CPDA at 30% of the blood/CPDA volume. This solution was mixed by inverting the blood bag several times. The bag was suspended for 60 minutes to allow the blood solution to separate by gravity. The white cell rich plasma was expelled into the secondary bag that was centrifuged at 750 x g for 20 minutes to sediment the cellular fraction. The plasma was expressed off leaving between 20-25 ml in which to cell pellet was resuspend. The volume of cells was adjusted to contain 10% DMSO (CryoSure-DEX40) prior to controlled rate freezing at –1°C/minute.
Sepax 2 (Centrifugal-based automated device)
Sepax 2 is an automated separation system, controlled by computer software. The machine concentrates the haematopoietic stem cell rich buffy coat from 35-290 ml of cord blood to a final volume of 10-50 ml. In this study a final volume of 20 ml was used. Each cord blood unit was separated with a single use kit, inserted into the apparatus. Each sample was spun at 1900 x g and processed according to the manufacturer’s instructions. As previous, pre-and post-processing samples were acquired for downstream analysis. DMSO, to a final concentration of 10% (CryoSure-DEX40), was added to the samples prior to controlled rate freezing at –1°C/minute.
MacoPress smart
MacoPress is an automated volume reduction system utilising optics to determine the position of the erythrocyte fraction. The machine concentrates the buffy coat from blood volumes >50 ml. In summary VRT0000XU (Top and bottom bag system Macopharma) was connected to the collection bag via spike and the contents drained into central bag of the three-bag system. The bag was centrifuged at 2200 x g for 18 minutes. The MacoPress parameters were then set according to the manufacturer’s instructions to give a final volume of 21ml. The resulting sample was adjusted to 10% DMSO (CryoSure-DEX40) prior to freezing.
AXP
The AXP platform also uses optical sensor technology to formulate precision separation and retention of the target mononuclear cell population. The minimum volume that can be processed is 40ml. Volume reduction was conducted as described in the manufacturer’s instructions to give a final volume of between 20-21 ml. The resulting sample was adjusted to 10% DMSO (CryoSure-DEX40) prior to controlled rate freezing at –1°C/minute.
Haematocrit measurement
Pre- and post-processed bloods were drawn into a haematocrit tube until the tube was approximately two-thirds full. The tube was inverted slowly to allow the blood to migrate towards the bottom of the tube. The base of the tube was sealed with sealant. The tube was assessed to ensure little to no air was interspersed in the column of blood. The tubes were centrifuged at 5000 x g for 5 minutes. Using a ruler, the length of the column of the packed red cells was measured and divide by the length of the whole column of blood (cells and plasma). To obtain the haematocrit, this value was multiplied by 100%.
Flow cytometry and cell enumeration
Nucleated cell recovery was assessed using Haematology Analyser (Horiba, UK) according to the manufacturer’s instructions. Flow cytometric analysis was carried out using Becton Dickinson (BD) FACS Caliber apparatus employing TruCount tubes. For blood samples, the gates were set based on Stem cell control CD34+ whole blood process control (BD Catalogue No: 340991). Samples were prepared as follows: 100 µl blood was added to a TruCount tube containing 20 µl of CD34+/CD45+ antibody mix together with viability dye 7AAD (5 µl). Tubes were then incubated at room temperature, in the dark for 20 min. Cells were then lysed using BD FACS lysis buffer.
Post-thaw analysis and Colony forming unit (CFU) assays
Frozen aliquots (1.5 ml) of the processed samples were rapidly thawed at 37°C. FACS analysis was conducted to establish the number of viable CD45+ and CD34+ cells post thaw. CFU assays were conducted to evaluate the content of the stem and progenitor cell compartments. In the case of AXP, Sepax 2 and MacoPress Smart, Lymphoprep was used to remove the contaminating erythrocyte fraction followed by flow cytometry to determine viable CD45+ cells post extraction. Viable CD45+ cells were then diluted in DMEM-F12 (Gibco UK) containing 2% foetal bovine serum (Gibco UK) to a final concentration of 1.5x105 cells/ml. A 0.3ml aliquot was combined with 3ml of Methocult (StemCell, UK) according to the manufacturer’s instruction. The resulting sample was pipetted equally across two wells of a 6-well plate. The remaining empty wells were filled with sterile distilled water. The plates were incubated at 37°C, 5% CO2 and >95% humidity for 14 days. The colonies were then characterised based on morphology into: BFU-E – Burst-forming unit-erythroid. Consisting of over 200 erythroblasts in single or multiple clusters. CFU-GM – colony forming unit-granulocyte, macrophage. In which colonies contain at least 20 granulocytes and or macrophages. CFU-GEMM – colony forming unit granulocyte, erythroid, macrophage, megakaryocyte. These consist of progenitors that produce erythroblasts and at least two other recognisable lineages. CFU-GEMM tend to produce large colonies of >500 cells.
Data analysis
In order to determine variability, mean recoveries of CD45+ and CD34+ for both post processing and post thaw were expressed relative to the whole blood starting material, which was considered as 100%. For colony forming unit assays, the assumption was made, that one viable CD34+ was capable of generating a single colony. This allowed a ratio of actual CFU/predicted CFU to be calculated. In order to remove bias when comparing ratios, only predicted CFU above 100 were used for downstream comparative analyses.
Statistical analysis
Test for normality was conducted using Kolmogorov-Smirnov and Shapiro-Wilk. Outlier analysis was conducted using ROUT. For multiple comparisons, Dunnett’s test was performed by computing a Student’s t-statistic for each treatment, where the statistic compares the treatment group to a single control group, in this case TotiCyte
Results
TotiCyte effectively recovers total nucleated and mononucleated cells
In order to determine the ability of TotiCyte to recover nucleated cells from human blood, samples (n=3) were processed and assessed for various cell population using a haematology analyser. Total nucleated cell recovery was >90%, of which 100% monocyte, 80% lymphocyte and 90% of neutrophils were evident (Supplementary Figure S1).
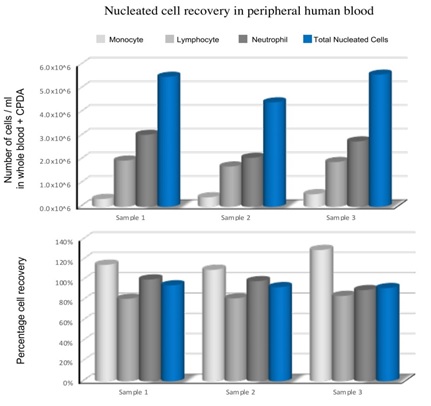 Supplementary Figure S1: TotiCyte effectively recovers total nucleated and mononucleated cells.
Supplementary Figure S1: TotiCyte effectively recovers total nucleated and mononucleated cells.
Comparison of the post-processing recovery of viable CD45+/CD34+ cells to examine haematological stem and progenitor cell compartments
Comparison of the viable recovery of CD45+ and CD34+ cells using TotiCyte, AXP, HES, MacoPress Smart or Sepax 2 was conducted using flow cytometry. The minimum volume of blood/CPDA processed using TotiCyte or HES was 30 ml, for AXP the minimum volume was 40ml and for MacoPress Smart and Sepax 2, 50 ml. All data are expressed in relation to the whole blood prior to processing. The average viable CD45+ recoveries (Figure 1A) were: TotiCyte (84.79%, SD ± 12.6, n=24), AXP (80.90%, SD ± 19.4, n=18), HES (76.48%, SD ± 9.3, n=19), MacoPress Smart (71.97%, SD ± 22.7, n=11) and Sepax 2 (99.38%, SD ± 11.9, n=10). No significant difference in post-process recovery of viable CD45+ cells was evident with the exception of Sepax 2 (p=0.02).
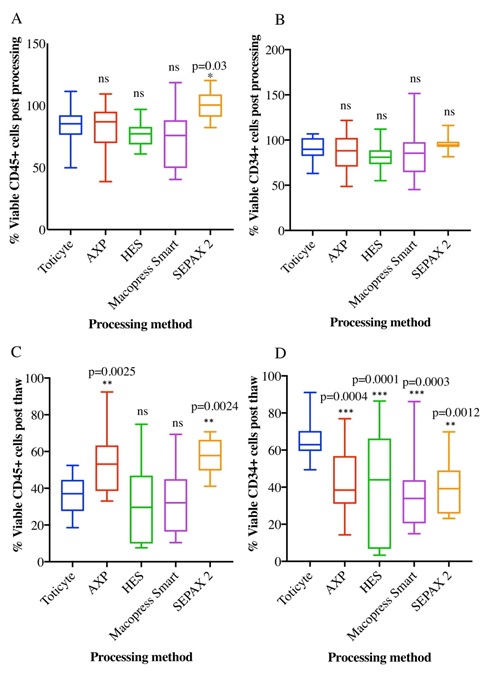 Figure 1: Comparison of TotiCyte processing with AXP, Sepax 2, HES and MacoPress Smart. Box and whisker plots show post processing recoveries for A. CD45+ and B. CD34+ cell recoveries for TotiCyte (n=24), AXP (n=18), HES (n=19), Sepax 2 (n=10) and MacoPress Smart (n=11). Post thaw recoveries for C. CD45+ and D. CD34+ for Post thaw recoveries for TotiCyte (n=24), AXP (n=18), HES (n=19), Sepax 2 (n=10) and MacoPress Smart (n=11). Adjusted p values are shown in relation to TotiCyte.
Figure 1: Comparison of TotiCyte processing with AXP, Sepax 2, HES and MacoPress Smart. Box and whisker plots show post processing recoveries for A. CD45+ and B. CD34+ cell recoveries for TotiCyte (n=24), AXP (n=18), HES (n=19), Sepax 2 (n=10) and MacoPress Smart (n=11). Post thaw recoveries for C. CD45+ and D. CD34+ for Post thaw recoveries for TotiCyte (n=24), AXP (n=18), HES (n=19), Sepax 2 (n=10) and MacoPress Smart (n=11). Adjusted p values are shown in relation to TotiCyte.
Assessment of post-processing viable recovery for CD34+ cells showed no significant difference between the technologies with average viable recoveries for TotiCyte (89.76, SD ± 11.9, n=24), AXP (87.28%, SD ± 19.6, n=18), HES (82.15%, SD ± 13.6, n=19), MacoPress Smart (86.77%, SD ± 27.9, n=11) and Sepax 2 (95.02%, SD ± 8.9, n=10) (Figure 1B). Of note, the overall loss of the hematopoietic progenitor cell population for all technologies tested in this study was on average 11.8%, which is below previous reported estimates of 14-42% [14].
Taken together these data suggest TotiCyte post-processing is equally as effective as current industry and public health cord blood bank standard technologies and capable of dealing with blood/CPDA volumes as small as 30 ml.
Comparison of viable post-thaw recovery of CD45+/CD34+ cells
Post-thaw cell recovery was assessed by flow cytometry for viable CD45+ and CD34+ as previously described. Comparisons were assessed for TotiCyte (n=24), AXP (n=18), HES (n=19), MacoPress Smart (n=11) and Sepax 2 (n=10). No significant difference in post-thaw viable CD45+ cell recovery was evident between TotiCyte (36.28%, SD ± 9.5), HES (31.13%, SD ± 21.7) or MacoPress Smart (32.32%, SD ± 17.8). It is noteworthy, that the variability in samples processed using HES was particularly marked (Figure 1C). However, AXP (53.98%, ± SD 17.3 adjusted p=0.025) and Sepax 2 (57.7%, SD ± 9.4 adjusted p=0.0024) showed higher post-thaw CD45+ recovery compared to the other technologies.
Contrastingly, assessment of viable CD34+ cell recovery was significantly improved when comparing TotiCyte with the other methodologies. Mean post-thaw viable cell recovery for TotiCyte processed samples (66.24%, SD ± 9.9), AXP (42.42%, SD ± 17.1, adjusted p=0.0004), HES (41.05%, SD ± 27.4, adjusted p=0.0001), MacoPress Smart (37.92%, SD ± 20.9, adjusted p=0.0003) and Sepax 2 (40.02%, SD ± 14.0, adjusted p=0.0012) (Figure 1D). This equated to TotiCyte giving a 1.6-fold improvement in CD34+ cell recovery compared to AXP and HES, and 1.7-fold compared with MacoPress Smart and Sepax 2.
Colony formation assays post-thaw
As post-thaw viability of cryopreserved cord blood does not guarantee functional activity, we conducted Colony Forming Unit (CFU) assays. In order to remove any potential impact of downstream processing used to remove contaminating erythrocytes from Sepax 2, MacoPress Smart and AXP samples, flow cytometry was used to re-determine viable CD45+ and viable CD34+ cells prior to plating. Viable CD45+ cells (2 x104) were plated and the number of viable CD34+ within the given population calculated. This provided the absolute expected colony number on the assumption 1 CFU arose per CD34+ cell. By dividing the absolute number of colonies by the predicted number the recovery rate could be calculated. In order to remove ratio bias, only samples with predicted CFU >100, were analysed. TotiCyte was significantly better compared with all systems tested producing on average CFU/viable CD34+ cell (0.47, SD ± 0.09) compared to AXP (0.34, SD ± 0.11, adjusted p=0.025), HES (0.32, SD ± 0.12, adjusted p=0.0012), MacoPress Smart (0.28, SD ± 0.11, adjusted p<0.0001) and Sepax 2 (0.27, SD ± 0.09 adjusted p=0.0053) (Figure 2). This equated to a 1.4-fold increase in CFU compared to AXP, 1.5-fold compared to HES, 1.7-fold compared to MacoPress Smart and Sepax 2.
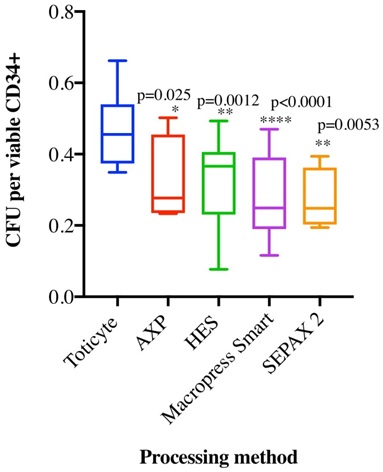 Figure 2: Post thaw colony unit forming assay. Viable CD45+ (1.5 x104) extracted using TotiCyte (n=20), AXP (n=7), HES (n=13), Sepax 2 (n=4) and MacoPress Smart (n=10) were cultured for 14 days. Colony unit formation shown is relative to the calculated number of colonies assumed if 1CFU was derived from 1 CD34+ viable cell. Adjusted p values are shown in relation to TotiCyte.
Figure 2: Post thaw colony unit forming assay. Viable CD45+ (1.5 x104) extracted using TotiCyte (n=20), AXP (n=7), HES (n=13), Sepax 2 (n=4) and MacoPress Smart (n=10) were cultured for 14 days. Colony unit formation shown is relative to the calculated number of colonies assumed if 1CFU was derived from 1 CD34+ viable cell. Adjusted p values are shown in relation to TotiCyte.
Depletion of erythrocytes and haemoglobin
One of our major aims in developing TotiCyte was the effective removal of the contaminating erythrocyte population from UCB without compromising stem cell recovery. We measured depletion using a standard capillary assay. TotiCyte deleted >99% of the haematocrit, post processing haematocrit: TotiCyte (0.9%, SD ± 0.06); AXP (54.5%, SD ± 9.2); HES (1.8%, SD ± 0.4), MacoPress Smart (27.6%, SD ± 9.8); Sepax 2 (38.2%, SD ± 4.4). TotiCyte was significantly superior to Sepax 2, MacoPress Smart and AXP (adjusted p<0.0001) but was similar to HES, as expected (Figure 3).
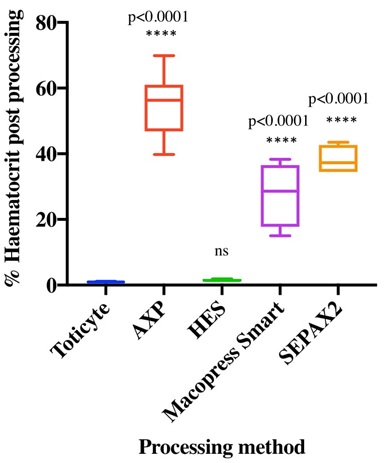 Figure 3: Haematocrit level expressed as % remaining after red cell depletion compared to whole blood control. TotiCyte (n=10), AXP (n=9), HES (n=4), Sepax 2 (n=4) MacoPress Smart (n=4). Adjusted p values are shown in relation to TotiCyte.
Figure 3: Haematocrit level expressed as % remaining after red cell depletion compared to whole blood control. TotiCyte (n=10), AXP (n=9), HES (n=4), Sepax 2 (n=4) MacoPress Smart (n=4). Adjusted p values are shown in relation to TotiCyte.
Summary of TotiCyte recoveries compared to the most common systems used in UCB processing
By combining the information from our analyses (Table 1), TotiCyte was capable of separating blood/CPDA volumes as low as 30 ml, showed a 2.2-fold improvement in CD34+ viable cell recovery compared to AXP, 2.3-fold increase compared to HES, 3.0-fold compared to MacoPress Smart and 2.8-fold increase compared to Sepax 2. Importantly, TotiCyte depleted >99% of the contaminating erythrocytes population. These observations have clinical relevance as the significant reduction in erythrocytes is likely to avoid ABO/Rh incompatibilities whilst the superior CD34+ cell viability and recovery would be expected to impact positively by enhancing “take-rates”.
|
Processing method |
Post-thaw viable CD34+ recovery |
CFUs |
Overall viable post- thaw CD34+ and CFUs relative to TotiCyte |
|
TotiCyte |
1.00 |
1.00 |
1.00 |
|
AXP |
0.64 |
0.72 |
0.46 |
|
HES |
0.62 |
0.69 |
0.43 |
|
MacoPress Smart |
0.57 |
0.59 |
0.34 |
|
Sepax 2 |
0.60 |
0.58 |
0.35 |
Table 1: Overall comparison of TotiCyte versus industry standard technologies for volume reduction on CD34+ recovery at point of use.
Discussion
Many methods of processing UCB prior to cryopreservation have been used since the first cord blood banks were established at the New York Blood Centre, Düsseldorf and Milan in 1992 [12,14-21]. One of the key issues for storage of cryoprotected whole cord blood units is the necessity for large amounts of costly liquid nitrogen storage space [17]. As such, to establish an adequate panel of hematopoietic cells, cord blood units need to be concentrated into a much smaller volume. It is for this reason that cord blood volume reduction technologies, which remove the bulk of the erythrocytes and plasma have been developed. However, all processing methods that reduce the erythrocyte fraction, suffer significant loss of nucleated cells and more importantly, the hematopoietic progenitor cell population, with loss quoted between 14-42% dependent on the technology used [14]. This is critically important as it is well established that the success of a cord blood transfer is dependent on cell dose, and insufficient cell dose is widely regarded as the most important limitation for umbilical cord blood transplantation, especially for adults and larger children [4,5,7,8,10,12 24-27]. This fact is now compounded by the introduction of DCC which has further impacted the volumes of cord blood procured.
We sought to develop a new processing methodology to remove the erythrocyte fraction and allow enrichment of the total nucleated cell population. To this end we developed TotiCyte reagent. TotiCyte causes rapid formation of rouleaux in less than 30 minutes without significant loss of the white cell fraction. The resulting volume of cord blood product post-processing with TotiCyte, is broadly similar to the volumes obtained by alternative processing methods (approximately 23 ml) but residual haematocrit is less than 1% as opposed to >40% for AXP, Sepax 2 and MacoPress Smart. In our hands, HES performed similarly although there is discrepancy in the literature as to the level of erythrocyte contamination using this methodology. More importantly, a dramatic improvement in post thaw recovery of CD34+ cells was evident with TotiCyte compared to the other systems tested, with greater than 66% overall viable recovery. Furthermore, TotiCyte recoveries were less variable particularly in comparison with HES and AXP. One important feature of our study is that it reports percent recovery post processing and post thaw in relation to the un-processed whole blood at the start. This allows absolute losses at each stage to be reported unlike other studies, which report post thaw in relation to post processing thereby artificially elevating reported recoveries.
It is clear that TotiCyte improves total nucleated cell recoveries post-thaw. However, it has been suggested that the measurement of total nucleated cell dose as measure of the efficiency of processing methods can be misleading [28] and that a ‘correction factor’ of 0.75 should be applied to the reported total nucleated cell count of erythrocyte replete units, as they will contain non-critical nucleated red cells and neutrophils, hence the likelihood of engraftment success may be overestimated due to the higher nucleated erythrocyte content, which in turn could lead to the assumption of a higher haematopoietic stem cell content. However, it has been countered that if this is incorrect, it could lead to underestimation of the progenitor cell content resulting in the inappropriate rejection of suitable units by transplant centres [29].
An alternative measure to determine the engraftment potential of a cord blood unit is the Colony Forming Unit (CFU) assay. Post-thaw CFU dose has been shown to be a strong predictor of engraftment success [23]. Similarly, our study shows that post-thaw viability does not directly correlate with CFU potential. This further supports the need for post-thaw CFU analysis as an important pre-requisite for the validation of processing methods and for the ongoing validation of processing platforms utilised by cord blood banks.
Conclusion
We found that TotiCyte dramatically improved the viable recovery of haemopoietic stem cells compared to standard industry methods. TotiCyte is cost effective, allowing storage of reduced volumes, whilst removing >99% of the erythrocyte content with little loss of the white cell fraction. The importance of the improved post-thaw recoveries cannot be over emphasised and could result in transplants with dramatically improved outcomes. In addition, the results of the CFU assays clearly indicate that these should become standard for the validation of cord blood processing methodologies. Our data clearly demonstrate that TotiCyte processing of UCB provides a significantly more efficient method of harvesting the valuable progenitor cell populations.
Acknowledgement
The authors would like to thank Dr. Xavier Fontana and Dr. Tim Chevassut for technical assistance and advice.
Author’s Contribution
Study Concept: JD, Experimental design and conduct: JD, LAM, RS, WH, MW, Data analysis: AK, LAM, JD, WC. Manuscript preparation: LAM, JD, Manuscript review: WC, CR, JD, LAM, AK.
Competing Interest
This study was funded in its entirety by CyteTech Ltd. All authors are, or were employees of CyteTech.
References
- Rocha V, Labopin M, Sanz G, Arcese W, Schwerdtfeger R, et al. (2004) Transplants of umbilical-cord blood or bone marrow from unrelated donors in adults with acute leukemia. N Engl J Med 351: 2276-2285.
- Atsuta Y, Suzuki R, Nagamura-Inoue T, Taniguchi S, Takahashi S, et al. (2009) Disease-specific analyses of unrelated cord blood transplantation compared with unrelated bone marrow trans- plantation in adult patients with acute leukemia. Blood 113: 1631-1638.
- Eapen M, Wagner JE (2010) Transplant outcomes in acute leukemia. I Semin Hematol 47: 46-50.
- Wagner JE, Barker JN, DeFor TE, Baker KS, Blazar BR, et al. (2002) Transplantation of unrelated donor umbilical cord blood in 102 patients with malignant and non-malignant diseases: influence of CD34 cell dose and HLA disparity on treatment-related mortality and survival. Blood 100: 1611-1618.
- Barker JN, Scaradavou A, Stevens CE (2010) Combined effect of total nucleated cell dose and HLA match on transplantation outcome in 1061 cord blood recipients with hematologic malignancies. Blood 115: 1843-1849.
- Cutler C, Multani P, Robbins D Kim HT, Le T, et al. (2013) Prostaglandin-modulated umbilical cord blood hematopoietic stem cell transplantation. Blood 122: 3074-3081.
- Barker JN, Weisdorf DJ, DeFor TE, Blaza BR, McGlave PB, et al. (2005) Transplantation of 2 partially HLA-matched umbilical cord blood units to enhance engraftment in adults with hematologic malignancy. Blood 105: 1343-1347.
- Ballen KK, Spitzer TR, Yeap BY, McAfee S, Dey BR, et al. (2007) Double unrelated reduced-intensity umbilical cord blood transplantation in adults. Biol Blood Marrow Transplant 13: 82-89.
- Wagner JE, Eapen M, Carter S, Wang Y, Schultz KR, et al. (2014) One-unit versus two-unit cord-blood transplantation for hematologic cancers. N Engl J Med 371: 1685-1694.
- Fernandez MN, Regidor C, Cabrera R, García-Marco JA, Forés R, et al. (2003) Unrelated umbilical–cord blood transplants in adults: Early recovery of neutrophils by supportive co-transplantation of a low number of highly purified peripheral blood CD34+ cells from an HLA–haploidentical donor. Exp Hematol 31: 535-544.
- Barker JN, Weisdorf DJ, DeFor TE, Blazar BR, Miller JS, et al. (2003) Rapid and complete donor chimerism in adult recipients of unrelated donor umbilical cord blood transplantation after reduced intensity conditioning. Blood 102: 1915-1919.
- Chow R, Nademanee A, Rosenthal J, Karanes C, Jaing TH, et al. (2007) Analysis of hematopoietic cell transplants using plasma-depleted cord blood products that are not red blood cell reduced. Biol Blood Marrow Transplant 13: 1346-1357.
- Barker JN, Abboud M, Rice RD, Hawke R, Schaible A, et al. (2009) A “no-wash” albumin-dextran dilution strategy for cord blood unit thaw: High rate of engraftment and a low incidence of serious infusion reactions. Biol Blood Marrow Transplant 15: 1596-1602.
- Takahashi TA, Rebulla P, Armitage S, van Beckhoven J, Eichler H, et al. (2006) Multi-laboratory evaluation of procedures for reducing the volume of cord blood: influence on cell recoveries. Cytotherapy 8: 254-264.
- Denning-Kendall P, Donaldson C, Nicol A, Bradley B, Hows J (1996) Optimal processing of human umbilical cord blood for clinical banking. Exp Hematol 24: 1394-1401.
- Regidor C, Posada M, Monteagudo D, Garaulet C, Somolinos N, et al. (1999) Umbilical cord blood banking for unrelated transplantation: evaluation of cell separation and storage methods. Exp Hematol 27: 380-385.
- Rubinstein P, Dobrila L, Rosenfield RE, Adamson JW, Migliaccio G, et al. (1995) Processing and cryopreservation of placental/umbilical cord blood for unrelated bone marrow reconstitution. Proc Natl Acad Sci USA 92: 10119-10122.
- Lasky LC, Lane TA, Miller JP, Lindgren B, Patterson HA, et al. (2002) In utero or ex utero cord blood collection: Which is better? Transfusion 42: 1261-1267.
- Alonso JM 3rd, Regan DM, Johnson CE, Oliver DA, Fegan R, et al. (2001) A simple and reliable procedure for cord blood banking, processing, and freezing: St. Louis and Ohio cord blood bank experiences. Cytotherapy 3: 429-433.
- Lapierre V, Pellegrini N, Bardey I, Malugani C, Saas P, et al. (2007) Cord blood volume reduction using an automated system (Sepax) vs. a semi-automated system (Optipress II) and a manual method (hydroxyethyl starch sedimentation) for routine cord blood banking: a comparative study. Cytotherapy 9: 165-169.
- Dazey B, Duchez P, Letellier C, Vezon G, Ivanovic Z, et al. (2005) Cord blood processing by using a standard manual technique and automated closed system "Sepax" (Kit CS-530). Stem Cells Dev 14: 6-10.
- Tanaka M, Yanagisawa R, Yamanaka M, Konno S, Takemura K, et al. (2020) Transfusion outcome for volume- and plasma-reduced platelet concentrates for pediatric patients. Transfus Apher Sci 59: 102776.
- Morgenstern DA, Ahsan G, Brocklesby M, Ings S, Balsa C, et al. (2016) Post-thaw viability of cryopreserved peripheral blood stem cells (PBSC) does not Guarantee functional activity: important implications for quality assurance of stem cell transplant Br J Haematol 174: 942-951.
- Jaing TH, Yang CP, Hung IJ, Chen SH, Sun CF, et al. (2007) Transplantation of unrelated donor umbilical cord blood utilizing double-unit grafts for five teenagers with transfusion-dependent thalassemia. Bone Marrow Transplant 40: 307-311.
- Barker JN, Kempenich J, Kurtzberg J, Brunstein CG, Delaney C, et al. (2019) CD34+ cell content of 126 341 cord blood units in the US inventory: implications for transplantation and banking. Blood Adv 3: 1267-1271.
- Eapen M, Rubinstein P, Zhang MJ, Stevens C, Kurtzberg J, et al. (2007) Outcomes of transplantation of unrelated donor umbilical cord blood and bone marrow in children with acute leukaemia: a comparison study. Lancet 369: 1947-1954.
- Ruggeri A, Eapen M, Scaravadou A, Cairo MS, Bhatia M, et al. (2011) Umbilical cord blood transplantation for children with thalassemia and sickle cell disease. Biol Blood Marrow Transplant 17: 1375-1382.
- Barker JN, Byam C, Scaradavou A (2011) How I treat: the selection and acquisition of unrelated cord blood grafts. Blood 117: 2332-2339.
- Petz LD, Chow R (2011) Selection of unrelated cord blood grafts. Blood 118: 478- 479.
Citation: Drew J, Slaughter R, Klimentov A, Channon WM, Rees C, et al. (2021) TotiCyte, a Paradigm Shift in Stem Cell Isolation and Storage from Umbilical Cord Blood. J Stem Cell Res Dev Ther 7: 073.
Copyright: © 2021 Jeff Drew, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.

