
Use of a Novel Respiratory Muscle Training Device Increases Pulmonary Vital Capacity in Real-World Application
*Corresponding Author(s):
Jeremy OllerenshawAnthem Biotechnologies, Atlanta, GA, United States
Email:ollerenj@anthembiotech.com
Abstract
A respiratory rehabilitation training device to gather data in a Real-World study setting over a five-year period. Study subjects were 18,949 volunteers who had purchased the Airofit PRO™ device from the manufacturer and whom had met the study inclusion criteria. Repeated measurements of respiratory performance parameters for a minimum of 28-days were made by the device in users, which yielded average improvements of 41% in user lung capacity over the study period. In addition, 14% and 59% of users showed increases of up to 10% and up to 100% in lung capacity respectively. These data strongly suggest the Airofit PRO™ device may be a beneficial training device in a broad range of individuals including athletes and in the respiratory rehabilitation of patients with respiratory illness including chronic obstructive pulmonary disease (COPD) and coronavirus disease 2019 caused by the SARS-CoV-2 virus.
Introduction
COPD is a condition caused by inflammatory damage and weakening of the lungs and respiratory muscles which limits airflow and gas exchange in the lung alveoli. Dynamic hyperinflation present in COPD is associated with an overload of respiratory muscles, which perpetuates the cycle of events [1]. Symptoms of COPD include trouble breathing, cough and wheezing. Inspiratory muscle weakness is frequently found [2] along with significant exercise limitation, deconditioning, decrease in athletic performance [3,4], and quality of life [5].
The best-known risk factors in developing COPD are exposure to tobacco smoke, although other factors such as environmental pollution or exposure to biomass combustion, together with individual predisposition, have also been correlated [6,7]. Emphysema and chronic bronchitis are the two most common types of COPD. These two conditions usually occur together and can vary in severity in COPD. Currently, COPD is one of the first causes of worldwide morbimortality.
Although COPD is a progressive condition, it is preventable and treatable so that quality of life is increased, and with appropriate management, the risk of COPD associated conditions, such as heart disease and lung cancer can be minimized. In the 1980s, [8] highlighted inspiratory muscle weakness in COPD patients and the potential benefits of targeted training. Respiratory exercise devices that employ inspiratory muscle training (IMT) are widely used to improve respiratory capacity in individuals with COPD and other respiratory diseases to improve respiratory function and quality of life. [9-12]. Respiratory rehabilitation (RR) as part of IMT is postulated as being the most cost-effective treatment strategy with widely accepted, reduction in hospital admissions and mortality in patients.
The Airofit Pro breathing trainer has been designed as a novel IMT device to improving lung capacity among its users and when used as part of a respiratory rehabilitation program, may have significant benefit in the treatment of COPD. A real-world evidential study in the use of the Airofit Pro breathing trainer has been performed in a cohort of more than16,000 self-selected subjects over a five-year period of enrollment with highly promising respiratory benefits including increases in lung capacity that could significantly benefit individuals suffering with COPD.
Methods
Test device description
The Airofit PRO™ [Airofit, Copenhagen, Denmark]) is a small, portable, lightweight, noninvasive manometer with a rubber-flanged mouthpiece designed for assessment of the respiratory muscles during a training program to improve lung capacity (Figure 1). The Airofit PRO™ E-unit contains an air flow sensor from which air pressure data is calculated for inhaled and exhaled air using an integrated computational circuit. The resistances of both inhaled and exhaled air are independently adjustable on the unit by the user. Data is transmitted to a smart-phone Airofit PRO™ Sport mobile application via a Bluetooth transmitter during use which is also present on the device and continuous calculation of air pressures and air flow rates in the device is made by the mobile phone software allowing for measurement and visualization of breathing patterns (Figure 2). The ability for the user to adjust airflow resistances on the Airofit PRO™ breathing trainer enables the user to train respiratory muscles to work harder for both inhalation and exhalation during breathing cycles and allows for specific training protocols to be implemented which include resistance level and duration of training sessions to suit the needs of each individual user to gain more efficient and stronger breathing. The device has previously been validated in use aging predicted values of respiratory function [13].
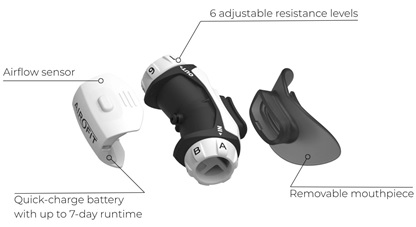 Figure 1: Detail Schematic Representation of the Airofit Pro Breathing Trainer.
Figure 1: Detail Schematic Representation of the Airofit Pro Breathing Trainer.
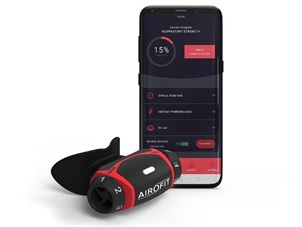 Figure 2: The Airofit Breathing Trainer Together with Smart-Phone Software Application.
Figure 2: The Airofit Breathing Trainer Together with Smart-Phone Software Application.
Study subjects
The study subjects included in the data analysis comprised self-selected individuals who had purchased the Airofit Pro breathing trainer from the manufacturer and had used the Airofit smart-phone software application to collect their user data during a five-year enrollment period. Data was collected anonymously through use of the Airofit smart-phone software application and stored on a cloud-based repository from users whom had given Airofit permission to use their data for scientific research.
For inclusion in the study, only data collected from individual users who had completed at least 28 days of training with the Airofit Pro breathing trainer, and whom had provided data from more than three training sessions per week was used. Additionally, an initial baseline lung capacity calculated as between 0.5L and 14L was required for users to be included in the study. This broad range for baseline lung capacity was chosen so that users with impaired lung function, healthy individuals and elite athletes were all represented in the study group. Changes in calculated lung capacity, either increased or decreased, were made by the Airofit smart-phone software application over time during multiple uses by the study participants. Users were excluded from inclusion in the study if supplied lung capacity changes while using the device were outside of the range of -20% to 100% from baseline as such values were considered to be unrealistic. Following selection of users for the study, given the inclusion criteria, the resulting data subset would provide consistent and reliable data, ensuring robust study analysis and conclusions.
User Training And Automated Data Collection
All training user sessions were performed while users were sitting and at rest in a quiet room, and according to the device manufacturers instructions. The calculated maximal inspiratory pressure (MIP) and maximal expiratory pressure (MEP) were recorded by the Airofit PRO™ device. The measurements were made in accordance with the American Thoracic Society (ATS)/European Respiratory Society (ERS) guidelines [13]. For each trial, MIP and MEP values were calculated according to formulas published by [14].
Vital lung capacity was calculated by way of the Rohrer three-parameter model [15,16] to calculate volumetric flow rate from pressure measurements across three differing sized orifices selected on the Airofit device. Following calibration of the device against known flow with a mechanical lung test system, pre-determined coefficients, k1,k2 and k3, representing the flow-pressure characteristics of each orifice size were used to calculate inhalation and exhalation volumetric air flow rate, Q (liters/second). From the Rohrer model:
ΔP = k1·Q + k2·Q2 + k0 : ΔP represents the pressure anomaly.
Solving for flow rate, Q (L/s)
k2·Q2 + k1·Q + (k0 ΔP) = 0 :
Finally, Volume, V (liters), is determined through time-integration of flow rate, Q
V (L) = ∫Qdt
Study Design and Analysis
The study used a Real-World Evidence approach (FDA (n.d.)) represents outcomes observed in actual users rather than controlled clinical settings. The data presented here is derived from self-selected customers who purchased and utilized the Airofit Pro device. Data collection was automated and captured via the Airofit software application and stored in a cloud-based repository, ensuring accurate, anonymized and unbiased data logging.
Results
Study Users
From the users whose data was collected for possible inclusion in the study, 16,490 participants met the study inclusion criteria as outlined above, and these users were subsequently included in the data analysis. The final study group comprised users that had trained for at least 28-days with the device following an initial familiarization period, provided consistent data for this training period, had a minimum of three recorded training sessions per week, and had initial lung capacity measurements between 0.5L and 14L with improvements in lung capacity during the study period of between -20% to 400% as calculated by the device software. Users showing inconsistencies in data logging or providing data that significantly deviated from physiological norms were excluded.
Changes in User Lung Capacity: Changes in lung capacity within the study group of more than 16,000 study subjects is shown in Figure 3 and Table 1. The data collected from all users was analyzed using a standardized process to ensure the results were consistent and without bias. The data has been processed such that each user’s percentage change in lung capacity terms, carried equal weight, regardless of their initial baseline lung capacity and average changes in lung capacity has been calculated by summing all percentage improvements and dividing by the total number of users.
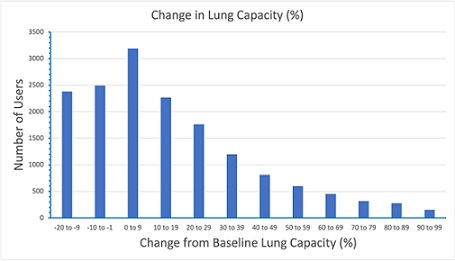 Figure 3: Histogram Showing Change in User Lung Capacity Following use of the Airofit Pro Breathing Trainer
Figure 3: Histogram Showing Change in User Lung Capacity Following use of the Airofit Pro Breathing Trainer
Each bar represents a 10% interval of improvement in lung capacity compared to their baseline data.
|
User Parameter |
Calculated Result |
|
Number of Users included in Study |
16,490 |
|
Users with up to 10% benefit in lung capacity |
2,723 (17%) |
|
Users Showing Increases in Lung Capacity of up to 100% |
11,123 (67%) |
|
Average Change in User Lung Capacity |
17% |
Table 1: Summary Results in Lung Capacity in Users Included in Study.
The improvements in lung capacity across compliant users are represented in Figure 3 and Table 1. The histogram of changes in lung capacity for users included in the study (Figure 3) shows a benefit to lung capacity in over 2,700 users of up to 10% over individual baseline data, with users represented at slightly lower numbers as benefit rates increase. Table 1 shows, the number of users benefitting in lung capacity between 1% and 100% of their baseline data was in excess of 11,000, representing almost 60% of the users in the study.
Real-World Study Applicability
The study outcomes reflect practical, everyday usage of the Airofit PRO™ by a diverse user base (including all ages, genders and ethnicities), making the findings highly relevant to users seeking tangible respiratory health improvements. The technology built into the device, coupled with the study design enables the data interpretation to bridge the gap between controlled clinical studies and real-world application.
Discussion
In using the Airofit PRO™ device, this large Real-World Evidence study performed over five years has shown improvements in lung capacity of actual users. The device has previously also been shown to be well suited to recording inhalation and exhalation data without modifications or adjustments to the device during an exercise session (Stavrou (2021)). Stavrou etal in their clinical study in athletes, also showed the Airofit device to be comparable in performance to the gold standard electronic spirometer, and further was easier to operate and provided more information than the gold standard device (Stavrou (2021)). The device is therefore accurate, not complex to operate and it is capable of being used with a range of resistance settings that can benefit the respiratory education of a broad range of individuals, including the general population, athletes and patients with chronic respiratory illness.
This study has shown the potential benefits of using the Airofit PRO™ device, with COPD patients to be significant. However, use of the device as respiratory rehabilitation in other respiratory illnesses and infections such as SARS-CoV-2 likely also has significant potential. Finally, the Airofit PRO™ device is well suited as a tool in respiratory tele-exercise and/or tele-rehabilitation for patients with difficulties in attending remote clinics because of geographic or financial limitations.
References
- Anzueto A, Miravitlles M (2017) Pathophysiology of dyspnea in COPD. Postgrad Med 129: 366-374.
- Gloeckl R, Marinov B, Pitta F (2013) Practical recommendations for exercise training in patients with COPD. Eur Respir Rev 22:178-186.
- Stavrou VT, Tourlakopoulos KN, Daniil Z, Gourgoulianis KI (2021) Respiratory Muscle Strength: New Technology for Easy Assessment. Cureus 13: 14803.
- McConnell AK, Koutedakis Y, McNaughton L, Backx K, et al. (2001) Inspiratory muscle training improves rowing performance. Med Sci Sports Exerc 33: 803-809.
- Seixas MB, Almeida LB, Trevizan PF, Martinez DG, Laterza MC, et al. (2020) Effects of inspiratory muscle training in older adults. Respir Care 65: 535-544.
- GICOLD (2025) Global Strategy for Prevention, Diagnosis and Management of COPD: 2025 Report.
- Chen HI (1985) Inspiratory muscle training in patients with chronic obstructive pulmonary disease. In: Am Rev Respir Dis 131: 251-255.
- Gloeckl R, Marinov B, Pitta F (2013) Practical recommendations for exercise training in patients with COPD. Eur Respir Rev 22:178-186.
- Geddes EL (2005) Inspiratory muscle training in adults with chronic obstructive pulmonary dis- ease: a systematic review. Respir Med 99: 1440-1458.
- Geddes EL, O'Brien K, Reid WD, Brooks D, Crowe J (2008) Inspiratory muscle training in adults with chronic obstructive pulmonary disease: an update of a systematic review. Respir Med 102: 1715-1729.
- Gosselink R, De Vos J, van den Heuvel SP, Segers J, Decramer M, et al. (2011) Impact of inspiratory muscle training in patients with COPD: what is the evidence? Eur Respir J 37: 416-425.
- Buysse DI, Reynolds CF, Monk TH, Berman SR, Kupfer DJ (1989) The Pittsburgh Sleep Quality Index: a new instrument for psychiatric practice and research. Psychiatry Res 28: 193-213.
- Cooke NT, Edwards TH, Spiro SG (1984). “Predicted normal values for maximal respiratory pressures in caucasian adults and children. Thorax 39: 535-538.
- Guerin C, Richard J-C (2007) Measurement of respiratory system resistance during mechanical ventilation. Intensive Care Med 33: 1046-1049.
- Lötters F, van Tol B, Kwakkel G, Gosselink R (2002) Effects of controlled inspiratory muscle training in patients with COPD: a meta-analysis. Eur Respir J 20: 570-577.
- FDA (2016) Real-World Evidence. US Food and Drug Administration, USA.
Citation: Ollerenshaw J, Rodrigues GD, Lesslar O, Richardson B, Dvorácek M, et al. (2025) Use of a Novel Respiratory Muscle Training Device Increases Pulmonary Vital Capacity in Real-World Application. J Pulm Med Respir Res 11: 094.
Copyright: © 2025 Jeremy Ollerenshaw, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.

