
Use of Exhaled Nitric Oxide (FENO) as an Indirect Measure of Compliance in Asthma
*Corresponding Author(s):
Dutt TSDepartment Respirologist, Petrborough City Hospital, Cambridgeshire, Bretton Gate, NHS, United Kingdom
Tel:+44 01733678000,
Email:tiyassendutt@gmail.com
Abstract
Aim
Difficult-to-treat asthma is often explained by poor adherence with controller medication. Failure to detect non-compliance, could result in inappropriate interventions; non-compliant patients who would benefit from education, adherence monitoring may instead receive invasive, expensive treatment including monoclonal therapies, bronchial thermoplasty. A clinic-based-test of compliance would be valuable.
Exhaled Nitric Oxide (eNO) is a simple, relatively inexpensive measure of airways inflammation with marked elevation in untreated asthma. We, and others, have shown previously that eNO values drop within hours to days of inhaled corticosteroid therapy.
We undertook a pilot study to test the hypothesis that eNO and compliance with controller therapy would be inversely correlated.
Methods
This was a cross sectional study reviewing pharmacy data of drug consumption and eNO values, of patients who visited the Asthma & Airway Centre from February 2015 till 19th October 2015.
Patients were eligible for inclusion if they had a diagnosis of asthma as per Canadian Thoracic Society guidelines, with a confirmed variable expiratory airflow limitation like a post-bronchodilator response of FEV1 >12% and >200ml from baseline, after 200-400 mcg of salbutamol inhalation, positive methacholine challenge study (PC20 < 8 mg/ml) or FEV1 variability >20% over time.
Patients were excluded if they have smoked tobacco within the last 3 months, or has an exhaled carbon monoxide level > 6 parts per million, if they were on daily oral steroids > 5mg of prednisone for >3 months, those with poor inhalation technique, Churg-Strauss Vasculitis, COPD, ILD.
We defined non-compliance when the controller prescription filling was <= 80% over 12 months’ time. We calculated compliance in percentage with the numerator being the number of doses the patient had actually taken, the denominator being the number of doses prescribed for the 12 month interval.
Results
There were 300 patients who were screened; 125 met the study criteria. Higher e NO value was associated with a suboptimal compliance. With the cut off level of e NO >=25 ppb, the sensitivity of the test is 98.61%, specificity 98.11% and positive predictive value 98.61% , to confirm sub-optimal compliance <=80%. Thus the study hypothesis that eNO and compliance has an inverse correlation could be demonstrated.
Conclusion
In this study we have tried for the first time to detect sub-optimal compliance to controller therapy using an objective test. In difficult-to-treat asthma patients, we can use available technology i.e. eNO, in a novel fashion to ensure that the treatment with controller therapy is optimal.
Keywords
Asthma; Compliance; Exhaled nitric oxide
INTRODUCTION
Asthma is a common disease affecting around 300 million individuals worldwide [1]. The reported incidence of asthma in adults is 3.6 and 4.6 cases per 1000 person-years for men and women, respectively [2]. There are about 489,000 deaths attributable to asthma annually [3]. Therefore the burden of the disease remains substantial for both the patients and for the health care system.
Despite the availability of effective and safe controller medications, for many patients asthma remains uncontrolled. Poorly controlled asthma increases the risks of severe and recurrent asthma exacerbations. This can further impair various aspects of daily living along with an overall decrease in the quality of life [4].
Difficult-to-treat asthma is often explained by poor adherence with controller medication with Inhaled Corticosteroid (ICS). Non-adherence is known to be associated with poor asthma outcomes [5]. Failure to detect such non-compliance could result in inappropriate interventions and treatment.
Although it is known since long that non-adherence to controller therapy is a major contributing factor for treatment failure, it has been a real challenge to ascertain this. Even an expensive clinic-based test of compliance would be undoubtedly valuable.
OBJECTIVE
Nitric Oxide (NO) is recognized to play a key role as a vasodilator, bronchodilator, and an inflammatory mediator [6]. Patients with untreated asthma have high levels of NO in their exhaled breath along with an increased expression of Nitric Oxide Synthase (NOS2) enzyme in the epithelial cells of their airways [7].
Exhaled Nitric Oxide (FENO) is a simple, non-invasive and relatively inexpensive measure of airway inflammation. We and others have shown previously that FENO values drop within hours to days of corticosteroid treatment of asthma, likely reflecting a reduction in inflammation [8,9].
STUDY HYPOTHESIS
We undertook this pilot study to test the hypothesis that Exhaled Nitric Oxide (FENO) and compliance with controller therapy would be inversely correlated.
MATERIALS AND METHODS
This was a cross sectional study reviewing pharmacy data of drug consumption and FENO values, of consecutive asthma patients who visited the Asthma & Airway Centre of Toronto Western Hospital (TWH), University Health Network (UHN) from February 23rd 2015 till December 1st, 2015.
Patients were eligible for inclusion if they had a diagnosis of asthma as per Canadian Thoracic Society (CTS) guidelines, with a confirmed variable expiratory airflow limitation like a post bronchodilator response of FEV1 >12% and > 200 ml from baseline, 10-15 minutes after 200-400 mcg of salbutamol inhalation, positive methacholine challenge study (PC20 < 8 mg/ml) or FEV1 variability > 20 % over time.
The exclusion criteria included patients who had smoked tobacco within the last 3 months or has an exhaled carbon monoxide level (eCO) > 6 ppm (parts per million): As because FENO is less accurate in smokers. The other exclusion criteria included patients who were on daily oral steroids > 5 mg of oral prednisone per day continuously or monthly triamcilone injections for > 3 months and/or demonstrated poor inhalation technique. Patients with other significant diseases including Churg-Strauss vasculitis, Chronic Obstructive Pulmonary Disease (COPD), Interstitial Lung Disease (ILD) were also excluded from the study. We defined non-compliance when the controller prescription filling was <80% over 12 months. We calculated compliance in percentage (%) with the numerator being the number of doses dispensed and the denominator being the number of doses prescribed for the 12-month interval.
Exhaled NO (FENO) measurements were done using the NIOX MINO machine, prior to spirometry and methacholine challenge, by a technique as recommended by the American Thoracic Society/ European Respiratory at a 50ml/s flow rate [10]. The FENO test was done three times for one patient and the average of the 3 values was taken as an objective measure of airway inflammation expressed in Parts Per Billion (ppb) units. Symbicort (ICS+LABA) was never prescribes as prn or as needed inhaler, even when there was an exacerbation. Any trigger was managed with Short Acting Beta Agonist (SABA) on a as needed basis.
RESULTS
We screened 356 patients from February 23rd 2015 till December 1st, 2015. Out of these patients 143 of them met the study criteria. The normal FENO values remain between 20 to 25 ppb (Parts Per Billion). This pilot study revealed that sub-optimal compliance was associated with higher FENO values.
For compliance between 100 % and 80 %, the FENO values remain within the upper limit of the normal range, i.e. up to 25 ppb. When the compliance decreases from 80 % to 40 %, the FENO values start rising from 25 ppb to 40 ppb to 60 ppb. As the compliance drops to < 40 %, the FENO values remain higher > than 25 ppb but can in some patients rise sharply to > 60 ppb and can increase even up to > 100 ppb. Thus, the study hypothesis that Exhaled Nitric Oxide (FENO) and compliance with controller therapy has an inverse correlation could be demonstrated as in figure 1.
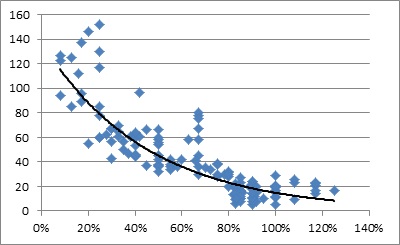
Figure 1: Scatter diagram of the findings, where the “X” axis determines the compliance in percentage (%) and the “Y” axis determine the e NO level in Parts Per Billion (ppb), thus demonstrating a curvilinear relationship.
With the cut off level of FENO > 25 ppb, the sensitivity is 98.80%, the specificity is 96.61%, the positive predictive value (PPV) is 97.64% and the Negative Predictive Value (NPV) is 98.27%to confirm a sub-optimal compliance < 80% as discussed in table 1. We calculated the sensitivity, specificity, Positive Predictive Value (PPV) and negative predictive value (NPV)using the 2/2 table as described in table 1. In 19 patients out of 143 i.e., ~ in 13% of our cases, the compliance has been > 100 %, which is sometimes seen in the asthma population. Our study showed no co-relation between the FENO values and the amount of ICS doses per day that the patients were on. We calculated the sensitivity, specificity, positive predictive value (PPV) and negative predictive value (NPV) using the 2/2 table as described in table 1.
|
Test |
Compliance <=80% |
Compliance >80% |
|
|
e NO>=25ppb |
83 patients (True Positive) |
2 patients (False Positive) |
PPV: 97.64% |
|
e NO < 25ppb |
1 patients (False Negative) |
57 patients (True Negatives |
NPV: 98.27% |
|
|
Sensitivity: 98.80% |
Specificity: 96.61% |
Total :143 patients |
Table 1: We calculated the sensitivity, specificity, Positive Predictive Value (PPV) and Negative Predictive Value (NPV) using the 2/2 table as described in table 1.
Adherence of >100 % is when patient kept additional supply of inhalers, particularly during the winter months for logistic issues.
DISCUSSION
In this pilot study we could demonstrate that Exhaled Nitric Oxide (FENO) can be used as a clinic-based tool to identify patients who are non-adherent with their treatment with controller Inhaled Corticosteroid (ICS) therapy. These patients do not take the medication optimally as prescribed.
So, the use of FENO test addresses the challenging issue of measuring non-compliance, which has been known to be a major contributor of treatment failure in difficult-to-treat- asthma. This test would help us classify non-compliant asthma patients who would need patient education, awareness and adherence monitoring to improve their asthma control from those who have refractory asthma in spite of being compliant on regular ICS therapy and would actually benefit from additional invasive, expensive interventions like monoclonal antibody therapies or bronchial thermoplasty.
In our study we did not include patients with a poor inhaler technique, so that we can pinpoint the non-adherence issue alone. Also, because we know that the FENO levels remain suppressed after treatment with systemic steroids, we excluded the subset of difficult-to-treat asthma patients who are on regular steroid treatment regimen with either oral prednisone or monthly triamcinolone injections.
In pediatric asthma population, there have been studies describing the inverse correlation between levels of FENO and adherence to steroid inhalation therapy [11-13]. These studies highlight the use of FENO as a useful tool to monitor adherence to steroids, which is often poor in asthmatics and thus significantly contribute to the management of anti-inflammatory treatment [11]. Our present study emphasises similar observation with higher FENO values co-relating with sub-optimal compliance in the adult asthmatics.
Various studies looking into compliance with respiratory medications in clinical trials &practice reveal that compliance in asthma hovers around 35 to 40 %, suggesting that patients may be titrating their consumption of controller therapy intelligently or at least based on some aspect of asthma control [14-16]. Compliance might be considered to be the interface between effective therapy and effective disease management. The most effective asthma drug will not be useful unless patients take it in optimal amounts to control their disease. In our current study we had this observation that when the compliance drops to < 80 % there is a rise in FENO value above the upper limit of normal, but as the compliance drops further < 40 % - 50 %, the FENO values can sharply rise.
We did not find any co-relation between the FENO values and the various doses of ICS therapy either low dose or medium dose or higher doses.
As a corollary to our current observation, in patients with a high FENO level~100ppb and poor compliance < 40 %, we also did a directly Observed Inhaled Corticosteroid Treatment (DIOS) with nebulised Budesonide for 5 days. Repeat FENO measurement after the DIOS treatment is done showed a significant drop in their FENO. So, we will be taking this test further in a future study, to stratify patients who would actually benefit with optimization of controller inhaler therapy from those who would not.
The limitation of the study is that it is done from a single, busy, tertiary care referral centre, so a certain amount of selection bias could have been there. Secondly, using pharmacy records alone has its own limitation. For example, we took into consideration the doses of ICS dispensed from the pharmacy in 12 months’ time as the numerator. We would not know for sure however, whether these doses were actually taken by the patient, or would it be left unused.
In this pilot study we have tried for the first time to detect sub-optimal compliance to controller therapy using an objective test. In patients with difficult-to-treat asthma, we can use available technology i.e. Exhaled Nitric Oxide (FENO), in a novel fashion to ensure that the treatment with controller therapy is optimal.
CONCLUSION
Point of care FENO measurement is useful for the detection of non-compliance with ICS-containing controller medication in difficult to treat asthma. Therefore, as FENO is currently not recommended in Canadian guidelines, as a marker of non-adherence to therapy in difficult asthma patients: we suggest based on our observations in this pilot study, that it should be included in future iterations.
REFERENCES
- Global Initiative for Asthma (2014) Global Strategy for Asthma Management and Prevention. Global Initiative for Asthma, USA.
- Eagan TML, Brøgger JC, Eide GE, Bakke PS (2005) The incidence of adult asthma: A review. International Journal of Tuberculosis and Lung Disease 9: 603-612.
- GBD 2013 Mortality and Causes of Death Collaborators (2015) Global, regional, and national age-sex specific all-cause and cause-specific mortality for 240 causes of death, 1990-2013: A systematic analysis for the Global Burden of Disease Study 2013. Lancet 385: 117-171.
- O'Byrne PM, Pedersen S, Schatz M, Thoren A, Ekholm E, et al. (2013) The poorly explored impact of uncontrolled asthma. Chest 143: 511-523.
- McNicholl DM, Stevenson M, McGarvey LP, Heaney LG (2012) The utility of fractional exhaled nitric oxide suppression in the identification of nonadherence in Difficult Asthma. Am J RespirCrit Care Med 186: 1102-1108.
- Nathan C, Xie QW (1994) Nitric oxide synthases: Roles, tolls, and controls. Cell 78: 915-918.
- Guo FH, Comhair SA, Zheng S, Dweik RA, Eissa NT, et al. (2000) Molecular mechanisms of increased nitricoxide (NO) in asthma: Evidence for transcriptional and posttranslational regulation of NO synthesis. J Immunol 164: 5970-5980.
- Kharitonov SK, Alving, Barnes PJ (1997) Exhaled and nasal nitricoxide measurements: Recommendations. Eur Respir J 10: 1683-1693.
- Chatkin JM, Ansarin K, Silkoff PE, Mcclean P, Gutierrez C, et al. (1999) Exhaled Nitric Oxide as a Noninvasive Assessment of Chronic Cough. Am J RespirCrit Care Med 159: 1810-1813.
- American Thoracic Society, European Respiratory Society (2005) ATS/ERS recommendations for standardized procedures for the online and offline measurement of exhaled lower respiratory nitric oxide and nasal nitric oxide. Am J RespirCrit Care Med 171: 912-930.
- Beck-Ripp J, Griese M, Arenz S, Koring C, Pasqualoni B, et al. (2002) Changes of exhaled nitric oxide during steroid treatment of childhood asthma. EurRespir J 19: 1015-1019.
- Delgado-Corcoran C, Kissoon N, Murphy SP, Duckworth LJ (2004) Exhalednitric oxide reflects asthma severity and asthma control. PediatrCritCare Med 5: 48-52.
- Cano-Garcinuno A, Carvajal-Uruena I, Diaz-Vazquez CA, Dominguez-Aurrecoechea B, Garcia-Merino A, et al. (2010) Clinical correlates and determinants of airway inflammation in pediatric asthma. J Investig Allergol Clin Immunol 20: 303-310.
- Gibson NA, Ferguson AE, Aitchison TC, Paton JY (1995) Compliance with inhaled asthma medication in preschool children. Thorax 50: 1274-1279.
- Chmelik F, Doughty A (1994) Objective measurements of compliance in asthma treatment. Ann Allergy 73: 527-532.
- Cochrane GM, Horne R, Chanez P (1999) Compliance in asthma. Respiratory Medicine 93: 763-769.
Citation: Dutt TS, Chapman KR, Boparai G (2020) Use of Exhaled Nitric Oxide (FENO) as an Indirect Measure of Compliance in Asthma. J Pulm Med Respir Res 6: 049.
Copyright: © 2020 Dutt TS, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.

