
Vitamin D and Pneumonia in Children: A Case Control Study
*Corresponding Author(s):
Kana Ram JatDepartment Of Pediatrics, All India Institute Of Medical Sciences, Government Medical College And Hospital, Delhi 110029, India
Tel:+91 9868152426,
Email:drkanaram@gmail.com
Abstract
Objectives
To evaluate Vitamin D levels and its correlation with severity and outcome of pneumonia in children.
Design
It is a case-control study.
Setting
The study was conducted at tertiary care centre from Northern India from June 2013 to June 2014.
Participants
Fifty children of 1 month to 12 years of age admitted with pneumonia were enrolled as cases. Fifty age and sex matched children without pneumonia were taken as controls.
Outcome measures
Vitamin D levels were compared between the groups and were correlated with severity and outcome of pneumonia.
Results
The median (IQR) age of cases and controls was 12 (5,39) months and 12 (5,39) months respectively. Overall, 76 (86.4%) children were Vitamin D deficient. The median (IQR) Vitamin D levels [cases 6.8 (2.0, 14.5), controls 7.3 (4.7, 14.6) ng/ml; p=0.362] and proportions of children having Vitamin D deficiency were similar between the groups. Vitamin D levels were not different among children with pneumonia, severe pneumonia, and very severe pneumonia (p=0.985). Odds ratio of children with pneumonia being Vitamin D deficient was 3.7 (95% CI: 0.930, 14.770; p-value 0.066). Vitamin D levels were not correlated with requirement of intensive care management, mechanical ventilation and duration of hospital stay.
Conclusion
Majority (86.4%) of children were Vitamin D deficient. Vitamin D levels were not different in cases and controls and were not related to severity and outcome of pneumonia.
The study is registered with Clinical Trials Registry of India (Reg. No: CTRI/2013/09/004021).
Keywords
Hospital stay; Pneumonia; Vitamin D
INTRODUCTION
There are numerous risk factors for pneumonia in children e.g., malnutrition, poverty, ethnicity, incomplete vaccination, smoke exposure, and underlying diseases like bronchopulmonary dysplasia and congenital heart diseases [4,5]. Despite interventions to control some or more of these factors, pneumonia continues to be a major threat to child health. There is growing interest to identify other risk factors that may contribute to incidence and severity of pneumonia.
Vitamin D had many functions other than bone metabolism. Recently, Vitamin D is found to have anti-infective property through modulation of innate immune system [6,7]. Vitamin D enhances the expression of Vitamin D Receptors (VDR) and increases production of cathelicidin and beta-defensin-2 peptides which acts through toll-like receptors to elicit antimicrobial function against infections [8-13]. Vitamin D also regulates phagocytosis dependent and antibody-dependent macrophages which protect from respiratory infections [14]. In Vitamin D deficiency, there is decreased expression of VDR resulting in impaired clearance of microbes and uncontrolled inflammation that leads to more lung damage with impaired oxygenation. Vitamin D levels are correlated with respiratory infections. Low Vitamin D production during winter season may be one of the reasons for increased respiratory infections during winter [15,16]. The hospitalization rate for children with acute respiratory infections was also correlated to skin pigmentation, one of factor determining Vitamin D production in human body [16].
Vitamin D is associated with risk and severity of pneumonia in various studies from world. Higher maternal Vitamin D levels were associated with lower risk of pneumonia in one study [17] but not in other study [18]. In observational studies from Iran [19], Kuwait [20], Egypt [21], Ethiopia [22] and Jordan [23] children with rickets had higher risk of pneumonia compared to children without rickets. In case-control studies, mean Vitamin D levels were significantly lower in children with pneumonia compared to controls [13,24-26]. There are very limited studies from India. We could find only one study from Indapur, India in 2004, where higher Vitamin D levels were associated with lower risk of pneumonia [27]. The study did not assess correlation between Vitamin D levels and outcome of pneumonia [27]. The available studies had mixed results and Vitamin D levels may vary according to regions, therefore, it is worthy to conduct more studies on Vitamin D and pneumonia. If Vitamin D is found to have correlated with pneumonia in children, further studies with emphasis on appropriate method for correction of Vitamin D deficiency may be considered. Thus, this study was planned to assess difference in Vitamin D levels in children with pneumonia and without pneumonia and to correlate Vitamin D levels with severity and outcome of pneumonia.
METHODS
Venous blood samples were collected from both cases and controls and were used to measure serum 25[OH]D levels by Chemiluminiscence assay. Serum 25[OH]D levels of less than 20 ng/ml (50 nmol/L), between 20-30 ng/ml (50-75 nmol/L), and levels of 30-80 ng/ml (75-200 nmol/L) were considered Vitamin D deficiency, insufficiency and sufficiency respectively [29-32].
Statistical methods
RESULTS
| Parameters | Cases (n=50) | Controls (n=50) | P value | |
| Age (months) median (IQR) | 12 (5, 39) | 12 (8, 48) | 0.537 | |
| Male: Female | 31:19 | 31:19 | 1.000 | |
| Month of enrolment | February | 13 | 3 | 0.070 |
| March | 7 | 8 | ||
| April | 7 | 11 | ||
| May | 17 | 17 | ||
| June | 6 | 11 | ||
| State of residence | Haryana | 13 | 24 | 0.133 |
| Chandigarh | 19 | 10 | ||
| Punjab | 11 | 11 | ||
| Uttar Pradesh | 2 | 2 | ||
| Himachal Pradesh | 3 | 1 | ||
| Other | 2 | 2 | ||
| Number of siblings (median, IQR) | 1 (1, 2) | 2 (1, 3) | 0.089 | |
| Duration of exclusive breast feeding (months); median, IQR | 6 (4, 6) | 6 (6, 8) | 0.143 | |
| Number of children with clinical rickets | 07 | 10 | 0.266 | |
| Number of children having complete immunization | 46 | 42 | 0.525 | |
Vitamin D levels were not available for 12 patients (5 cases and 7 controls) due to inadequate sample or spilling during processing. Median (IQR) Vitamin D levels for all participants were 6.9 (2, 14.5) ng/ml with a minimum of 2.0 ng/ml to maximum of 40.9 ng/ml levels. Overall, 76 (86.4%), 8 (9.1%), and 4 (4.5%) children were Vitamin D deficient, insufficient and sufficient respectively. A total of 17 (7 cases, 10 controls) children had clinical rickets and difference between cases and controls was not statistically different (Table 1). The Vitamin D levels were not different between cases and controls (Table 2). Similarly, proportions of children having Vitamin D deficiency were similar among the groups (Table 2). Odds ratio of children with pneumonia being Vitamin D deficient and Vitamin D deficient or insufficient was 3.7 (95% confidence interval: 0.930, 14.770; p-value 0.066) and 3.3 (95% confidence interval: 0.330, 33.024; p-value 0.355) respectively. Hemoglobin levels were also similar between the groups (Table 2).
| Parameters | Cases (n=50) | Controls (n=50) | P value | |
| Vitamin D levels (ng/ml); median, IQR | 6.8 (2.0, 14.5) | 7.3 (4.7, 14.6) | 0.362 | |
| Hemoglobin (gm%); mean±SD | 9.6±2.3 | 9.5±2.9 |
0.827 (95% CI: -1.174, 1.463) |
|
| Calcium (mg/dl); mean±SD | 9.4±1.7 | 8.8±1.8 |
0.106 (95% CI: -0.125, 1.295) |
|
| Phosphate (mg/dl); mean±SD | 4.7±1.1 | 4.6±1.1 |
0.679 (95% CI: -0.349, 0.534) |
|
| Alkaline phosphatase (IU/L); median, IQR | 162 (127, 238) | 204 (137, 274) | 0.302 | |
| Vitamin D status*; n (%) | Deficient (42 (93.3%)34 (79.1%)0.150 | |||
| Insufficient (20-30 ng/ml) | 2 (4.4%) | 6 (14.0%) | ||
| Sufficient (>30 ng/ml) | 1(2.2%) | 3 (6.9%) | ||
| Calcium levels (n=cases 49, control 47) | Normal | 25 (51.0%) | 32 (68.1%) | 0.087 |
| Hypo | 18 (36.8%) | 14 (29.8%) | ||
| Hyper | 06 (12.2%) | 1 (2.1%) | ||
| Phosphate levels (n=cases 49, control 47) | Normal | 38 (77.6%) | 40 (85.1%) | 0.523 |
| Hypo | 8 (16.3%) | 6 (12.8%) | ||
| Hyper | 3 (6.1%) | 1 (2.1%) | ||
| Alkaline phosphatase levels (n=cases 49, control 45) | Normal | 28 (57.1%) | 30 (66.7%) | 0.615 |
| normal range | 16 (32.7%) | 12 (26.7%) | ||
| Above normal range | 5 (10.2%) | 3 (6.7%) | ||
| *Vitamin D levels could not be assessed in 5 cases and 7 controls. | ||||
Calcium, phosphate and alkaline phosphatase levels were similar between cases and controls (Table 2). The levels were categorized as normal, increased or decreased based on laboratory values provided by Nelson textbook of Pediatrics (19th Edition) and again there was no difference among groups (Table 2).
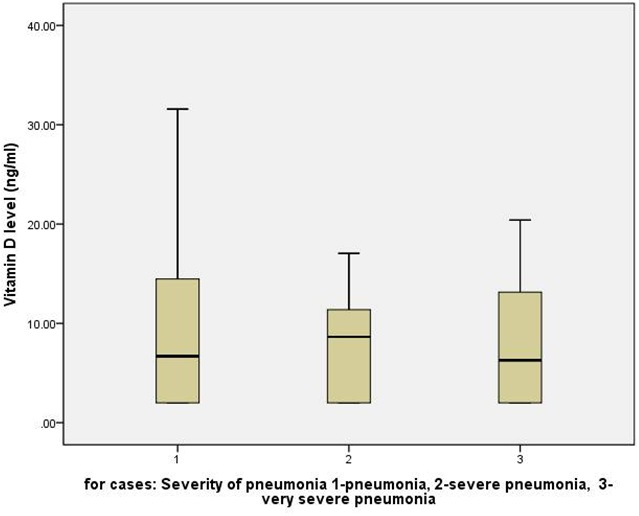
Among cases; 24 (48.0%), 16 (32%), and 10 (20%) children had pneumonia, severe pneumonia, and very severe pneumonia respectively. The median (IQR) Vitamin D levels in three category of pneumonia severity were 6.7 (2.0, 14.6), 8.6 (2.0, 11.8), 6.3 (2.0, 13.7) ng/ml respectively (p=0.985, Kruskal Wallis Test). Thus, Vitamin D levels were not different in children with increasing severity of pneumonia (Figure 1). Thirteen (26%) children among cases required intensive care management and four (8%) children required mechanical ventilation. Again, Vitamin D levels were not different in children who required intensive care management and/or mechanical ventilation compared those who did not require it.There was no mortality among enrolled participants. Finally, duration of hospital stay among cases had no relation with Vitamin D levels (Pearson Correlation -.082, p-value 0.739).
DISCUSSION
The previously reported case-control studies on Vitamin D and Lower Respiratory Tract Infection (LRTI) in children are shown in (Table 3). Karatekin et al., [13] compared neonates admitted with ALRI in NICU to healthy neonates and found that Vitamin D levels were significantly lower in neonates with ALRI (9.12±8.88 v/s 16.33±13.42 ng/ml; p=0.011) and Vitamin D levels 0.05). Roth et al., [26,34] reported no difference in Vitamin D levels in children of 1 month to 2 years of age admitted for pneumonia compared to controls in the study conducted at Canada [34] but Vitamin D levels were significantly lower in children with pneumonia in the study conducted at Bangladesh [26]. Wayse et al., [27] from India observed significantly lower Vitamin D levels in children of 2 months to 5 years of age with severe pneumonia compared to healthy controls (22.8, 95% CI 21.0-24.7 v/s 38.4, 95% CI 34.2-43.1 nmol/l; p<0.001). There are few Randomized Controlled Trials (RCTs) evaluating the effect of Vitamin D supplementation in Lower Respiratory Tract Infection (LRTI) in children [35-38]. All except one [38] RCT concluded that Vitamin D supplementation was not beneficial in preventing or treating LRTI in children.
There may be several reasons for Vitamin D being similar among cases and controls in present study. First is overall high prevalence of Vitamin D deficiency among studied children; a large proportion of both case and controls were Vitamin deficient. Secondly we took admitted children without pneumonia as controls instead of healthy controls. As Vitamin D had anti-inflammatory and anti-infective properties and children with other diseases may have Vitamin D deficiency also. We did this intentionally to evaluate the role of Vitamin D in pneumonia specifically; therefore, we wanted cases and controls to be similar in almost all aspects except pneumonia. The ideal study to see association between Vitamin D and pneumonia should measure Vitamin D at birth and than at regular intervals and to follow children for development of pneumonia; but we could not do this because of lack of funding.
The strength of study is age and sex matched controls and prospective study. One limitation of study is lack of healthy controls.
CONCLUSION
The trial is registered at Clinical Trial Registry of India (CTRI) with registration No. CTRI/2013/09/004021.
CONFLICT OF INTEREST
None.
ETHICS COMMITTEE APPROVAL
ACKNOWLEDGEMENT
REFERENCES
- Bhutta ZA (2007) Dealing with childhood pneumonia in developing countries: how can we make a difference? Arch Dis Child 92: 286-288.
- Wardlaw T, Salama P, Johansson EW, Mason E (2006) Pneumonia: the leading killer of children. Lancet 368: 1048-1050.
- Tessa MW, White JE, Matthew H (2006) Pneumonia: The Forgotten Killer of Children. UNICEF, WHO, Geneva, Switzerland.
- Welliver RC (2003) Review of epidemiology and clinical risk factors for severe Respiratory Syncytial Virus (RSV) infection. J Pediatr 143: 112-117.
- Hassan MK, Al-Sadoon I (2001) Risk factors for severe pneumonia in children in Basrah. Trop Doct 31: 139-141.
- Holick MF (2007) Vitamin D deficiency. N Engl J Med 357: 266-281.
- Adams JS, Hewison M (2008) Unexpected actions of Vitamin D: new perspectives on the regulation of innate and adaptive immunity. Nat Clin Pract Endocrinol Metab 4: 80-90.
- Walker VP, Modlin RL (2009) The Vitamin D connection to pediatric infections and immune function. Pediatr Res 65: 106-113.
- Belderbos ME, Houben ML, Wilbrink B, Lentjes E, Bloemen EM, et al. (2011) Cord blood Vitamin D deficiency is associated with respiratory syncytial virus bronchiolitis. Pediatrics 127: 1513-1520.
- Mohamed WA, Al-Shehri MA (2013) Cord blood 25-hydroxy Vitamin D levels and the risk of acute lower respiratory tract infection in early childhood. J Trop Pediatr 59: 29-35.
- Haider N, Nagi AG, Khan KM (2010) Frequency of nutritional rickets in children admitted with severe pneumonia. J Pak Med Assoc 60: 729-732.
- Inamo Y, Hasegawa M, Saito K, Hayashi R, Ishikawa T, et al. (2011) Serum Vitamin D concentrations and associated severity of acute lower respiratory tract infections in Japanese hospitalized children. Pediatr Int 53: 199-201.
- Karatekin G, Kaya A, Saliho?lu O, Balci H, Nuho?lu A (2009) Association of subclinical Vitamin D deficiency in newborns with acute lower respiratory infection and their mothers. Eur J Clin Nutr 63: 473-477.
- Raloff J (2006) The antibiotic Vitamin. Deficiency in Vitamin D may predispose people to infection. Science News 170: 312-317.
- Yorita KL, Holman RC, Steiner CA, Effler PV, Miyamura J, et al. (2007) Severe bronchiolitis and respiratory syncytial virus among young children in Hawaii. Pediatr Infect Dis J 26:1081-1088.
- Grant WB (2008) Variations in Vitamin D production could possibly explain the seasonality of childhood respiratory infections in Hawaii. Pediatr Infect Dis J 27: 853.
- Morales E, Romieu I, Guerra S, Ballester F, Rebagliato M, et al. (2012) Maternal Vitamin D status in pregnancy and risk of lower respiratory tract infections, wheezing, and asthma in offspring. Epidemiology 23: 64-71.
- Carroll KN, Gebretsadik T, Larkin EK, Dupont WD, Liu Z, et al. (2011) Relationship of maternal Vitamin D level with maternal and infant respiratory disease. Am J Obstet Gynecol 205: 215.
- Salimpour R (1975) Rickets in Tehran. Study of 200 cases. Arch Dis Child 50: 63-66.
- Lubani MM, al-Shab TS, al-Saleh QA, Sharda DC, Quattawi SA, et al. (1989) Vitamin-D-deficiency rickets in Kuwait: the prevalence of a preventable disease. Ann Trop Paediatr 9: 134-139.
- Lawson DE, Cole TJ, Salem SI, Galal OM, el-Meligy R, et al. (1987) Etiology of rickets in Egyptian children. Hum Nutr Clin Nutr 41: 199-208.
- Auss-Kettis A, Bjornesjo KB, Manneimer E, Cvibach T, Clark P, et al. (1965) Rickets in Ethiopia. The occurrence and clinical picture of the disease in the experiences of a Pediatric Clinic in Addis Ababa. Ethiopia Med J 3: 109-121.
- Najada AS, Habashneh MS, Khader M (2004) The frequency of nutritional rickets among hospitalized infants and its relation to respiratory diseases. J Trop Pediatr 50: 364-368.
- McNally JD, Leis K, Matheson LA, Karuananyake C, Sankaran K, et al. (2009) Vitamin D deficiency in young children with severe acute lower respiratory infection. Pediatr Pulmonol 44: 981-988.
- Muhe L, Lulseged S, Mason KE, Simoes EA (1997) Case-control study of the role of nutritional rickets in the risk of developing pneumonia in Ethiopian children. Lancet 349: 1801-1804.
- Roth DE, Shah R, Black RE, Baqui AH (2010) Vitamin D status and acute lower respiratory infection in early childhood in Sylhet, Bangladesh. Acta Paediatr 99: 389-393.
- Wayse V, Yousafzai A, Mogale K, Filteau S (2004) Association of subclinical Vitamin D deficiency with severe acute lower respiratory infection in Indian children under 5 y. Eur J Clin Nutr 58: 563-567.
- WHO (1995) The Management of Acute Respiratory Infection in Children: Practical Guidelines for Outpatient Care. WHO, Geneva, Switzerland.
- Holick MF (2007) Vitamin D deficiency. N Engl J Med 357: 266-281.
- Thomas MK, Lloyd-Jones DM, Thadhani RI, Shaw AC, Deraska DJ, et al. (1998) HypoVitaminosis D in medical inpatients. N Engl J Med 338: 777-783.
- Heaney RP, Dowell MS, Hale CA, Bendich A (2003) Calcium absorption varies within the reference range for serum 25-hydroxy Vitamin D. J Am Coll Nutr 22: 142-146.
- Vieth R, Bischoff-Ferrari H, Boucher BJ, Dawson-Hughes B, Garland CF, et al. (2007) The urgent need to recommend an intake of Vitamin D that is effective. Am J Clin Nutr 85: 649-650.
- Oduwole AO, Renner JK, Disu E, Ibitoye E, Emokpae E (2010) Relationship between Vitamin D levels and outcome of pneumonia in children. West Afr J Med 29: 373-378.
- Roth DE, Jones AB, Prosser C, Robinson JL, Vohra S (2009) Vitamin D status is not associated with the risk of hospitalization for acute bronchiolitis in early childhood. Eur J Clin Nutr 63: 297-299.
- Choudhary N, Gupta P (2012) Vitamin D supplementation for severe pneumonia--a randomized controlled trial. Indian Pediatr 49: 449-454.
- Manaseki-Holland S, Maroof Z, Bruce J, Mughal MZ, Masher MI, et al. (2012) Effect on the incidence of pneumonia of Vitamin D supplementation by quarterly bolus dose to infants in Kabul: a randomised controlled superiority trial. Lancet 379: 1419-1427.
- Manaseki-Holland S, Qader G, Isaq Masher M, Bruce J, Zulf Mughal M, et al. (2010) Effects of Vitamin D supplementation to children diagnosed with pneumonia in Kabul: a randomised controlled trial. Trop Med Int Health 15: 1148-1155.
- Camargo CA Jr, Ganmaa D, Frazier AL, Kirchberg FF, Stuart JJ, et al. (2012) Randomized trial of Vitamin D supplementation and risk of acute respiratory infection in Mongolia. Pediatrics 130: 561-567.
Citation: Jat KR, Kaur J, Guglani V (2016) Vitamin D and Pneumonia in Children: A Case Control Study. J Pulm Med Respir Res 2: 004.
Copyright: © 2016 Kana Ram Jat, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.

