
Journal of Practical & Professional Nursing Category: Clinical
Type: Case Report
Zika Virus Disease Detection - A Case Report
*Corresponding Author(s):
Vicki L ChandlerNursing, Health Promotion & Disease Prevention Department, College Of Nursing, University Of Tennessee Health Science Center, Memphis, United States
Tel:+1 901-647-0518,
Fax:+1 901-448-4121
Email:VLChand2@bellsouth.net
Received Date: Aug 21, 2017
Accepted Date: Sep 26, 2017
Published Date: Oct 12, 2017
Abstract
A 36-year-old white male presents to a southeastern, metropolitan clinic complaining of symptoms suspicious for Zika Virus (ZIKV). After a thorough history, physical exam and appropriate laboratory tests, the patient is diagnosed with ZIKV. The ZIKV is mainly transmitted by a bite from an infected Aedes aegyptl or Aedes aegyptl mosquito. The number of cases of ZIKV may be underestimated since symptoms are often mild and self-limiting and health care is not always sought. The most frequent symptoms of ZIKV include fever, arthralgias, maculopapular rash and conjunctivitis. It is increasingly known as the disease that can cause fetal microcephaly in pregnant women infected with the virus. The history and epidemiology of the ZIKV, symptoms, diagnosis, treatment, prevention efforts, and implications for health care providers are discussed in this article.
Keywords
Microcephaly; Mosquito; Pregnancy; Rash; Zika virus infection
INTRODUCTION
A 36-year-old white male presented to a southeastern, metropolitan clinic in the late summer complaining of body aches, especially in the neck and shoulders, sweats, headache and a swollen lymph node just below the right ear lobe for 3 days. He decided to seek medical care when he awakened with a non-pruritic rash on his neck. By the time he was examined the rash had spread to the shoulders, upper chest and back. His past medical history includes hypertension and hypercholesterolemia, for which he takes triamterene/hydrochlorothiazide, 37.5 mg/25 mg and atorvastatin, 20 mg, respectively. He denied tobacco, alcohol or any illicit drug use and has no known food or medication allergies. His occupation is sales related. He returned from a church mission trip to Guatemala five days before he presented to the clinic. His symptoms began two days after his return. He denied sore throat, nasal congestion, cough, appetite loss, nausea or vomiting. He reported that he had slightly loose stools from last 12 hours.
Physical examination revealed normal vital signs (weight 292 pounds, height 74 inches, body mass index 37.5, blood pressure 120/81 mmHg, pulse 74, respirations 16, temperature 98.6), an enlarged, slightly tender right parotid node, a red, confluent maculopapular rash behind the ears radiating to the neck, shoulders, upper chest and upper back. Conjunctiva was unaffected. Heart and lung sounds were normal. Abdominal and testicular exam were normal. The only abnormality on the Complete Blood Count (CBC) was a slightly decreased white blood cell count (3.7). Complete Metabolic Profile (CMP) was unremarkable (Table 1).
Physical examination revealed normal vital signs (weight 292 pounds, height 74 inches, body mass index 37.5, blood pressure 120/81 mmHg, pulse 74, respirations 16, temperature 98.6), an enlarged, slightly tender right parotid node, a red, confluent maculopapular rash behind the ears radiating to the neck, shoulders, upper chest and upper back. Conjunctiva was unaffected. Heart and lung sounds were normal. Abdominal and testicular exam were normal. The only abnormality on the Complete Blood Count (CBC) was a slightly decreased white blood cell count (3.7). Complete Metabolic Profile (CMP) was unremarkable (Table 1).
| Symptoms | Body aches, sweats, headache, swollen lymph node in neck below ear, non-pruritic rash, loose stools |
| Labs | White blood cell count 3.7 (slightly decreased) |
| Complete metabolic profile unremarkable | |
| Physical findings | Red, confluent, maculopapular rash on neck, shoulders, upper chest, upper back |
| Single enlarged, slightly tender parotid lymph node on right | |
| Remainder of exam normal, including conjunctiva |
HISTORY OF ZIKA VIRUS
The Zika Virus (ZIKV) is mainly transmitted by a bite from an infected Aedes aegypti or Aedes aegyptlmosquito [1]. Intrauterine, perinatal, laboratory-acquired and transfusion-associated transmission cases also have been reported [2]. Moreover, it can be transmitted via vaginal or anal sex of an infected person [3]. ZIKV RNA has been found in semen up to 6 months and in vaginal secretions for 11 days [2]. It was first detected in a Rhesus monkey in 1947 in the Zika Forest of Uganda and in a human in Uganda in 1952 [1]. It was considered benign until it caused a large outbreak of mild illness in the Pacific Islands in 2007 [1]. In 2015 the virus invaded Brazil and Latin America with serious effects including microcephaly, brain disorders and other neurological disorders such as Guillain-Barre Syndrome [1]. On February 26, 2016 ZIKV became a reportable condition in the United States by the Council of State and Territorial Epidemiologists [4]. By September 2016 there were 2,382 cases of ZIKV reported in 48 of the 50 states and the District of Columbia in the United States [5]. However, this number excluded infants with congenital ZIKV [5]. Also, the number of cases may be underestimated since the infection is often mild, self-limiting and health care is not sought [5]. In fact, ZIKV does not produce symptoms in 80% of infected individuals [6]. Four states reported half of all cases, which were New York (23%), Florida (20%), California (6%) and Texas (5%) [5]. Local transmission has occurred in Puerto Rico and within a 4.5 square mile area in Miami Beach, Florida [7]. Of the 2,382 cases that were reported, 99% were from direct travel to an endemic area or secondary to sexual intercourse with someone who had travelled to those areas [5]. The areas which were attributed to the most cases of ZIKV transmission are the following: Caribbean (65%), Central America (18%), South America (9%), North America (5%), Southeast Asia, the Pacific Islands and Africa (<1%) [5]. The other 1% of patients with ZIKV contracted the disease locally [8]. Twenty-six patients were bitten by infected mosquitos in Southern Florida, one acquired ZIKV from a needle stick in a laboratory setting, and it is unknown how the other patient contracted the disease [8]. However, the patient did have close personal contact with a family member with the ZIKV. This family member had a 100,000 times higher than average level of viremia and died from septic shock [8]. The most frequent symptoms of ZIKV are fever, arthralgias, maculopapular rash and conjunctivitis [2]. However, it can also cause abdominal pain, constipation or diarrhea, aphthous ulcers and itching [2]. There have been reported neurological effects such as Guillain-Barre syndrome [1]. It is increasingly known as the disease that causes microcephaly during pregnancy, but it causes many other serious problems such as deficiently developed or missing fetal brain structures, miscarriage, stillbirth, hearing and vision defects and poor growth [2].
Diagnosing ZIKV can be challenging because false positive tests can occur, and serologic testing fails to determine when the infection actually occurred [7]. Due to his recent travel to Guatemala and suspecting the patient may have ZIKV, the lab provider was contacted, who recommended obtaining ZIKV RNA QL real time RT PCR, which is only for use under the FDA’s emergency use authorization. It identifies Zika viral RNA, and is usually detectable in serum during the acute phase of infection. The patient had a positive result which indicated current infection. Labs are required to report all positive results to public health authorities. The epidemiologist from the local county health department contacted the clinic and provided a Zika Virus Laboratory Testing Decision Tree for use with future patients suspected of having ZIKV (Figure 1).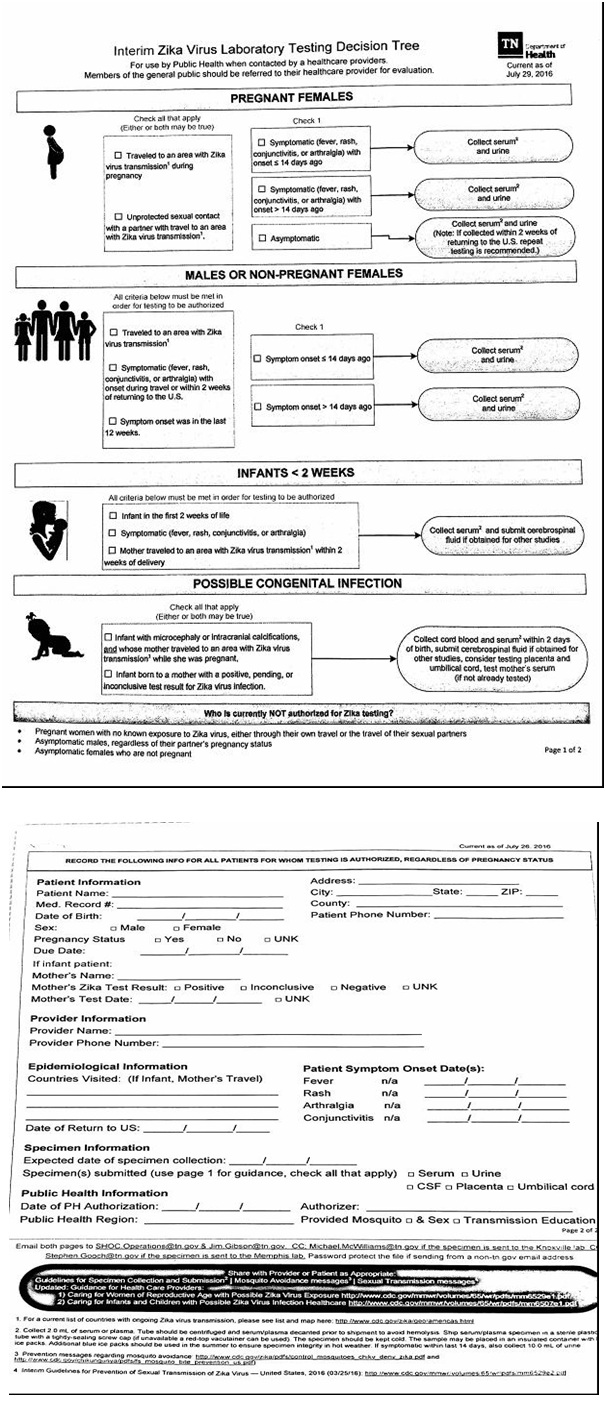
Dengue fever antibody (IgM) and Chikungunya antibody (IgM) were also obtained because of the patient’s symptoms and recent travel to Guatemala. Dengue fever is transmitted to humans by a mosquito bite and can cause a high fever, headache, pain behind the eyes, joint pain, muscle and/or bone pain, rash, mild bleeding of the nose or gums, petechiae, easy bruising and a low white count [7]. It can be found in the Indian subcontinent, Southeast Asia, Southern China Taiwan, the Pacific Islands, the Caribbean (except Cuba and the Cayman Islands), Mexico, Africa, and Central and parts of South America [9]. Chikungunya is also a mosquito-borne alphavirus associated with large outbreaks in Asia, Africa and the Caribbean [10]. Local transmission in the United States has been documented. Symptoms include severe arthritis pain, fever, rash and headache [10]. Both the Dengue and Chikungunya antibody titers were negative.
Diagnosing ZIKV can be challenging because false positive tests can occur, and serologic testing fails to determine when the infection actually occurred [7]. Due to his recent travel to Guatemala and suspecting the patient may have ZIKV, the lab provider was contacted, who recommended obtaining ZIKV RNA QL real time RT PCR, which is only for use under the FDA’s emergency use authorization. It identifies Zika viral RNA, and is usually detectable in serum during the acute phase of infection. The patient had a positive result which indicated current infection. Labs are required to report all positive results to public health authorities. The epidemiologist from the local county health department contacted the clinic and provided a Zika Virus Laboratory Testing Decision Tree for use with future patients suspected of having ZIKV (Figure 1).

Figure 1: Zika virus decision tree.
Negative test results do not rule out ZIKV and should not be used as the sole basis for patient management decisions. Clinical examination, patient history and epidemiological information should also be taken into consideration when caring for a patient suspected of having ZIKV.
Dengue fever antibody (IgM) and Chikungunya antibody (IgM) were also obtained because of the patient’s symptoms and recent travel to Guatemala. Dengue fever is transmitted to humans by a mosquito bite and can cause a high fever, headache, pain behind the eyes, joint pain, muscle and/or bone pain, rash, mild bleeding of the nose or gums, petechiae, easy bruising and a low white count [7]. It can be found in the Indian subcontinent, Southeast Asia, Southern China Taiwan, the Pacific Islands, the Caribbean (except Cuba and the Cayman Islands), Mexico, Africa, and Central and parts of South America [9]. Chikungunya is also a mosquito-borne alphavirus associated with large outbreaks in Asia, Africa and the Caribbean [10]. Local transmission in the United States has been documented. Symptoms include severe arthritis pain, fever, rash and headache [10]. Both the Dengue and Chikungunya antibody titers were negative.
TREATMENT
At this time treatment remains supportive [11]. Acetaminophen or NSAIDS are recommended to control fever and body aches. Adequate hydration and rest is encouraged for the acute infection. For those patients with complications due to ZIKV, such as various neurological manifestations, appropriate treatments are prescribed.
Avoidance to areas where ZIKV has been detected is ideal especially for women of childbearing age, but not always realistic. If travel to ZIKV prone areas is necessary, a mosquito repellant with 20-30% concentration of DEET should be used. Light colored long sleeved clothing and long pants can help prevent mosquito bites. Also, stagnant water removal and rinsing out the container to rid mosquito eggs is recommended. In June 2016 the Food and Drug Administration (FDA) approved 2 newly developed vaccines for ZIKV phase 1 trials in human subjects [12]. The FDA predicts it will take approximately 2-3 years for vaccines to finish clinical trials and be available to be given to women of childbearing age [12]. Researchers predict there may not be enough cases of ZIKV to test the vaccine’s effectiveness by the time the vaccine is available [12]. Some researchers believe the current ZIKV epidemic will be over in the next 1-3 years [12]. A live-attenuated vaccine (10-del ZIKV) is currently being evaluated. A single injection of 10-del ZIKV produced immunity in mice and prevented viremia when challenged with ZIKV [13]. Medications may soon be available to treat active ZIKV. Sofosbuvir, which is used to treat Hepatitis C virus, was discovered to decrease ZIKV levels in infected mice and prevented neuro-motor impairment by inhibiting ZIKV [14]. Ribavirin, another drug used for Hepatitis C, has been found to inhibit ZIKV replication and induce ZIKV cell death in infected mice [15]. In August 2016 the FDA produced a publication for agencies that collect blood in areas with local transmission of the ZIKV by mosquitos stating that the ZIKV was a relevant transfusion-transmitted infection [2]. Specific recommendations such as testing blood donations and screening potential donors for specific ZIKV risk factors were provided to decrease the risk of transmitting the ZIKV via transfusions [2]. For those patients, who developed symptoms of ZIKV, the virus can be spread 3-12 days before symptoms develop [6]. ZIKV can be detected in whole blood up to 58 days after symptoms have begun [2].
Avoidance to areas where ZIKV has been detected is ideal especially for women of childbearing age, but not always realistic. If travel to ZIKV prone areas is necessary, a mosquito repellant with 20-30% concentration of DEET should be used. Light colored long sleeved clothing and long pants can help prevent mosquito bites. Also, stagnant water removal and rinsing out the container to rid mosquito eggs is recommended. In June 2016 the Food and Drug Administration (FDA) approved 2 newly developed vaccines for ZIKV phase 1 trials in human subjects [12]. The FDA predicts it will take approximately 2-3 years for vaccines to finish clinical trials and be available to be given to women of childbearing age [12]. Researchers predict there may not be enough cases of ZIKV to test the vaccine’s effectiveness by the time the vaccine is available [12]. Some researchers believe the current ZIKV epidemic will be over in the next 1-3 years [12]. A live-attenuated vaccine (10-del ZIKV) is currently being evaluated. A single injection of 10-del ZIKV produced immunity in mice and prevented viremia when challenged with ZIKV [13]. Medications may soon be available to treat active ZIKV. Sofosbuvir, which is used to treat Hepatitis C virus, was discovered to decrease ZIKV levels in infected mice and prevented neuro-motor impairment by inhibiting ZIKV [14]. Ribavirin, another drug used for Hepatitis C, has been found to inhibit ZIKV replication and induce ZIKV cell death in infected mice [15]. In August 2016 the FDA produced a publication for agencies that collect blood in areas with local transmission of the ZIKV by mosquitos stating that the ZIKV was a relevant transfusion-transmitted infection [2]. Specific recommendations such as testing blood donations and screening potential donors for specific ZIKV risk factors were provided to decrease the risk of transmitting the ZIKV via transfusions [2]. For those patients, who developed symptoms of ZIKV, the virus can be spread 3-12 days before symptoms develop [6]. ZIKV can be detected in whole blood up to 58 days after symptoms have begun [2].
CONCLUSION
The patient tested positive for ZIKV RNA. The remainder of his illness was uneventful and he did not suffer any complications from contracting the virus. He returned to work a week and a half later. The physician from the local health department called the patient and suggested to use a condom to prevent possible ZIKV exposure to the patient’s wife, stay indoors, empty any standing water outside around his home, and avoid parks and use insect repellent if he had to go outdoors. Health department workers sprayed his neighborhood and set bait around his house for mosquitos. Incidentally, the patient reported upon follow-up that a female in her 60’s on the same mission trip to Guatemala tested positive for ZIKV. She lived in another town and was reportedly much sicker.
Healthcare providers should educate their patients regarding tips to avoid ZIKV. This includes avoidance of areas where mosquito-borne transmission occurs, using insect repellent with at least 20-30% concentration of DEET, wearing long sleeved shirts and long pants, and emptying any containers with standing water. Healthcare providers should also educate their patients regarding the signs and symptoms of ZIKV, which include acute onset of fever, a maculopapular rash, arthralgias, and/or purulent conjunctivitis, and to seek medical attention immediately if symptoms develop and have positive risk factors for contracting the ZIKV [5]. Pregnant patients and women of childbearing age should be educated to avoid travel to affected areas due to the risk of fetal loss, microcephaly, vision or hearing complications or other serious abnormalities of the brain. Also, all patients who are pregnant should be questioned regarding the potential of having been exposed to ZIKV, such as travelling to an area known to have ZIKV or having sex without a condom. Pregnant women with symptoms and possible exposure should be tested for ZIKV [7]. Testing is no longer routinely recommended to pregnant women without ZIKV symptoms who have had a recent ZIKV exposure, but is recommended to pregnant women who have ongoing ZIKV exposure [7]. Patients who have travelled to or live in an area with ZIKV transmission or who have had unprotected sex with an individual who visited those areas or has ZIKV should be tested for ZIKV.
Healthcare providers should educate their patients regarding tips to avoid ZIKV. This includes avoidance of areas where mosquito-borne transmission occurs, using insect repellent with at least 20-30% concentration of DEET, wearing long sleeved shirts and long pants, and emptying any containers with standing water. Healthcare providers should also educate their patients regarding the signs and symptoms of ZIKV, which include acute onset of fever, a maculopapular rash, arthralgias, and/or purulent conjunctivitis, and to seek medical attention immediately if symptoms develop and have positive risk factors for contracting the ZIKV [5]. Pregnant patients and women of childbearing age should be educated to avoid travel to affected areas due to the risk of fetal loss, microcephaly, vision or hearing complications or other serious abnormalities of the brain. Also, all patients who are pregnant should be questioned regarding the potential of having been exposed to ZIKV, such as travelling to an area known to have ZIKV or having sex without a condom. Pregnant women with symptoms and possible exposure should be tested for ZIKV [7]. Testing is no longer routinely recommended to pregnant women without ZIKV symptoms who have had a recent ZIKV exposure, but is recommended to pregnant women who have ongoing ZIKV exposure [7]. Patients who have travelled to or live in an area with ZIKV transmission or who have had unprotected sex with an individual who visited those areas or has ZIKV should be tested for ZIKV.
REFERENCES
- Kindhauser MK, Allen T, Frank V, Santhana RS, Dye C (2016) Zika: the origin and spread of a mosquito-borne virus. Bull World Health Organ 94: 675-686.
- Food and Drug Administration (2016) Revised Recommendations for Reducing the Risk of Zika Virus Transmission by Blood and Blood Components. US Department of Health and Human Services, Food and Drug Administration, Center for Biologics Evaluation and Research, Silver Spring, MD, USA.
- Brooks JT, Friedman A, Kachur RE, LaFlam M, Peters PJ, et al. (2016) Update: interim guidance for prevention of sexual transmission of Zika virus-United States, July 2016. MMWR Morb Mortal Wkly Rep 65: 745-747.
- Council of State and Territorial Epidemiologists (2016) Zika virus disease and congenital Zika virus infection interim case definition and addition to the Nationally Notifiable Disease. Council of State and Territorial Epidemiologists, Atlanta,USA.
- Walker WL, Lindsey NP, Lehman JA, Krow-Lucal ER, Rabe IB, et al (2016) Zika virus disease cases-50 states and the District of Columbia, January 1-July 31, 2016. MMWR Morb Mortal Wkly Rep 65: 983-986.
- Petersen LR, Jamieson DJ, Powers AM, Honein MA (2016) Zika virus. N Engl J Med 374: 1552-1563.
- Oduyebo T, Polen KD, Walke HT, Reagan-Steiner S, Lathrop E, et al. (2017) Update: Interim Guidance for Health Care Providers Caring for Pregnant Women with Possible Zika Virus Exposure-United States (Inclulding U.S. Territories), July 2017. MMWR Morb Mortal Wkly Rep 66: 781-793.
- Brent C, Dunn A, Savage HM, Faraji A, Rubin M, et al. (2016) Preliminary findings from an investigation of Zika virus infection in a patient with no known risk factors-Utah, 2016. MMWR Morb Mortal Wkly Rep 65: 981-982.
- Centers for Disease Control and Prevention (2016) Dengue Fever. Centers for Disease Control and Prevention, Atlanta,USA
- Centers for Disease Control and Prevention (2016) Chikungunya Virus. Centers for Disease Control and Prevention, Atlanta, USA
- Florida Department of Health (2016) Zika Virus. Florida Department of Health, Florida, USA
- Maurice J (2016). The Zika virus public health emergency: six months on. Lancet 388: 449-450.
- Shan C, Murato AE, Nunes BTD, Luo H, Xie X, et al. (2017) A live attenuated Zika virus vaccine candidate induces sterilizing immunity in mouse models. Nat Med 23: 763-767.
- Ferreira AC, Zaverucha-do-Valle C, Reis PA, Barbosa-Lima G, Vierira YR, et al. (2017) Sofosbuvir protects Zika virus-infected mice from mortality, preventing short- and long-term sequelae. Sci Rep 7: 9409.
- Kamiyama N, Soma R, Hidano S, Watanabe K, Umelkita H, et al. (2017) Ribavirin inhibits Zika Virus (ZIKV) replication in vitro and suppresses viremia in ZIKV-infected STAT1-deficient mice. Antiviral Res 146: 1-11.
Citation: Chandler VL, Reed LT, Collins TM (2017) Zika Virus Disease Detection - A Case Report. J Pract Prof Nurs 1: 001.
Copyright: © 2017 Vicki L Chandler, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.

Journal Highlights
© 2025, Copyrights Herald Scholarly Open Access. All Rights Reserved!
