
First Trials on Hatchery and Larval Development of the Sea Cucumber, Holothuria scabra (Jaeger 1883) In Oman
*Corresponding Author(s):
Khalfan M Al-RashdiAquaculture Center, Ministry Of Agriculture And Fisheries Wealth, Muscat, Oman
Tel:+968 24736618,
Email:khalfanrashdi@gmail.com
Abstract
Although sea cucumber Holothuria scabra has always been part of the traditional exploitation of the benthos in Mahout Bay of Arabian Sea, the foreign demand for the product and its high price have put increased pressure on the resource leading rapidly to overfishing. Aquaculture of this species has developed as a response to the overfishing problem but has not been yet studied in Oman. As a first step to evaluate its potential for aquaculture in Oman it was thus necessary to conduct hatchery trials. Four hatchery runs were conducted to evaluate the quality of the local broodstock, the response and efficiency of in vitro maturation and fertilization and the success of larval development and rearing. Collected animals of 200-600g were transported by road for 5 hours with zero evisceration to the Hatchery station. In vitro maturation and fertilization success of more than 90% were achieved using maturation inducing fractions method leading to the development of mature eggs and normal embryos larvae. Auricularialarval stages were completed within 15 days and fed normally on microalgae Phaeodactylum tricornutum, Chaetoceros sp. and Nanochloropsis sp. Settlement rate was reached 9% for pentactula larvae. However, a high mortality of 70% was observed during early larval development caused by ciliated protozoans and copepods attack. The culture of Holothuria scabra population in Oman is thus promising but further research is needed to ensure higher survival rates, particularly in the early larval stages where adequate water filtration and sterilization is essential.
Keywords
INTRODUCTION
The natural spawning of this species in Oman has been recently documented and showed, as in many other locations, an annual reproductive cycle [11]. Natural maturation occurred in February and March followed by spawning in April and May, which is correlated to high sea surface temperature and precipitation as both may serve as exogenous synchronizing cues for gamete maturation and spawning [11]. Alarming signs of the of the Omani population of H. scabra overfishing have been observed with stock densities decreasing to less than 1 individual per ha [10], a regular decrease in the size of animal processed and a progressive switch to less valuable species in only a few years of exploitation. Therefore, restocking and stock enhancement in addition to fisheries management policies are needed to ensure the sustainability of this resource. This article documents the first attempts made to develop hatchery techniques for the Omani sea cucumber Holothuria scabra population as initial steps towards aquaculture development and stock enhancement programs.
MATERIALS AND METHODS
Culture site and system
Broodstock collection and transportation
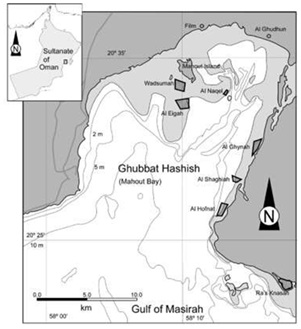 Figure 1: Location of the Holothuria scabra population used for the spawning experiments. Individuals were collected from various locations within Mahout Bay.
Figure 1: Location of the Holothuria scabra population used for the spawning experiments. Individuals were collected from various locations within Mahout Bay.Induced maturation and fertilization
Body fluid was drained and the gonads of males and females were gently removed using forceps and placed in separate beakers with filtered sea water. Gonads were then cut separately into small pieces using scissors to release oocytes and sperm cells from gonadal tubules. The oocytes were then sieved on 60µm mesh with filtered sea water and placed in petri dishes at concentrations of 100 oocytes/ml. Maturation of the oocytes was induced with the MIF solution for 2 hours, and then rinsed with filtered sea water to remove MIF residuals. Fertilization was achieved by adding few drops of sperm suspension to the mature oocytes. The fertilized eggs were kept in a shallow basin without aeration until hatching of gastruled embryos. Observations and photography of embryonic development stages were carried out on random samples every 30-60 minutes using a light microscope at 40X magnification.
Larval development and rearing
H. scabra larvae were fed daily from the second day PF with different types of micro-algae until settlement. Three mixed algae were cultured under laboratory conditions with artificial light and aeration. A temperate species Phaeodactylum tricornutum was cultured at low temperature from 19 to 24°C in the laboratory. Chaetoceros sp. and Nanochloropsis sp. were cultured both under laboratory and outdoor conditions (29-30°C) and fertilized with Guillard’s F/2 medium. Initially, the developing larvae were fed twice daily with P. tricornutum at a final concentration of approximately 2500 cells per liter. On day 8 PF, the final concentration of P. tricornutum was increased to 5000 cells per liter. During the Doliolaria stage (a non-feeding stage), the feeding was temporarily suspended and resumed after metamorphosis (typically on day 20 PF). At this stage both microalgae (Chaetoceros, Nanochloropsis and Phaeodactylum) and particulate diet (dried and powdered Sargassum sp.) were added to favor settlement and feed the newly settled juveniles.
RESULTS
Maturation induction and fertilization
|
Fertilization number |
Dates |
Broodstock size (g) |
Fertilized oocytes |
Produced larvae |
|
|
Male |
Female |
||||
|
F1 |
September 12th, 2009 |
287 (1) |
287&346 (2) |
160000 |
139200 |
|
F2 |
April 15th, 2010 |
245 (1) |
457&375 (2) |
140000 |
96000 |
|
F3 |
July 13th, 2010 |
319 (1) |
320&425 (2) |
85000 |
59,766 |
|
F4 |
November 17th, 2010 |
200&260 (2) |
600&375 (2) |
120000 |
105,400 |
|
Total |
505000 |
400366 |
|||
Embryonic and larval development
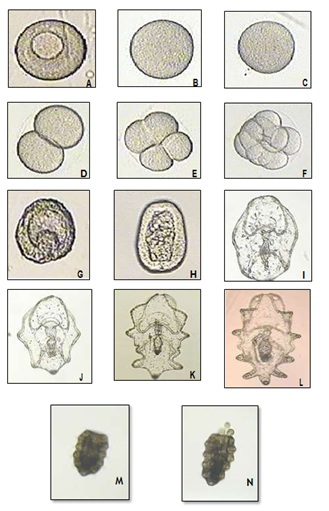 Figure 2: A. Immature oocyte; B. Mature oocyte; C. Fertilized egg; D. Two cell stage (1 h 30 mn); E. Four-cell stage (2 h); F. Multi- cells (3 h); G. Blastula (12 h); H. Gastrula (16 h); I. Newly hatched Auricularia (36 h); J. Early Auricularia (2.5 d); K. Mid Auricularia (6 d); L. Late Auricularia (13 d); M. Doliolaria (15); N. Early Pentactula with tentacles (20).
Figure 2: A. Immature oocyte; B. Mature oocyte; C. Fertilized egg; D. Two cell stage (1 h 30 mn); E. Four-cell stage (2 h); F. Multi- cells (3 h); G. Blastula (12 h); H. Gastrula (16 h); I. Newly hatched Auricularia (36 h); J. Early Auricularia (2.5 d); K. Mid Auricularia (6 d); L. Late Auricularia (13 d); M. Doliolaria (15); N. Early Pentactula with tentacles (20).There was a high mortality during the first few days PF during all four trials. With the exception of trial 1 for which there were no microalgae to feed the larvae, from day 4 to day 15 mortality remained quite low. A second mortality was observed between day 15 and day 21 in all three remaining trials (Figure 3). During the daily microscopic observations of the larvae, abundant ciliate protozoans and copepods were observed in the larval tanks. However, after the 4th day PF, survival was stable.
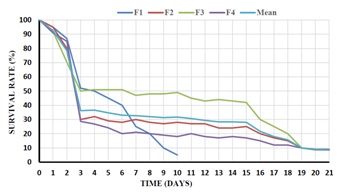 Figure 3: Survival rates of H. scabra over 4 hatchery runs and the mean of the 3 runs during 21 days of embryonic and larval development and rearing.
Figure 3: Survival rates of H. scabra over 4 hatchery runs and the mean of the 3 runs during 21 days of embryonic and larval development and rearing.DISCUSSION
There are several national management measures applied on this fishery, including total ban as the current case here in Oman; however, these seem to be insufficient for a sustainable use of the resources [6]. A sustainable and efficient solution so far to enhance production as well as depleted natural stock of this species is through developing aquaculture [5]. To ensure H. scabra aquaculture sustainability and affordability, the production of fertilizable eggs and larvae in large quantities and at any time is a key element [14]. Several methods have been described to induce H. scabra spawning, mainly thermal shock [15-17] alone or combination with water pressure and drying treatments [18]. Spawning can also be induced using chemical such as spirulina. However, these methods may not be reliable to produce mature fertilizable oocytes outside the natural reproductive season of the organism [14,19]. Such critical point has been addressed through a new methodology based on in vitro maturation using artificial inducers [13,14] which recently have been identified [20]. The use of such maturation inducers allows holothurians farmers to obtain clean, fertilized eggs year-round. This method was tested in Oman H. scabra population and achieved more than 90% oocyte maturation and fertilization, which is consistent with Léonet et al. [13] and Eeckhaut et al. [14], results on Madagascar H. scabra populations. The method requires the sacrifice of several large adult specimens which may be difficult to justify for a locally endangered species, although these few specimens will produce 1000s of juveniles [21]. Despite larval developmental timing variations among various H. scabra populations, the embryos underwent normal development processes up to the pentactula stage over 21 days PF which is consistent with published values for larval development in H. scabra as it is reviewed by Junus et al. [3].
The high mortality observed during the first few days PF took place before feeding and is thus unlikely the result of a contamination by the feeding solutions. However, the abundant copepods and ciliates may be responsible for this temporary mortality [22]. Copepods attack larvae either directly or by repeated collisions causing bodily damage to the larvae [15]. Asha & Muthia [23] observed 65% mortality due to ciliates on the 9th day of rearing H. spinifera larvae. Since the water used for larval rearing was filtered and UV-treated, it is unlikely the source of the copepods and ciliates, but some of the equipment used to siphon the water, transfer the larvae from one container to another, the container themselves may have been contaminated by a concomitant fish hatchery and emphasize the need for high levels of training in sterilization and decontamination and a sepsis. Unfortunately, separating copepods from sea cucumber larvae is difficult as their sizes are similar [24]. To control copepod population, Pitt & Duy [15] recommended the application of trichlorofon (Dipterex), an insecticide which may be effective against some arthropod infestation. Alternative solutions using either naturally occurring substances or better management practices are needed [25]. In addition, optimization of the diet using locally available species is also necessary [26].
CONCLUSION
ACKNOWLEDGMENT
REFERENCES
- Purcell SW, Hair CA, Mills DJ (2012) Sea cucumber culture, farming and sea ranching in the tropics: Progress, problems and opportunities. Aquaculture 368-369: 68-81.
- Conand C (2004) Present status of world sea cucumber resources and utilisation: An international overview. Advances in sea cucumber aquaculture and management Pg no: 14-18.
- Junus S, Kwong JP, Khoo G (2018) A review on the recent advances in the biology and aquaculture technology of Holothuria scabra. Journal of Survey in Fisheries Sciences 4: 5-25.
- Purcell SW, Williamson DH, Ngaluafe P (2018) Chinese market prices of beche-de-mer: Implications for fisheries and aquaculture. Marine Policy 91: 58-65.
- Hamel JF, Conand C, Pawson DL, Mercier A (2001) The sea cucumber Holothuria scabra(Holothuroidea: Echinodermata): Its biology and exploitation as Beche-de-mer. Advances in Marine Biology 41: 129-223.
- Lovatelli A, Conand C, Purcell S, Uthicke S, Hamel JF, et al. (2004) Advances in sea cucumber aquaculture and management, FAO Fisheries Technical Paper 463, Rome, Italy.
- Eriksson H, de la Torre-Castro M, Purcell S, Olsson P (2014) Lessons for resource conservation from two contrasting small-scale fisheries. Ambio 44: 204-213.
- Watanabe S, Sumbing JG, Lebata-Ramos MJ (2014) Growth pattern of the tropical sea cucumber, Holothuria scabra, under captivity. JARQ 48: 457-464.
- Al-Rashdi KM, Al-Busaidi SS, Al-Rassadi IH (2007) Status of the sea cucumber fishery in the Sultanate of Oman. SPC Beche-de-mer Information Bulletin 25: 17-21.
- Al-Rashdi KM, Claereboudt MR (2010) Evidence of rapid overfishing of sea cucumbers in the Sultanate of Oman. SPC Beche-de-mer Information Bulletin 30: 10-13.
- Al-Rashdi KM, Claereboudt MR (2018) Reproductive biology of the sea cucumber Holothuria scabra(Jaeger 1883) in Mahout Bay, Arabian Sea, Oman. International Journal of Fisheries and Aquatic Studies 6: 100-108.
- Al-Rashdi KM (2012) Project final report on diversity, stock and aquaculture potential of sea cucumber in Oman. Ministry of Agriculture and Fisheries Wealth, Aquaculture Centre, Muscat, Sultanate of Oman. Pg no: 150.
- Léonet A, Rasolofonirina R, Wattiez R, Jangoux M, Eeckhaut I (2009) A new method to induce oocyte maturation in holothuroids (Echinodermata). Invertebrate Reproduction and Development 53: 13-21.
- Hair CA, Pickering TD, Mills DJ (2012) Asia-Pacific tropical sea cucumber aquaculture: Proceedings of an international symposium held in noumea, New Caledonia, 15-17 February, 2011. ACIAR Proceedings 136: 1-209.
- Pitt R, Nguyen DQD (2004) Breeding and rearing of the sea cucumber Holothuria scabra in Vietnam. FAO Fisheries Tech Pap 463: 333-346.
- Duy NDQ (2010) Seed production of sandfish (Holothuria scabra) in Vietnam. Aquaculture Department, Southeast Asian Fisheries Development Center, Iloilo, Philippine.
- Hair C, Kaure T, Southgate PC, Pickering T (2011) Potential breakthrough in hatchery culture of Sandfish Holothuria scabra by using algal concentrate as food. SPC Bêche-de-mer Inf. Bull 31: 60-61.
- Agudo N (2007) Sandfish Hatchery Techniques. Australian Center for International Agricultural Research (ACIAR), Thynne St, Australia. Pg no: 42.
- Battaglene SC, Seymour JE, Ramofafia C, Lane I (2002) Spawning induction of three tropical sea cucumbers, Holothuria scabra, H. fuscogilva and Actinopyga mauritiana. Aquauculture 207: 29-47.
- Léonet et al. (2019) in press.
- Purcell SW, Ngaluafe P, Foale SJ, Cocks N, Cullis BR, et al. (2016) Multiple factors affect socioeconomics and wellbeing of artisanal sea cucumber fishers. PLoS One 11: 0165633.
- James BD (2004) Captive breeding of the sea cucumber, Holothuria scabra, from India. FAO Fisheries Technical Paper 463: 385-398.
- Asha PS, Muthia P (2002) Spawning and larval rearing of sea cucumber Holothuria (Theelothuria) spinifera Theel. SPC Beche-de-mer Information Bulletin 16: 11-15.
- James BD, Rajapandian ME, Basker BK, Gopinathan CP (1988) Successful induced spawning and rearing of the holothurian Holothuria (Metriatyla) scabra Jaeger at Tuticorin. Marine Fisheries Infor Ser 87: 30-33.
- McLean E, Ibrahim FS, Al-Rashdi KM (2011) Better management practices for Omani aquaculture. Ministry of Agriculture & Fisheries Wealth, Aquaculture Centre, Muscat, Sultanate of Oman. Pg no: 80.
- Militz AT, Leini E, Duy ND, Paul GS (2018) Successful large-scale hatchery culture of sandfish (Holothuria scabra) using micro-algae concentrates as a larval food source. Aquaculture Reports 9 :25-30.
Citation: Al-Rashdi KM, Claereboudt MR, Eeckhaut I (2019) First Trials on Hatchery and Larval Development of the Sea Cucumber, Holothuria scabra (Jaeger 1883) In Oman. J Aquac Fisheries 3: 014
Copyright: © 2019 Khalfan M Al-Rashdi, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.

