
Maintenance of Two-Spotted Goby, Gobiusculus flavescens (Fabricius, 1779) (Perciformes, Gobiidae), In Captivity as a Resource for Ornamental Fishkeeping
*Corresponding Author(s):
Paulo MaranhãoMarine And Environmental Sciences Centre, ESTM, Polytechnic Institute Of Leiria, Peniche, Portugal
Tel:+351 262783607/ +351 919935275,
Email:pmaranhao@ipleiria.pt
Abstract
The survival and growth of semipelagic two-spotted goby (Gobiusculus flavescens) were investigated in the laboratory to appreciate the ability of this species to be kept in captivity as a resource for ornamental fishkeeping. The survival of individuals was tested under three temperatures (23°C, 25°C and 27°C) during 12 days. The growth of individuals was tested using two diets: Diet 1-“SERA®vipan” + “SERA®discus granulat” and Diet 2-“Mysis and Artemia brine shrimp” (Ocean Nutrition®), at 21°C, during 5 weeks. The low death rate at 23°C (10%) can indicate some potential for this species to be used in ornamental aquariophilia. The diet consisting of Mysis sp. and Artemia sp. brine shrimp (Diet 2) appears to be well accepted be the fishes and promotes a good growth. The two-spotted goby proved to be a species very easy to handle and to maintain at the appropriate temperatures.
Keywords
INTRODUCTION
Although there is no accurate information regarding the figures of the aquarium industry in the international scenario, it is estimated to generate revenues over US$ 300 million with an annual growth rate of 14%. Approximately US$ 28 to 44 million of this amount is generated by the ornamental marine fish trade. However, the trade in marine species has aroused controversy regarding the extraction of almost all traded marine organisms from wild populations and, in many cases, illegally [3]. The cyanide is a toxic chemical used to capture aquarium fish. The use of cyanide on coral reefs to capture aquarium fish is illegal and cause damage in fish as well in coral reef. The use of cyanide to capture juvenile angelfish in Komodo, Indonesia, is an example of an illegal capture of aquarium fish and cause of damage in ambience [4].
In recent years, the acquisition of marine ornamental fish has greatly increased. Recent advances in fish husbandry and aquarium equipment technology have further facilitated the hobby. The vast majority of ornamental fishes in the aquarium trade is of freshwater origin and farm-raised. Colombia, Peru and Brazil are the countries that export more wild-caught freshwater aquarium fish [5].
The purpose of managing a fishery is to maintain the resource so that it is renewable and therefore sustainable. Responsible aquaculture practices rely on sustainable production systems, to minimize impacts on the natural environment, and support resource conservation. In other words, the harvest of fish from the wild or their domestic culture, if performed with sound foundations in ecological and economic principles, can be sustainable and self-reliant commercial industries [6]. Cultivation in captivity can help sustain the marine ornamental industry and mitigate the impact to the environment, thus reducing the need of collecting wild specimens of natural populations [7]. To adapt and standardize techniques of cultivation and propagation for each species or group of organisms has been achieved, will achieve greater diversification of species that meets the demand of the market and thus the decrease in the volumes of extraction [7,8].
Studies related to the biology of the species of interest, new techniques of cultivation, determination of alternative live foods, systems of water recycling and the state of health of cultivated organisms, may allow the production of organisms of higher quality than the extracted from the natural environment [9,10].
In that sense, it is important to understand if there are new species that are able to keep in captivity, and what are their requirements in terms of environmental conditions and food. It is also necessary to evaluate the possibility of cultivating these species in order to minimize the impacts on the ecosystem.
The two-spotted goby, Gobiusculus flavescens, is a common fish species along rocky shores in northern European waters [11]. It is a small (40-60 mm), semi pelagic marine fish [12], forming loose shoals in association with macroalgal vegetation and mussel beds growing on the rock surface [13]. It is a short-lived species, with a life span of 1-2 years [14]. Both sexes display courtship behavior and have sexual ornamentation during the breeding season. Male ornaments consist of large dorsal fins with iridescent blue lines, and iridescent blue spots along the sides of the body [15]. Females develop a conspicuous, bright orange belly at sexual maturity [16]. Due to these characteristics, this species could have a great interest for ornamental aquariums.
The process of keeping and breeding ornamental marine and freshwater fish is becoming one of the fast growing hobbies in the world. One of the biggest problems that beginners have is maintaining the temperature of the aquariums. Fishes are ectotherms, meaning their body temperature depends on ambient temperature. The fish’s body temperature remains more or less the same of water. Many of the ornamental fish do not support large changes in the environmental temperatures, which make them more vulnerable.
The aim of this study was to test the survival of adults two-spotted gobies reared under three different temperatures and to test the growth of individuals under two different diets at 21°C.
MATERIALS AND METHODS
The survival of individuals was tested under three temperatures, were groups of ten fishes (five males and five females) were submitted to 23°C, 25°C and 27°C maintained with thermostats (100 w Aquapor) during 12 days. The three experimental aquaria had a water volume of 20 L.
The growth of individuals was tested using two diets: Diet 1-“SERA®vipan” + “SERA®discus granulat” and Diet 2-“Mysis and Artemia brine shrimp” (Ocean Nutrition®), at 21°C. This experiment had a system consisting of six aquariums of 5 L each: Three for Diet 1 and other three for Diet 2. In each aquarium were placed five fishes. The fishes were weighed and measured (total length) once a week, during five weeks. Total length was measured on a millimeter grid to nearest 0.5 mm and wet body mass was determined to an accuracy of 0.01 g using an Ohaus Scout Pro Scale, after carefully blotting the fish to remove excess water. The fish were anaesthetized with 25 ml of 2-phenoxy-ethanol diluted in 1000 ml of sea water. After the measurement and weighting, each fish was transferred to fresh sea water.
During the experiments, all the aquaria were cleaned once a week and the water quality monitored regularly (pH, ammonia, nitrite and nitrate, dissolved oxygen, salinity, kH and phosphates). Feeding (ad libitum) and temperature control were made once a day.
For the two diets, the differences between the initial and final length and weight of fishes were tested with the Student’s test [17]. The measuring of the relationship between weight and length was conducted by simple linear regression analysis [17]. Where applicable, results are presented as mean±Standard-Deviation (SD). For all statistical tests, the significance level was set at P≤0.05. All calculations were performed with IBM SPSS Statistics 25.
RESULTS
|
Water temperature |
Fishes (N) |
Dead fishes (N) |
Death rate (%) |
|
23°C |
10 |
1 |
10 |
|
25°C |
10 |
5 |
50 |
|
27°C |
10 |
7 |
70 |
|
Experience |
Fishes (N) |
Dead fishes (N) |
Death rate (%) |
|
Diet 1 |
15 |
4 |
27 |
|
Diet 2 |
15 |
2 |
13 |
No statistically significant differences were found in initial weight and initial length of the fishes between the two diets at P > 0.05 level (initial weight: t (28) = 0.174; initial length: t (28) = -0.249; figures 1 and 2). Statistically significant differences were found in the final fish weight between the two diets (t (22) = -2.818, P = 0.01 < 0.05; figure 1), but not in the final length (t (22) = -0.697, P > 0.05; figure 2).
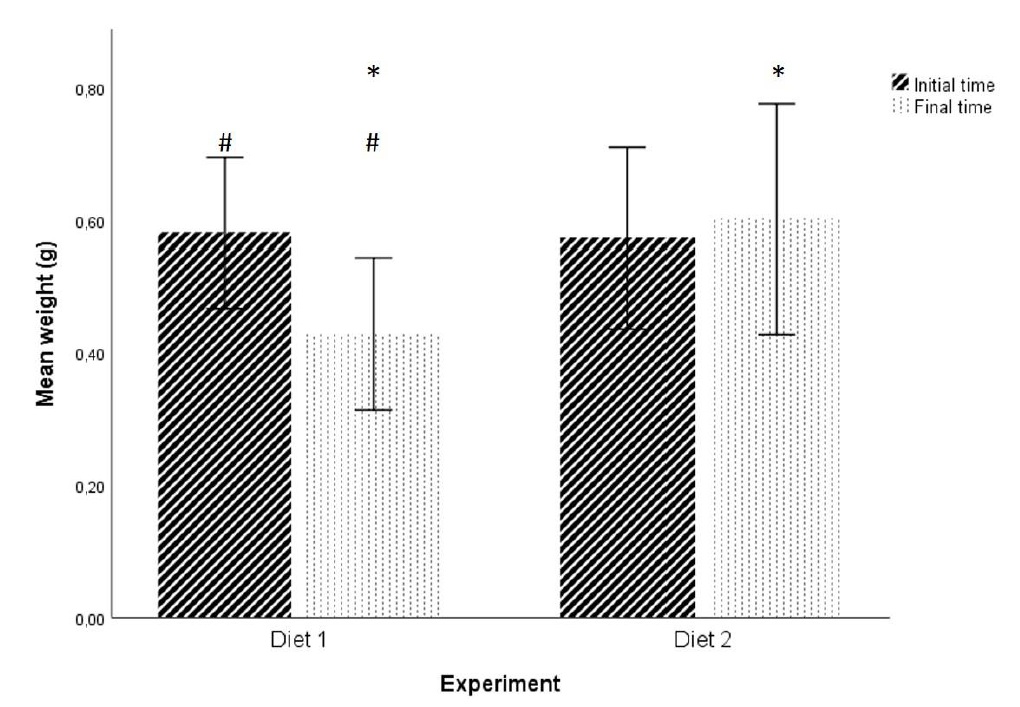 Figure 1: Effect of the two diets on mean weight of individuals. (* and #) Significant differences in planned comparisons (P < 0.05, Student’s test). Results are presented as mean ± SD.
Figure 1: Effect of the two diets on mean weight of individuals. (* and #) Significant differences in planned comparisons (P < 0.05, Student’s test). Results are presented as mean ± SD.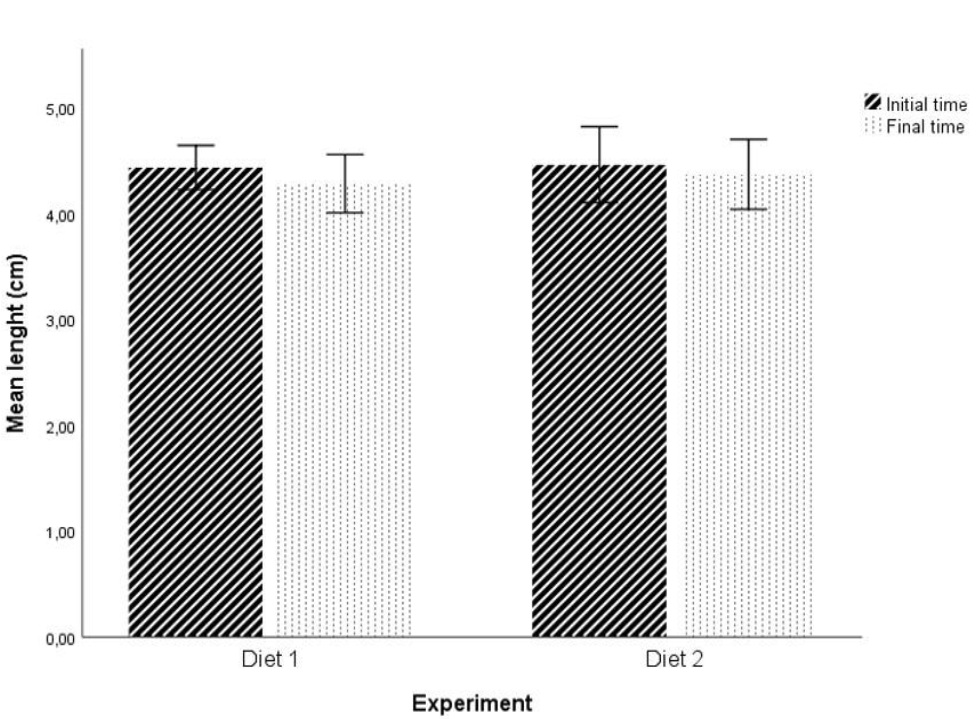 Figure 2: Effect of the two diets on mean length of individuals. Results are presented as mean ± SD.
Figure 2: Effect of the two diets on mean length of individuals. Results are presented as mean ± SD.In addition, for Diet 1, there were statistically significant differences between the initial weight and the final weight of the fish (t (24) = 3.355, P = 0.003 < 0.05; Figure 1). No statistically significant differences were found for the length (t (24) = 1.598, P > 0.05; Figure 2). For Diet 2 there were no statistically significant differences in any of the factors under study (weight and length) (weight: t (26) = -0.493, P > 0.05; length: t (26) = 0.693, P > 0.05; Figures 1 and 2).
A simple linear regression analysis was conducted and the results showed that 61 % (R2) of the variability of the data could be explained by the linear regression (r = 0.78) (Figure 3). Thus, length and weight of individuals are strongly and positively correlated, and for every 1 cm increase in length, the weight will increase on average by 0.37 g. On the other hand, the proportion of common variance shared between length and weight with Diet 1 is 49.3 % (r = 0.70) while for Diet 2 this coefficient is particularly higher, i.e., R2 = 73.5 % (r = 0.86). Therefore, both correlation coefficients confirm that the length and the weight are positively correlated, although for Diet 2 the strength of the relationship is characterized by better-quality correlation.
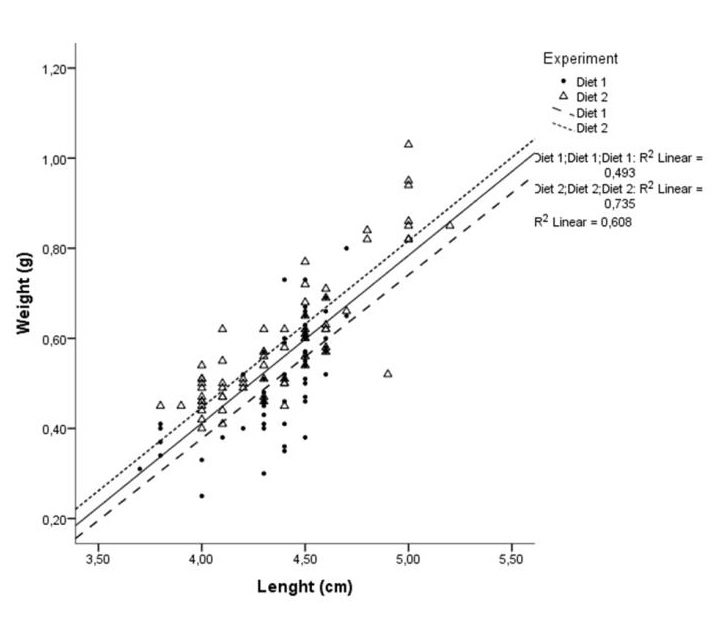 Figure 3: Scatter plot of the weight versus the length. The black regression line has the intercept equal to -1.1 and slope equal to 0.37. Effects of both the intercept and slope are statistical significant (P < 0.05), therefore there is a significant linear association between weight and length (correlation coefficient, r = 0.78 and R2 = 60.8%). In addition, for Diets 1 and 2, the correlation coefficient confirm that the length and the weight are correlated strongly and positively (R2Diet1 = 49.3 %; rDiet1 = 0.70 and R2Diet2 = 73.5 %; rDiet2 = 0.86).
Figure 3: Scatter plot of the weight versus the length. The black regression line has the intercept equal to -1.1 and slope equal to 0.37. Effects of both the intercept and slope are statistical significant (P < 0.05), therefore there is a significant linear association between weight and length (correlation coefficient, r = 0.78 and R2 = 60.8%). In addition, for Diets 1 and 2, the correlation coefficient confirm that the length and the weight are correlated strongly and positively (R2Diet1 = 49.3 %; rDiet1 = 0.70 and R2Diet2 = 73.5 %; rDiet2 = 0.86).DISCUSSION
The maintenance in captive of wild individuals, seeks to provide them with the adequate conditions for their survival. To ensure that these conditions are the best and most appropriate are necessary studies on the type of habitat, what temperatures provide fishes the better performance, what physical and chemical parameters allow them to have a higher survival rate and what is the best food in order to achieve a healthy growth. The two-spotted goby is a planktivorous species, and in the wild, the species feeds on small crustaceans such as copepods, amphipods and mysids [19,20]. There are several types of food available in aquarium trade that allows a better selection of food to be given to the individuals kept in captivity. Compared the two diets used, it was observed that diet consisting of Mysis sp. and Artemia sp. brine shrimp (Diet 2) appears to be well accepted be the fishes and promotes a good growth. This result can be explained by the fact that individuals are already accustomed to this kind of food, because, despite being inert, is the same type of food to which they are accustomed to eating in the wild [21]. The results obtained for the diet consisting of commercial flakes and granules (Diet 1) reflect the reactions observed when individuals were fed. The different appearance and texture of the food items may have resulted in the rejection of food by individuals. An appropriate diet allows obtaining improved growth and development of G. flavescens [22], which seems to be the case of our Diet 2, allowing its maintenance and growth in captivity.
The temperatures used could be a limitation to the use of this species on ornamental fishkeeping. However, the two-spotted goby proved to be a species very easy to handle and to maintain at the appropriate temperatures. The low death rate at 23°C can indicate some potential for this species to be used in ornamental fishkeeping.
In the future, it is interesting to do more studies about this specie. How the specie breeding and what is the optimum conditions to do that. It is interesting know how the temperature influence the courtship and the larvae development. In the future, it is important to develop a commercial food adequate for this specie.
ACKNOWLEDGEMENT
REFERENCES
- FAO (1996-2005) The numbers represent the average unit value of imports for 1994-2003. FAO, USA. Pg no: 83-97.
- Chapman FA, Fitz-Coy SA, Thunberg EM, Adams CM (1997) United States of America trade in ornamental fish. Journal of the World Aquaculture Society 28: 1-10.
- Reynoso FL, Castaneda-Chavez M, Zamora-Castro JE, Hernandez-Zarate G, Ramirez-Barragan MA, et al. (2012) Ornamental marine fishkeeping: A trade of challenges and opportunities. Latin American Journal of Aquatic Research, 40: 12-21.
- Wood EM (2001) Collection of coral reef fish for aquaria: Global trade, conservation issues and management strategies. Marine Conservation Society, UK. Pg no: 80.
- Chapman FA (2000) Ornamental fish culture, freshwater. In: RR Stickney (ed.). Encyclopedia of Aquaculture. John Wiley & Sons Inc, New York, USA.
- Vagelli AA, Erdmann MV (2002) First comprehensive ecological survey of the Banggai cardinal fish, Pterapogon kauderni. Environmental Biology of Fishes 63: 1-8.
- Tlusty M (2002) The benefits and risks of aquacultural production for the aquarium trade. Aquaculture 205: 203-219.
- Setu SK, Kumar TTA, Balasubramanian T, Dabbagh AR, Keshavarz M (2010) Breeding and rearing of regal damselfish Neopomacentrus cyanomos (Bleeker, 1856): The role of green water in larval survival. World Journal of Fish and Marine Sciences, 2: 551-557.
- Rajasekar J, Kant-Setu S, Ajithkumar TT, Balasubramanian T (2009) Development of hatchery technology for marine ornamental damsel fishes with special reference to in captivity. World Journal of Agricultural Sciences 5: 466-469.
- Olivotto I, Planas M, Simões N, Joan-Holt G, Alessandro-Avella M, et al. (2011) Advances in breeding and rearing marine ornamentals. Journal of the World Aquaculture Society, 42: 135-166.
- Miller PJ (1986) Gobidae. In: Whitehead PJP, Bauchot ML, Hureau JC, Nielsen J, Tortonese E, (eds.). Fishes of Northeastern Atlantic and the Mediterranean, UNESCO, Bungay, England.
- Wacker S, de Jong K, Forsgren E, Amundsen T (2012) Large males fight and court more across a range of social environments: An experiment on the two spotted goby Gobiusculus flavescens. J Fish Biol 81: 21-34.
- Folkstead H (2005) Stage dependent habitat use under conflicting predation pressure: An experimental test with larval and juvenile two-spotted gobies, Gobiusculus flavescens Fabricius. Journal of Experimental Marine Biology and Ecology 323: 160-171.
- Borg A, Forsgren E, Amundsen T (2006) Seasonal change in female choice for male size in the two-spotted goby. Animal Behavior 72: 763-771.
- de Jong K, Wacker S, Amundsen T, Forsgren E (2009) Do operational sex ratio and density affect mating behaviour? An experiment on the two-spotted goby. Animal Behavior 78: 1229-1238.
- Svensson PA, Forsgren E, Amundsen T, Sköld HN (2005) Chromatic interaction between egg pigmentation and skin chromatophores in the nuptial coloration of female two-spotted gobies. Journal of Experimental Marine Biology and Ecology 208: 4391-4397.
- Zar HJ (2010) Biostatistical analysis, (5th edn). Prentice-Hall, New Jersey, USA.
- Frommel A, Clemmesen C (2009) Use of biochemical indices for analysis of growth in juvenile two-spotted gobies (Gobiusculus flavescens) of the Baltic Sea. Scientia Marina 73: 159-170.
- Costello MJ, Edwards J, Potts GW (1990) The diet of the two-spot goby, Gobiusculus flavescens(Pisces). Journal of the Marine Biological Association of the United Kingdom 70: 329-342.
- Ehrenberg SZ, Hansson S, Elmgren R (2005) Sublittoral abundance and food consumption of Baltic gobies. Journal of Fish Biology 67: 1083-1093.
- Thetmeyer H (1997) Diel rhythms of swimming activity and oxygen consumption in Gobiusculus flavescens (Fabricius) and Pomatoschistus minutus (Pallas) (Teleostei: Gobiidae). Journal of Experimental Marine Biology and Ecology 218: 187-198.
- Thetmeyer H, Kils U (1995) To see and not be seen: The visibility of predator and prey with respect to feeding behaviour. Marine Ecology Progress Series 126: 1-8.
Citation: Teles V, Silva A, Mendes S, Maranhão P (2019) Maintenance of Two-Spotted Goby, Gobiusculus flavescens (Fabricius, 1779) (Perciformes, Gobiidae), In Captivity as a Resource for Ornamental Fishkeeping. J Aquac Fisheries 3: 018.
Copyright: © 2019 Vera Teles, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.

