
Population Biology of Manningis arabicum (Jones & Clayton, 1983; Decapoda: Brachyura, Ocypodidae) from Umm SA Mangrove Swamps, Qatar
*Corresponding Author(s):
Ralf RiedelGulf Coast Research Laboratory, University Of Southern Mississippi, Ocean Springs, Mississippi, United States
Tel:+1 2282150090,
Email:Ralf.Riedel@usm.edu
Abstract
Keywords
INTRODUCTION
Studies involving brachyuran crabs from the Arabian Gulf region have mostly focused on taxonomy. Early studies from the Arabian Gulf were reported by Stephensen [5] and Tirmizi [6]. Jones & Clayton [3], reported 17 species of tropical burrowing crabs along the coast of Kuwait and Al-Khayat & Jones [7], reported on new Brachyurans from the genera Manningis and Leptochryseus. Crabs were of the families Grapsidae and Ocypodidae, including 2 new species of the genera Cleistostoma and Paracleistostoma.
Although taxonomic studies have demonstrated the high diversity of brachyuran crabs along the coasts of the Arabian Gulf, few attempts have been made to examine the dynamics of their population. Population biology studies are of immediate importance, as brachyurian crabs in the Arabian Gulf inhabit mangrove swamps and intertidal mud flats, largely threatened environments [8]. An understanding of trends of crab populations in such environments may serve well in using M. arabicum as indicators of habitat health.
Of the few studies of Gulf of Arabia crab biology, Jones & Clayton [3], examined ecological relations among crabs of the genera Cleistostoma and Paracleistostoma on Kuwait mudflats. Clayton & Al-Kindi [9], studied the population structure and dynamics of Scopimera crabricauda in Omani estuaries. Similarly, Snowden et al. [10], studied the population biology of Ilyoplax stevensi, also on mudflats in Kuwait. Our research will provide additional information on Brachiuran crab population biology for environmental conservation efforts.
This study is the first for characterizing the population biology of M. arabicum from Qatar mangroves. Analyses of selected biological aspects of the crab M. arabicum were evaluated to better understand the interplay between this species and its environment. Such understanding may assist in developing future indices of habitat health using this species as an indicator.
MATERIALS AND METHODS
Monthly random samples of M. arabicum were collected from intertidal areas of Umm SA mangrove swamps between October 2013 and September 2014 (Figure 1). Swamps located in the study area are predominated by Avicennia marina (grey mangrove) and secondarily by Rhizophora mucronate, which provide nursery ground and cover for a vast array of fish, sea snakes, turtles, and birds.
 Figure 1: Sampling location in the Umm SA mangrove swamps, Qatar.
Figure 1: Sampling location in the Umm SA mangrove swamps, Qatar.
Air and surface water temperatures were recorded monthly using a mercury bulb thermometer. Salinity was determined using a portable salinometer and pH with a portable meter. Samples of the top 5 cm of sediment were collected for analysis of grain size, organic matter, and moisture content. Grain size was determined according to Buchanan [11]. Organic matter content was determined measuring the weight loss after 6hrs at 450?.
Crabs were collected during low tide from their burrows using a shovel. Crabs were sexed from their abdominal morphology and number of pleopods. Egg bearing females were recorded. Carapace width to the nearest 0.05 mm was measured with a Vernier caliper (CW=greatest Cephalothorax Width), and individual weights recorded to the nearest 0.001g.
Descriptive data on abundance and size by season and gender were recorded to examine crab population size structure. Sex ratios and proportion of egg-bearing females were determined to infer reproductive season and potential.
Carapace width data was grouped into 5 mm classes for estimation of population growth parameters. The von Bertalanffy Growth Function (VBGF) was used to describe growth. Growth parameters were estimated using FiSAT II [12] and ELEFAN-1 [13]. Estimated parameters were the asymptotic length (L∞) and the growth coefficient (K), the latter estimated using the K-scan routine to determine reliable estimate of the K value. The estimates of L∞ and K were used to estimate the φ' growth performance index [14] of M. arabicum, based on the equation φ'=2 log10 L∞+log10 K.
Crab carapace Width-Individual Weight Relationships (W-W) were calculated to describe body allometry as an indicator of population condition [15-18]. Estimates of W-W were obtained by gender and season. To assess allometry, the linear form of the length-weight relation power function was used [19-21]. The power function is of the form
W=a Lb (1)
Where is the intercept parameter, or shape coefficient, and b the allometric parameter. The linearized equation of (1) is of the form
ln(W)=ln(a)+b*ln(L) (2)
Parameter estimates and fit of (2) were done using ordinary linear least square regression. The parameter W in equation (2) for this study was the individual crab weight (g), and the parameter L the total carapace width (mm).
RESULTS
Physical data
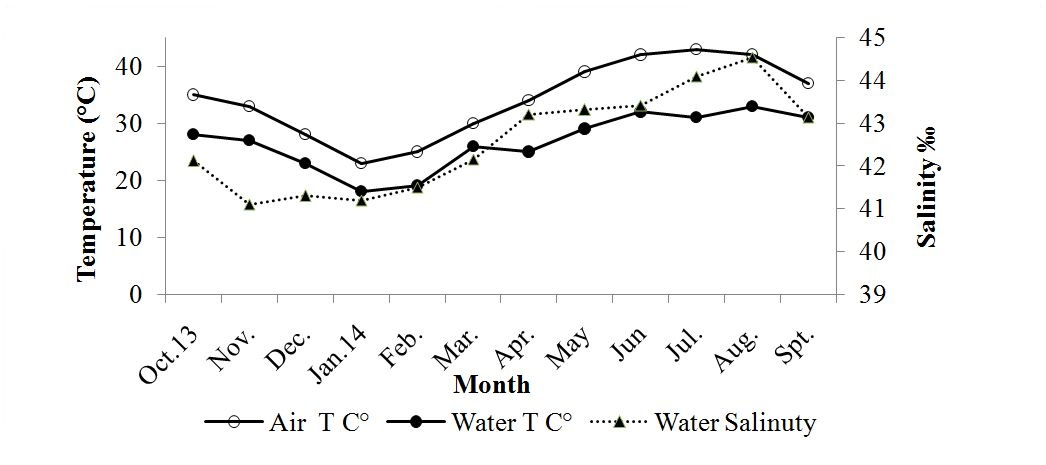 Figure 2: Air and surface sea water temperatures (?) and salinity (‰) at Umm SA (October 2013 to September 2014).
Figure 2: Air and surface sea water temperatures (?) and salinity (‰) at Umm SA (October 2013 to September 2014).Substrate at the collection site comprised mostly of clay (45.14 percent±0.4 SEM), followed by silt (37.36 percent±0.4 SEM) and sand (17.50 percent±0.4 SEM), with a mean grain size of 0.11 mm±0.01 SEM. Mean organic matter was at 3.71 percent±0.43 SEM, pH at 7.53±0.12 SEM, and moisture at 25.88 g±1.05 SEM.
Population structure
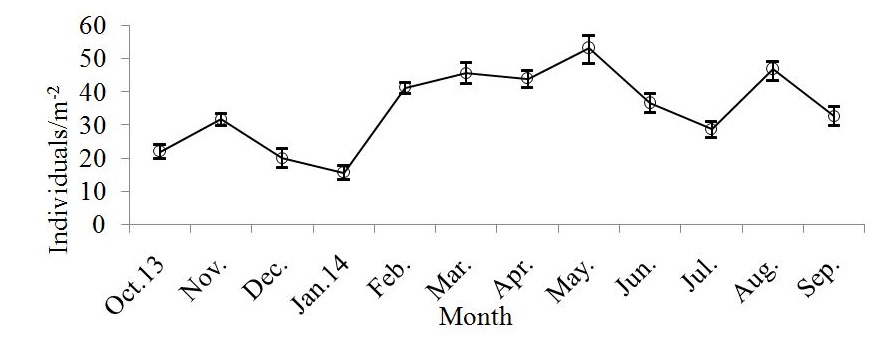 Figure 3: Monthly average of population abundance/m-2, for M. arabicum collected from Umm SA, October 2013-December 2014. Vertical bars are standard error of the mean.
Figure 3: Monthly average of population abundance/m-2, for M. arabicum collected from Umm SA, October 2013-December 2014. Vertical bars are standard error of the mean.Carapace width varied from 3.2 to 11.5 mm for males and from 3.8 to 11.7 mm for females. Mean size of females (8.4±0.08 mm) was not significantly different from that of males (7.8±0.09 mm; t-test, p>0.05). The size classes of 9.0 to 9.5 mm and 9.5 to 10.0 mm had the largest numbers of individuals for males and females, respectively. Most of the ovigerous individuals were between 3.8 and 11.7 mm CW (Figure 4).
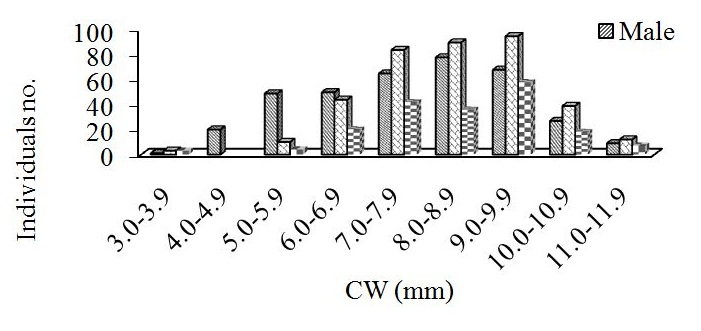 Figure 4: Carapace width frequency distributions for male and female Manningis arabicum collected from Umm SA mangrove swamp between October 2013 and September 2014.
Figure 4: Carapace width frequency distributions for male and female Manningis arabicum collected from Umm SA mangrove swamp between October 2013 and September 2014.Population growth
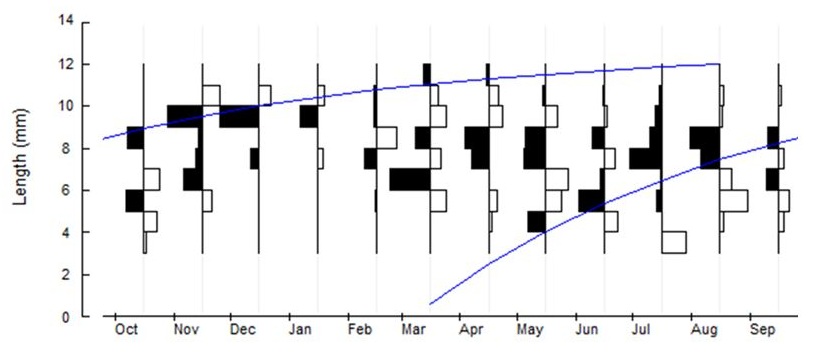 Figure 5: Restructured length-frequency (W-W) distribution with superimposed growth curves for Manningis arabicum collected from Umm SA mangrove swamp between October 2013 and September 2014.
Figure 5: Restructured length-frequency (W-W) distribution with superimposed growth curves for Manningis arabicum collected from Umm SA mangrove swamp between October 2013 and September 2014.Carapace width-individual weight relationships
|
N |
R |
a |
SEMa |
b |
SEMb |
p-Value a |
p-Value b |
|
|
Males |
||||||||
|
Fall |
71 |
0.337 |
2.94 |
0.388 |
-1.337 |
0.449 |
0.231 |
0.004 |
|
Winter |
81 |
0.678 |
<0.01 |
0.939 |
2.446 |
0.299 |
<0.001 |
<0.001 |
|
Spring |
91 |
0.969 |
<0.01 |
0.245 |
2.992 |
0.081 |
<0.001 |
<0.001 |
|
Summer |
124 |
0.8 |
<0.01 |
0.498 |
2.457 |
0.167 |
<0.001 |
<0.001 |
|
Females |
||||||||
|
Fall |
82 |
0.163 |
0.45 |
0.531 |
-0.85 |
0.575 |
0.133 |
0.143 |
|
Winter |
82 |
0.758 |
<0.00 |
0.71 |
2.304 |
0.222 |
<0.001 |
<0.001 |
|
Spring |
104 |
0.93 |
<0.00 |
0.339 |
2.819 |
0.11 |
<0.001 |
<0.001 |
|
Summer |
110 |
0.67 |
<0.00 |
0.688 |
2.084 |
0.222 |
<0.001 |
<0.001 |
Comparisons of the weight loss showed variation according to seasons (Table 2). Males of M. arabicum for a fixed width were heavier than females in the fall and winter. Both males and females lost weight during autumn and spring and gained weight during winter and summer. Males added 42 percent weight from fall to winter, dropped 36 percent from winter to spring and dropped 17 percent from spring to summer. Similarly, females added 30 percent weight from fall to winter, dropped 16 percent from winter to spring and dropped 6 percent from spring to summer (Table 2).
|
Season Mean Water Temperature ?±SE |
N |
Mean Weight Males |
SEM |
N |
Mean Weight Females |
SEM |
|
Fall 28.67±1.2 |
71 |
0.268 |
0.019 |
82 |
0.226 |
0.01 |
|
Winter 20.00±1.2 |
81 |
0.379 |
0.018 |
82 |
0.293 |
0.012 |
|
Spring 26.67±1.2 |
91 |
0.242 |
0.017 |
104 |
0.245 |
0.013 |
|
Summer 32.00±0.5 |
124 |
0.201 |
0.014 |
110 |
0.231 |
0.012 |
Sex ratio
|
Males |
Females |
Total |
Males % |
Females % |
Sex Ratio |
Chi Square |
|
|
October |
38 |
23 |
61 |
62.3 |
37.7 |
1.0:0.61 |
3.69* |
|
November |
33 |
59 |
92 |
35.9 |
64.1 |
1.0:1.79 |
7.35* |
|
December |
30 |
32 |
62 |
48.4 |
51.6 |
1.0:1.07 |
0.06 |
|
January |
21 |
20 |
41 |
51.2 |
48.8 |
1.0:0.95 |
0.02 |
|
February |
30 |
30 |
60 |
50 |
50 |
1.0:1.0 |
0 |
|
March |
29 |
32 |
61 |
47.5 |
52.5 |
1.0:1.10 |
0.15 |
|
April |
33 |
35 |
68 |
48.5 |
51.5 |
1.0:1.06 |
0.06 |
|
May |
29 |
37 |
66 |
43.9 |
56.1 |
1.0:1.28 |
0.97 |
|
June |
33 |
29 |
62 |
53.2 |
46.8 |
1.0:0.88 |
0.25 |
|
July |
27 |
26 |
53 |
50.9 |
49.1 |
1.0:0.96 |
0.02 |
|
August |
35 |
28 |
63 |
55.6 |
44.4 |
1.0:0.8 |
0.78 |
|
September |
29 |
27 |
56 |
51.7 |
48.2 |
1.0:0.93 |
0.07 |
|
Total |
367 |
378 |
745 |
49.3 |
50.7 |
1.0:1.02 |
13.42 |
Note: Test of heterogeneity: df (df=1, P<0.05)
Sum of 12 Chi-squares 11:13.42
Pooled Chi-square: 0.16
|
Size-Group (mm) |
Males |
Females |
Total |
Males % |
Females % |
Sex Ratio |
Chi Square |
|
3.0-0.5 |
1 |
0 |
1 |
100 |
|||
|
3.5-4.0 |
0 |
3 |
3 |
100 |
|||
|
4.0-4.5 |
8 |
0 |
8 |
100 |
|||
|
4.5-5.0 |
12 |
0 |
12 |
100 |
|||
|
5.0-5.5 |
15 |
0 |
15 |
100 |
|||
|
5.5-6.0 |
34 |
10 |
44 |
77.27 |
22.73 |
1:0.29 |
13.09* |
|
6.0-6.5 |
12 |
25 |
37 |
32.43 |
67.57 |
1:2.08 |
4.57* |
|
6.5-7.0 |
38 |
20 |
58 |
65.52 |
34.48 |
1:0.53 |
5.59* |
|
7.0-7.5 |
25 |
43 |
68 |
36.76 |
63.24 |
1:1.72 |
4.76* |
|
7.5-8.0 |
40 |
41 |
81 |
49.38 |
50.62 |
1:1.03 |
0.03 |
|
8.0-8.5 |
35 |
47 |
82 |
42.70 |
57..30 |
1:1.34 |
1.75 |
|
8.5-9.0 |
43 |
43 |
86 |
50.00 |
50.00 |
1:1.00 |
0.00 |
|
9.0-9.5 |
50 |
38 |
88 |
56.80 |
43.20 |
1:0.76 |
1.64 |
|
9.5-10.0 |
18 |
57 |
75 |
24.00 |
76.00 |
1:3.17 |
20.28* |
|
10-10.5 |
16 |
16 |
32 |
50.00 |
50.00 |
1:1.14 |
0.00 |
|
10.5-11 |
11 |
23 |
34 |
32.35 |
67.75 |
1:1.09 |
1.16 |
|
11-11.5 |
6 |
9 |
15 |
40.00 |
60.00 |
1:1.26 |
9.69* |
|
11.5-12 |
3 |
3 |
6 |
50 |
50 |
1:1.00 |
0 |
|
Total |
367 |
378 |
745 |
49.26 |
50.74 |
62.56 |
Note: Test of heterogeneity: df (df=1, P<0.05)
Sum of 14 Chi-squares 13:62.56
Pooled Chi-square: 0.16
*asterisks indicate significant differences (p<0.05; χ2 test) from the 1:1 ratio.
Reproductive season
 Figure 6: Percentage of ovigerous females M. arabicum collected from Umm SA mangrove swamp between October 2013 and September 2014.
Figure 6: Percentage of ovigerous females M. arabicum collected from Umm SA mangrove swamp between October 2013 and September 2014.DISCUSSION
Size frequency distribution is usually a population trait that varies throughout the year, due to varied reproduction times and wide-ranging larvae recruitment rates [22]. Size frequency distribution of M. arabicum, however, suggested that the recruitment was continuous throughout the year, not showing any intra-annual patterns. Recruitment for this species favored males.
Estimates of crab growth rates and performance from this study provide a baseline for future studies on growth trends for this species. Examples of such studies include assessing mud crab growth as indicators of habitat health or the effectiveness of mangrove rehabilitation programs [23]. Growth patterns from carapace width-body weight relationships, on the other hand, was consistent with other Brachyurans, according to Hartnoll [24-26]. In our study, the values for the exponent b, the allometric parameter, ranged from 2.08 to 2.30, which suggests negative allometric growth (organism becomes slenderer as it becomes longer). Moreover, male allometric parameter was estimated to be higher than that of females, indicating that the overall negative allometric growth for the population is even more pronounced for the latter. Similar results were found for crabs of the species Uca rapax in Brazil, indicating a general trend toward negative allometry for Ocypodidae crabs [27].
Seasonally, there was also a difference in the allometric parameter by gender of crabs examined in this study. Inconclusive data was observed for body allometry in the fall. The most pronounced difference was observed in spring for both genders, with the highest value for that parameter. Various factors may be responsible for observed differences, such as species density, shore level, temperature, salinity, food quantity and quality, sex, time of year and stage of maturity [28,29]. We surmise that the differences were mostly as a consequence of weight gains during the spring, potentially due to higher food availability from the higher rains during that season. Such data should be considered, when implementing conservation practices based on time-of-year.
Crab weight measurements indicated that males were heavier than females during the fall and summer, with females being heavier in the winter and spring. This should be expected, as M. arabicum does not show sexual dimorphism in chelae size. As M. arabicum have similar sized chelae, the observed differences could be due to differences in carapace density. Seasonal carapace weight differences were also observed, showing higher values for the spring. This could be explained by a loss of water starting in April and extending through October, as water salinity during this period was highest. Salinity values were below 20‰ during the winter and mid spring, rising to as high as 57‰ in the summer and fall. Water losses from high salinity waters has also been observed elsewhere [30].
Sex ratio of brachyuran crabs is usually close to unity [25,31,32], with some variations according to populations and year within the same population being reported by Sastry [33], Johnson [34], and Varisco & Vinuesa [35]. Sex ratios close to unity in ocypodids have also been reported by Simons and Jones [36] and Snowden et al, [10]. Sex ratios for crabs in this study favored males in October and females in November. Ratios by carapace width favored males over smaller widths and females over larger widths. As there is no evidence that these species change sex, behavioral differences related to breeding might be one explanation for the observed results. It is possible that either non-ovigerous females remain at deeper levels in burrows outside the breeding season, or males remain on the surface to exhibit territorial displays [37]. Ovigerous females may also have spent prolonged periods underground and, when on the surface, foraged closer to water sources, creating spatial separation from foraging males [38].
Most ovigerous females from this study were observed from October to April, with peaks in December and January. No ovigerous females were observed from June to September. Studies on the reproductive cycles of family Ocypodidae are very limited in the Gulf region. Apel [39], reported that the longest breeding period was of 10 months for M. depressus in Saudi Arabia.
The majority of tropical species tend to breed continuously throughout the year or have prolonged breeding seasons compared to species at higher latitudes [40], with some seasonality present in subtropical regions [41]. As latitude increases, breeding seasons tend to match periods of higher water temperature [33,42-44], with breeding starting later in the season [45]. Although temperature is most likely the controlling factor [46-49], showed that for species which spawn throughout the year, low breeding coincides with the SW monsoon season, possibly due to a low in food availability for crab larvae during that period. Similarly, Pillay & Nair in a study of the breeding biology of brachyuran crabs from the southwest coast of India, found that availability of food for the young during the planktotrophic life was a critical cue for breeding season timing, most likely determining the length of the planktonic phase and, therefore, risk of predation [50-53]. No information is available on size at sexual maturity for males M. arabicum. It is not unreasonable to assume that the size at first maturity, as for females in this study, occurs when individuals are small.
This study constitutes the first account on the population biology of M. arabicum. We provide baseline data to support and further conservation efforts of coastal and estuarine habitats, one of the most threatened, yet ecologically and economically important ecosystems. The data from this study may complement studies using mud crab density as indicators of habitat health [23], by offering data on growth and reproduction, as these latter parameters may more closely indicate habitat health than single estimates of number of individuals. Our information, thus, may be preferred for the often multidisciplinary and multifaceted studies on mangrove restoration and preservation [54].
CONCLUSION
REFERENCES
- Henmi Y (2000) Comparisons of life history traits among populations of theocypodid crab Macrophthalmus japonicus in habitats with constraining foodavailability. Crust Res 29: 109-120.
- Litulo C (2004) Reproductive Aspects of a Tropical Population of the Fiddler Crab Uca annulipes (H. Milne Edwards, 1837) (Brachyura: Ocypodidae) at Costa Do Sol Mangrove, Maputo Bay, southern Mozambique. Hydrobiologia 525: 167-173.
- Al Khayat JA, Jones D (1999) A comparison of the macrofauna of natural and replanted mangroves in Qatar. Estuarine, Coastal and Shelf Science 49: 55-63.
- Jones DA, Clayton D (1983) The systematics and ecology of crabs belonging to the genera Cleistostoma De Haan and Paracleistostoma De Man on Kuwait mudflats. Crustaceana 45: 183-199.
- Stephensen K (1944) The brachyura of the iranian gulf. With an appendix: The male pleopoda of the Brachyura. In: Jessen K, Spärck R (eds.). Danish scientific investigations in Iran, Part 4. E. Munksgaard, Denmark.
- Tirmizi NM (1974) A description of Callianassa martensi, Miers, 1884 (Decapoda, Thalassinidea) and its occurrence in the northern Arabian Sea. Crustaceana 26: 286-292.
- Al Khayat JA, Jones D (1996) Two new genera, Manningis and Leptochryseus (Decapoda: Camptandriinae), and descriptions of the first zoea of six Brachyurans from the Arabian Gulf. Journal of Crustacean Biology 16: 797-813.
- Milani AS (2018) Mangrove forests of the persian gulf and the gulf of oman. In: Makowski C, Finkl C (eds.). Threats to Mangrove Forests, Coastal Research Library, Springer, Cham, New York, USA.
- Clayton D, Al-Kindi A (1998) Population structure and dynamics of two scopimerine sand crabs Scopimera crabricauda, Alcock 1900 and Dotilla sulcate (Forskall 1775) in an estuarine habitat in Oman. Tropical Zoology 11: 197-215.
- Snowden RD, Clayton DA, Al-Taher EY (1991) Population biology of Ilyoplax stevensi (Brachyura: Ocypodidae) on Kuwait mudflat. Mar Ecol Prog Ser 71: 219-225.
- Buchanan J (1984) Sediment analysis. In: Holme N, McIntyre A (eds.). Methods for the Study of Marine Benthos. Blackwell, Oxford United Kingdom.
- Gayanilo FC (1996) Fisat: FAO-Iclarm stock assessment tools. Reference Manual. FAO, Rome. Pg no: 262.
- Pauly D, David N (1981) ELEFAN I, a BASIC program for the objective extraction of growth parameters from length-frequency data. Berichte der Deutschten Wissenschaftlichen Kommission fur Meeresforschung 28: 205-211.
- Pauly D, Munro J (1984) Once more on growth comparisons in fish and invertebrates. Fishbyte 2: 21.
- Stickney R (1972) Length-Weight relationships for several fishes and invertebrates in Georgia coastal wasters with condition factors for fish species. Skidaway Institute of Oceanography Savannah, Georgia, USA.
- Ricker WE (1975) Computation and interpretation of biological statistics of fish populations. Bulletin of the Fisheries Research Board of Canada, Ottawa, Canada.
- Jones RE, Petrell RJ, Pauly D (1999) Using modified length-weight relationships to assess the condition of fish. Aquacultural Engineering 20: 261-276.
- Quinn TJ, Deriso RB (1999) Quantitative fish dynamics. Oxford University Press, Oxford, UK. Pg no: 560.
- Peters R (1983) The ecological implications of body size. Cambridge University Press, Cambridge, UK.
- Calder W (1984) Size, function, and life history. Harvard University Press, Cambridge, USA.
- Reiss M (1989) The allometry of growth and reproduction. Cambridge University Press, Cambridge, UK. Pg no: 182.
- Thurman CL (1985) Reproductive biology and population structure of the fiddler crab Uca subcylindrica (Stimpson). Biol Bull 169: 215- 229.
- Ulfa M, Ikejima K, Poedjirahajoe E, Faida LRW, Harahap MM (2018). Effects of mangrove rehabilitation on density of Scylla spp. (mud crabs) in Kuala Langsa, Aceh, Indonesia. Regional Studies in Marine Science 24: 296-302.
- Hartnoll RG (1974) Variation in growth pattern between some secondary sexual characters in crabs (Decapoda Brachyura). Crustaceana 27: 131-136.
- Hartnoll RG (1978) The determination of relative growth in Crustacea. Crustaceana 34: 281-293.
- Bliss DE (1982) Growth. In: Bliss DE, Abele LG (eds.). The biology of Crustacea: Embryology, morphology, and genetics. Academic Press, New York, USA.
- Castiglioni D, Negreiros Fransozo M (2004) Comparative analysis of the relative growth of Uca rapax(Smith, 1870) (Crustacea, Ocypodidae) from two mangroves in São Paulo, Brazil. Revista Brasileira de Zoologia 21: 137-144.
- Pauly D (1984) Fish population dynamics in tropical waters: A manual for use with programmable calculators. ICLARM Stud Rev, Manila, Philippines. Pg no: 325.
- Sparre P, Venema SC (1998) Introduction to tropical fish stock assessment Part I: Manual. FAO Fisheries Technical Paper 306/1. Rev 2. Rome, Italy.
- Tagatz M (1965) The fishery for blue crabs in the St. Johns River, Florida, with special reference to fluctuations in yield between 1961 and 1962. U. S. Fish and Wildlife Service Special Scientific Report Fisheries Number 501. Fish and Wildlife Service, Washington, D. C. 11 pp.
- Wenner AM (1972) Sex ratio as a function of size in marine Crustacea. Am Nat 106: 321-350.
- Lawal-Are A (2010) Reproductive biology of the blue crab, Callinectes amnicola (De Rochebrune) in the Lagos Lagoon, Nigeria. Turk J Fish Aquat Sci 10: 1-7.
- Sastry AN (1983) Ecological aspects of reproduction. In: Vernberg FJ, Vernberg WB (Eds.). The Biology of Crustacea. Environmental adaptations. Academic Press, New York, USA. Pg no: 383.
- Johnson PTJ (2003) Biased sex ratios in fiddler crabs (Brachyura, Ocypodidae): A review and evaluation of the influence of sampling method, size class, and sex-specific mortality. Crustaceana 76: 559-580.
- Varisco M, Vinuesa J (2011) Reproductive biology of the spider crab Leucippa pentagona (Decapoda: Epialtidae), in Bustamante Bay, Argentina. Lat Am J Aquat Res 39: 471-480.
- Simons M, Jones M (1981) Population and reproductive biology of the mud crab Macrophthalmus hirtipes (Jacquinot, 1853) (Ocypodidae) from marine and estuarine habitats. J Nat Hist 15: 981-994.
- Clayton D (1986) Ecology of mudflats with particular reference to those of the northern Arabian Gulf. In: Halwagy R, Clayton D, Behbehani M (eds.). Marine environment and pollution, Proceedings of the First Arabian Gulf Conference on Environment and Pollution. Pg no: 83-96.
- Macia A, Quincardete I, Paula J (2001) A comparison of alternative methods for estimating population density of the fiddler crab Uca annulipes at Saco mangrove, Inhaca Island (Mozambique). Hydrobiologia 449: 213-219.
- Apel M (1994) Effects of the 1991 oil spill on the crab fauna (Crustacea: Decapode: Brachyura) of intertidal mudflats in the western persian gulf. Courier Forsch Inst Senckenberg 166: 40-46.
- Emmerson WD (1994) Seasonal breeding cycles and sex ratios of eight species of crabs from Mgazana, a mangrove estuary in Transkei, South Africa. J Crust Biol 14: 568-578.
- Costa TM, Negreiros-Fransozo ML (2002) Population Biology of Uca thayeri Rathbun, 1900 (Brachyura, Ocypodidae) in a Subtropical South American Mangrove Area: Results from Transect and Catch-Per-Unit-Effort Techniques. Crustaceana 75: 1201-1218.
- Giese AC, Pearse JS (1974) Reproduction of marine invertebrates: Acoelomate and pseudoacoelomate metazoans. In: Giese AC (ed.). Reproduction of Marine Invertebrates, Academic Press, Los Angeles. Pg no: 546.
- Spivak ED, Anger K, Bas CC, Luppi TA, Ismael D (1996) Size structure, sex ratio, and breeding season in two intertidal grapsid crab species from mar chiquita lagoon, Argentina. Neritica 10: 7-26.
- Litulo C, Mahanjane Y, Mantelatto FLM (2005) Population Biology and Breeding Period of the Sand-Bubbler Crab Dotilla fenestrata (Brachyura: Ocypodidae) from Southern Mozambique. Aquat Ecol 39: 305-313.
- Jones MB, Simons M (1983) Latitudinal variation in reproductive characteristics of a mud crab Helice grassa (Grapsidae). Bulletin of Marine Science, Miami 33: 656-670.
- Orton JH (1920) Sea temperatures, breeding and distribution of marine animals. J Mar Biol Assoc United Kingdom 12: 339-366.
- Gunter G (1957) Treatise on Marine Ecology and Paleoecology. Geol Soc Am Mem 1: 159-184.
- Kinne O (1970) Temperature: Animals-invertebrates. In: Kinne O (ed.). Marine Ecology, Environmental Factors, Wiley, London.
- Ahmed M (1980) The breeding and recruitment of marine animals of the coast of Pakistan bordering the Arabian Sea. Proc 1st Pakistan Cong Zool 55-96.
- Suklin SD (1975) The significance of diet in the growth and development of larvae of the blue crab, Callinectes sapidus (Rathbun) under laboratory conditions. J Exp Mar Bio Ecol 20: 119-135.
- Anger K (1983) Temperature and the larval development of Hyas araneus L. (Decapoda: Majidae) extrapolation of laboratory data to field conditions. J Exp Mar Biol Ecol 69: 203-215.
- Anger K, Queiroga H, Calado R (1983) Larval development and behavior strategies in Brachyura. In: Anger K (eds.). Treatise on zoology: Anatomy, taxonomy, biology 9: 317-374.
- Dawirs RR (1985) Temperature and larval development of Carcinus maenas (Decapoda) in the laboratory: Predictions of larval dynamics in the sea. Mar Ecol Prog Ser 24: 297-302.
- Lee SY, Khim JS (2017) Hard science is essential to restoring soft-sediment intertidal habitats in burgeoning East Asia. Chemosphere 168: 765-776.
Citation: Al-Khayat JA, Jawal L, Riedel R (2018) Population Biology of Manningis arabicum (Jones & Clayton, 1983; Decapoda: Brachyura, Ocypodidae) from Umm SA Mangrove Swamps, Qatar. J Aquac Fisheries 2: 012.
Copyright: © 2018 Jassim A Al-Khayat, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.

