
Study of the Age of Centropomus robalito by Otoliths Analysis of sagitta, asteriscus and lapillus in Mexican Central Pacific
*Corresponding Author(s):
Elaine Espino BarrInstituto Nacional De Pesca, Centro Regional De Investigación Pesquera Manzanillo, Playa Ventanas, Manzanillo, Mexico
Tel:+52 3141067756,
Email:elespino@gmail.com
Abstract
Morphology, morphometry and growth rings of the otoliths: Sagitta, asteriscus and lapillus of Centropomus robalito Jordan and Gilbert 1882 in Cuyutlan lagoon, Colima, Mexico were studied. Samples were taken from October 2015 to September 2016. Differences between sexes, as right and left of the three pairs of otoliths were analyzed. In all cases the growth of the otoliths was eccentric to the core. The relationship between total length of the fish and length of the sagitta showed that this structure is suitable to determine the age of this species. Nine growth rings were identified on sagittae. The identification of growth rings was carried out only in sagittae. Data on lengths obtained by polymodal curves of C. robalito were compared to the present results.
Keywords
INTRODUCTION
With regard to the yellowfin snook Centropomus robalito Jordan and Gilbert 1882 (Figure 1), its distribution is from Sinaloa state Mexico to northern Colombia [3,4].
 Figure 1: Yellowfin snook Centropomus robalito Jordan & Gilbert 1882.
Figure 1: Yellowfin snook Centropomus robalito Jordan & Gilbert 1882.Its fishery importance is medium in a local scale, it is delivered complete in ice and its commercial classification is of third category. Its price is of $40.00 Mexican pesos ($2.00 US dollars). It is fished with hand line and fish hook, cast net, and gill nets. Annual commercial captures go from 1.4 tons to 26.7 tons with an average of 12.8 tons in Colima during the last years (from 2013 to 2017) [5].
Studies have been carried out to determine age through the analysis of length frequency and growth lines on scales by Tovilla-Hernández and Castro-Aguirre [6], in the coast of Chiapas, Mexico. Gil-López et al. [7], studied the life cycle of C. robalito in the Mar Muerto lagoon system, Oaxaca and Chiapas. Analysis of length structures and biomass was done in Sinaloa and Nayarit, Mexico by Madrid-Vera et al. [8]. Analysis of feeding habits in juvenile in Barra de Navidad lagoon, Jalisco, Mexico was carried out by Flores-Ortega et al. [9].
We did not find studies on the age determination of C. robalito using otoliths. The following objectives of this study are: a) Description and analysis of the labyrinth system. b) Morphologic analysis of the sagittae, asterisci and lapilli. c) Morphometric study of the otoliths and its variation regarding age and sex. d) Identification of growth marks. e) Comparison with results by other authors.
Studies on the growth ring identification are necessary to determine the species growth and the assessment of the von Bertalanffy growth constants, necessary to calculate age groups of the population and analyze the population dynamics of the species, such as reproduction, feeding, maximum sustainable yield models, prediction and capture simulation. This information is important to achieve a rational management and prevent overexploitation of resources.
MATERIAL AND METHODS
In the laboratory, data were taken from each organism: Total Length (TL, cm), Standard Length (SL, cm), Total Weight (TW, g), Eviscerated Weight (EW, g) and sex.
Sagittae, asterisci and lapilli were obtained by doing a transversal cut in the organism’s skull, removing the brain, and extracting the semi-circular canals (left and right). Otoliths were liberated from the otic capsules, cleaned in water and dried. Samples were then preserved dry in Eppendorf tubes, and labeled accordingly.
Otoliths were analyzed with a dissecting microscope. The glossary terminology of Secor et al. [10], was used to describe the labyrinth system and the sagittae of this species. In the case of the asterisci and lapilli, similar concepts were used for description as in Gallardo-Cabello et al. [11-17], and Espino-Barr et al. [18-21].
Photographs from each otolith (internal and external aspect, and close ups) were taken with the scanning electronic microscope JEOL model JSM6360LV. Samples of otoliths were prior mounted on aluminum sample holder, on a two faced carbon tape and coated with a layer of gold of 20 Å for two minutes, in a vaporizer.
Measurements of the length and width of the three pairs of otoliths (right and left) were registered, with the help of a graduated measuring ocular in the microscope. Sample size was corroborated [22].
Regressions by least squares were used to calculate the relationship constants of the sagitta rostrum Length (SL) vs. Width (SW). In the case of the asterisci and lapilli the regression indexes were only used for Length vs. Width (AL-AW, LL-LW). The allometric relationships between total length of the fish and the length and width of each otolith were also obtained by least square regression.
A one way variance analysis (ANOVA, α=0.05) [23] was used to determine if there were morphometric differences between male and female otoliths, and between right and left otolith.
Growth ring identification was carried out on sagittae, observing rings by transparency in a stereoscopic microscope, using transmitted light. Average length for each growth ring was calculated.
RESULTS
Morphometrics of Centropomus robalito
|
StL (cm) |
TL (cm) |
Hi (cm) |
TW (g) |
EW (g) |
|
|
n |
335 |
335 |
335 |
334 |
335 |
|
Average |
15.90 |
20.78 |
4.78 |
91.30 |
82.67 |
|
Maximum |
27.0 |
34.0 |
8.0 |
390.0 |
353.0 |
|
Minimum |
5.0 |
6.5 |
1.6 |
2.7 |
2.5 |
|
SD |
3.70 |
4.69 |
1.16 |
59.22 |
53.73 |
Table 2 describes the measurements of the otoliths. The big structures are sagittae, 2.43 times bigger than the asterisci and 2.45 bigger than the lapillus. Compared sizes can be observed in figure 2.
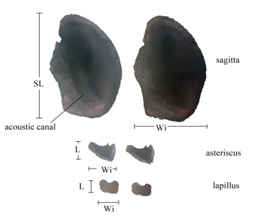 Figure 2: Relationship between the three pairs of otoliths in Centropomus robalito: Left side internal aspect, right side external aspect. SL=rostrum length, WI=Width, L=Length.
Figure 2: Relationship between the three pairs of otoliths in Centropomus robalito: Left side internal aspect, right side external aspect. SL=rostrum length, WI=Width, L=Length.|
TL (cm) |
SL (mm) |
SW (mm) |
AL (mm) |
AW (mm) |
LL (mm) |
LW (mm) |
|
|
n |
366 |
362 |
363 |
350 |
348 |
348 |
347 |
|
Average |
21.23 |
8.52 |
5.18 |
1.38 |
2.06 |
1.56 |
1.01 |
|
Maximum |
32.0 |
13.1 |
7.4 |
2.1 |
3.4 |
2.1 |
1.7 |
|
Minimum |
8.5 |
3.5 |
2.4 |
0.7 |
0.8 |
0.8 |
0.5 |
|
SD |
3.945 |
1.519 |
0.797 |
0.238 |
0.355 |
0.219 |
0.139 |
Labyrinth system of Centropomus robalito
 Figure 3: Section of the membranous labyrinth of Centropomus robalito (22 cm total length) (enlarged 14 times) showing the sacculus, which includes the sagitta, the lagena with the asteriscus; also the acoustic macula is shown penetrating the acoustic canal of each of these otoliths.
Figure 3: Section of the membranous labyrinth of Centropomus robalito (22 cm total length) (enlarged 14 times) showing the sacculus, which includes the sagitta, the lagena with the asteriscus; also the acoustic macula is shown penetrating the acoustic canal of each of these otoliths.The acoustic macula (inside each chamber) penetrates the otoliths at the level of the acoustic canal through the neuromas, which are nerve cells (Figures 3 and 4). Deposition of the calcium carbonate and protein takes place through the macula, leading the otoliths to grow and enlarge their size. Ramifications of the eighth cranial nerve can be observed in the macula, through which impulse transmission to the brain is carried out, by means of vibration of the otolith suspended in the endolymph [25-27]. Sagittae and asterisci are related with the sound perception, gravity and angular acceleration, while the lapilli are more related to the fishes’ equilibrium [28,29].
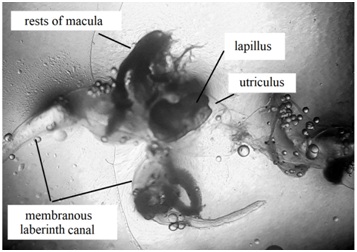 Figure 4: Section of the membranous labyrinth of Centropomus robalito (22 cm total length) (enlarged 14 times) showing the utriculus with lapillus, the membranous labyrinth canal and rests of macula.
Figure 4: Section of the membranous labyrinth of Centropomus robalito (22 cm total length) (enlarged 14 times) showing the utriculus with lapillus, the membranous labyrinth canal and rests of macula.The protein of which otoliths are made was denominated otoline by Degens et al. [30], and presents a high molecular weight. Other component that forms the otoliths is calcium carbonate in its form of aragonite [24,31-34].
Description of the sagitta
 Figure 5: Scanning photograph of: a) Left internal aspect with acoustic canal, b) The right sagitta external aspect of Centropomus robalito. R=Rostrum, P=Postrostrum, D=Dorsal margin, V=Ventral margin.
Figure 5: Scanning photograph of: a) Left internal aspect with acoustic canal, b) The right sagitta external aspect of Centropomus robalito. R=Rostrum, P=Postrostrum, D=Dorsal margin, V=Ventral margin.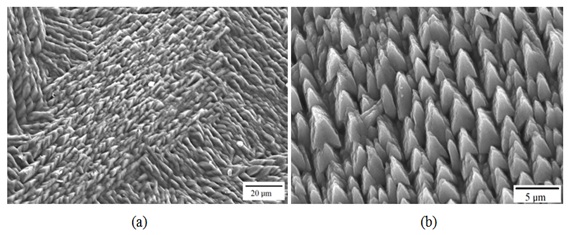 Figure 6: Details in a) and b) of crystals in the acoustic canal corresponding to the ostium of the sagitta of Centropomus robalito.
Figure 6: Details in a) and b) of crystals in the acoustic canal corresponding to the ostium of the sagitta of Centropomus robalito.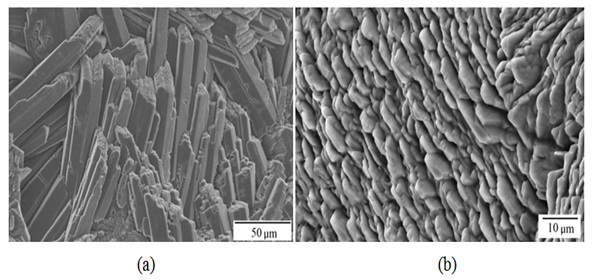 Figure 7: Details in a) and b) of crystals in the acoustic canal corresponding to the cauda of the sagitta of Centropomus robalito.
Figure 7: Details in a) and b) of crystals in the acoustic canal corresponding to the cauda of the sagitta of Centropomus robalito.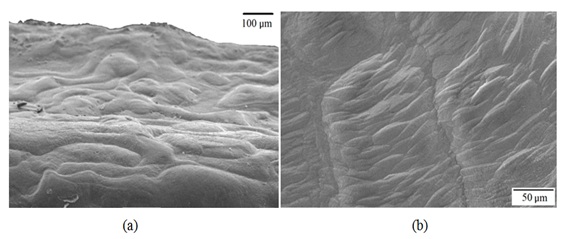 Figure 8: Details in a) and b) of growth rings corresponding to a lower periodicity than seasonal on the external aspect of the sagitta of Centropomus robalito.
Figure 8: Details in a) and b) of growth rings corresponding to a lower periodicity than seasonal on the external aspect of the sagitta of Centropomus robalito.Description of the asteriscus
The anterior margin of the asteriscus consists of two parts divided by a blunt projection, nominated as the dorsal area that exhibits a smaller surface and a ventral area of bigger surface (Figure 9). The dorsal and ventral margins exhibit sections that can be rounded or rectilinear.
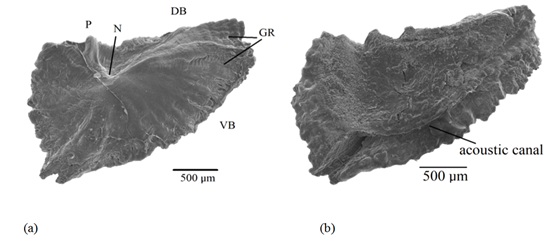 Figure 9: Scanning photograph of the right and left asteriscus, a) External and b) Internal aspect of Centropomus robalito: P=Projection, N=Core or primordia, DB=Dorsal Border, VB=Ventral Border, GR=Growth Rings of lower periodicity than seasonal, and acoustic canal.
Figure 9: Scanning photograph of the right and left asteriscus, a) External and b) Internal aspect of Centropomus robalito: P=Projection, N=Core or primordia, DB=Dorsal Border, VB=Ventral Border, GR=Growth Rings of lower periodicity than seasonal, and acoustic canal.The Asteriscus presents in its inner aspect an acoustic canal (Figures 9b and 10), where the acoustic macula captures impulse transmission of this otolith in the lagena and takes it through the eighth cranial nerve to the brain. Also, it is through this acoustic macula that the calcium carbonate and otolin are deposited in this structure, which nurture the asteriscus and allows that it develops and grows as the organism ages.
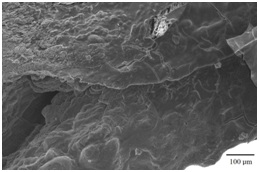 Figure 10: Details of the acoustic canal of the asteriscus of Centropomus robalito.
Figure 10: Details of the acoustic canal of the asteriscus of Centropomus robalito.Description of the lapillus
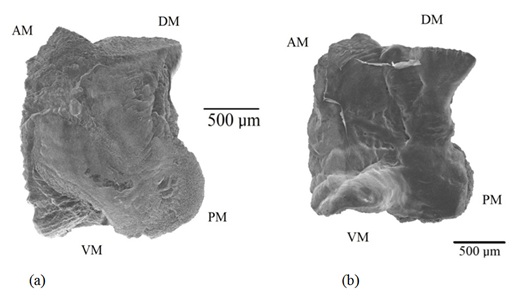 Figure 11: Scanning photograph of the right and left lapillus, a) External and b) Internal aspect of Centropomus robalito: AM=Anterior Margin, PM=Posterior Margin, DM=Dorsal Margin and VM=Ventral Margin.
Figure 11: Scanning photograph of the right and left lapillus, a) External and b) Internal aspect of Centropomus robalito: AM=Anterior Margin, PM=Posterior Margin, DM=Dorsal Margin and VM=Ventral Margin.The dorsal margin is significantly longer than the ventral and mostly rectilinear, while the ventral border shows dentitions. The inner aspect of the lapillus is concave and its curvature increments with age. The acoustic canal can be observed in the anterior border, this structure comes into contact with the acoustic macula. The external aspect of the lapillus is convex. The surfaces of the external and inner face are smooth.
On the surface of the external aspect, calcic carbonate crystals are observed in which otoline is disposed (Figures 12a and 12b). In the posterior border of the internal aspect, growth rings can be observed with a smaller periodicity than seasonal (Figures 13a and 13b).

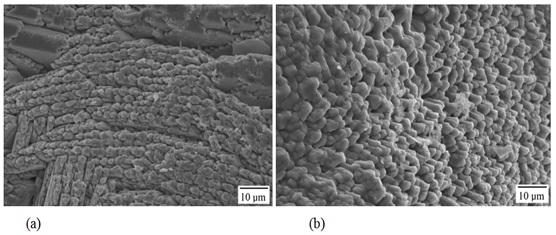 Figure 13: Details in a) and b) of crystals in the internal aspect of the lapillus of Centropomus robalito.
Figure 13: Details in a) and b) of crystals in the internal aspect of the lapillus of Centropomus robalito. Growth of the sagitta
|
|
All |
Females |
Males |
|||
|
Classes (cm) |
SL (mm) |
SW (mm) |
SL (mm) |
SW (mm) |
SL (mm) |
SW (mm) |
|
5 |
2.41 |
1.73 |
2.73 |
1.97 |
2.24 |
1.92 |
|
8 |
3.63 |
2.48 |
3.94 |
2.71 |
3.47 |
2.65 |
|
11 |
4.80 |
3.15 |
5.06 |
3.36 |
4.66 |
3.30 |
|
14 |
5.92 |
3.78 |
6.10 |
3.95 |
5.83 |
3.89 |
|
17 |
7.01 |
4.38 |
7.10 |
4.50 |
6.98 |
4.44 |
|
20 |
8.08 |
4.95 |
8.06 |
5.02 |
8.12 |
4.96 |
|
23 |
9.13 |
5.51 |
8.99 |
5.51 |
9.25 |
5.46 |
|
26 |
10.16 |
6.04 |
9.90 |
5.99 |
10.37 |
5.94 |
|
29 |
11.18 |
6.56 |
10.78 |
6.44 |
11.48 |
6.40 |
|
32 |
12.19 |
7.07 |
11.64 |
6.89 |
12.58 |
6.85 |
|
35 |
13.18 |
7.57 |
12.48 |
7.31 |
13.67 |
7.28 |
|
38 |
14.16 |
8.06 |
13.31 |
7.73 |
14.76 |
7.70 |
Table 4 shows the relationship between the rostrum length vs. width of the sagitta, both for species as a whole and for each sex. The relationship between the rostrum length and width of the sagitta is expressed by the value of the exponent b=0.802 which corresponds to a negative allometric growth, where the sagitta grows more lengthwise than widthwise. The determination index for these two series of data is R2=0.886, with an ANOVA result of F=2817. The relationships for sexes were b=0.609 for females, and b=0.801 for males, which is closer to an isometric index.
|
SL vs. SW |
a |
b |
R2 |
F’ |
n |
|
All |
0.929 |
0.802 |
0.886 |
2817 |
362 |
|
Females |
1.439 |
0.609 |
0.633 |
355 |
206 |
|
Males |
0.919 |
0.800 |
0.872 |
596 |
88 |
The relationship between total fish length, length of the rostrum and width of the sagitta is shown in table 5. In the case of males, values were closer to an isometric growth, with b=0.930, data for the species was b=0.873, and for females b=0.781. Values of the fish length vs. width of sagitta showed values of the allometric index: For all specimens b=0.757 (R2=0.872, F=2450), by sex the values were b=0.673 (females) and b=0.684 (males). These results show that as the organisms grow in length, sagittae decrease in size (length and width).
|
Total length (cm) |
a |
b |
R2 |
F’ |
n |
|
|
SL (mm) |
Both |
0.591 |
0.873 |
0.842 |
1919 |
360 |
|
Females |
0.777 |
0.781 |
0.527 |
225 |
202 |
|
|
Males |
0.501 |
0.930 |
0.970 |
2783 |
87 |
|
|
SW (mm) |
Both |
0.513 |
0.757 |
0.872 |
2450 |
361 |
|
Females |
0.668 |
0.673 |
0.580 |
285 |
207 |
|
|
Males |
0.640 |
0.684 |
0.743 |
253 |
88 |
Growth of the Asteriscus
|
|
All |
Females |
Males |
|||
|
Classes (cm) |
AL (mm) |
AW (mm) |
AL (mm) |
AW (mm) |
AL (mm) |
AW (mm) |
|
5 |
0.48 |
0.66 |
0.42 |
0.74 |
0.45 |
0.57 |
|
8 |
0.68 |
0.96 |
0.61 |
1.03 |
0.65 |
0.88 |
|
11 |
0.85 |
1.23 |
0.79 |
1.29 |
0.83 |
1.17 |
|
14 |
1.02 |
1.48 |
0.97 |
1.54 |
1.01 |
1.46 |
|
17 |
1.17 |
1.73 |
1.13 |
1.76 |
1.18 |
1.74 |
|
20 |
1.32 |
1.96 |
1.29 |
1.98 |
1.34 |
2.02 |
|
23 |
1.46 |
2.19 |
1.45 |
2.18 |
1.50 |
2.29 |
|
26 |
1.60 |
2.41 |
1.60 |
2.38 |
1.65 |
2.57 |
|
29 |
1.73 |
2.63 |
1.75 |
2.57 |
1.80 |
2.83 |
|
32 |
1.86 |
2.84 |
1.90 |
2.76 |
1.95 |
3.10 |
|
35 |
1.98 |
3.05 |
2.04 |
2.94 |
2.09 |
3.36 |
|
38 |
2.10 |
3.25 |
2.18 |
3.11 |
2.23 |
3.62 |
Table 7 shows the relationships of the allometric indexes between length and width of the asteriscus; for all individuals a value of b=0.838 (R2=0.621 and F=569) was obtained, which corresponds to a negative allometric growth, that is, the asteriscus grows more in width than in length as the fish ages. In the case of males the value of b=0.998 is very close to an isometric index, that is, the asteriscus grows in length and width in the same proportion. For females the b=0.525 corresponding a negative allometric index.
|
AL vs. AW |
a |
b |
R2 |
F’ |
n |
|
All |
1.568 |
0.838 |
0.621 |
569 |
348 |
|
Females |
1.796 |
0.525 |
0.411 |
138 |
198 |
|
Males |
1.495 |
0.998 |
0.616 |
136 |
85 |
The relationships between total length of the fish and length and width of the asteriscus for all the individuals as for sexes are shown in table 8.
|
Total length (mm) |
|
a |
b |
R2 |
F’ |
n |
|
AL (mm) |
All |
0.149 |
0.728 |
0.702 |
821 |
349 |
|
Females |
0.112 |
0.817 |
0.443 |
158 |
199 |
|
|
Males |
0.124 |
0.795 |
0.647 |
155 |
85 |
|
|
AW (mm) |
All |
0.186 |
0.786 |
0.726 |
919 |
347 |
|
Females |
0.237 |
0.708 |
0.502 |
199 |
198 |
|
|
Males |
0.133 |
0.909 |
0.524 |
94 |
85 |
The allometric index value between the fish length and the length of the asteriscus for all individuals is b=0.728 (R2=0.702 and F’=821), which represents a negative allometric index, where the asteriscus’ length grows less than the fish length. Similarly data were obtained for sexes where b=0.817 in females and b=0.795 in males.
The allometric index value between the fish length and the width of the asteriscus for all individuals is b=0.786 (R2=0.726 and F’=919), which corresponds to a negative allometric index, where the asteriscus’ width grows less than the fish length. Similarly, this relationship in females shows low values, that is, a negative allometric index: b=0.708. Nevertheless, in the case of the males there is a strong tendency to isometry represented by the value of the index b=0.909, where growth of the width of the asteriscus is proportional to the fish growth.
Growth of the lapillus
|
|
All |
Females |
Males |
|||
|
Classes (cm) |
LL (mm) |
LW (mm) |
LL (mm) |
LW (mm) |
LL (mm) |
LW (mm) |
|
5 |
0.65 |
0.50 |
0.62 |
0.54 |
0.71 |
0.52 |
|
8 |
0.86 |
0.63 |
0.84 |
0.66 |
0.92 |
0.65 |
|
11 |
1.05 |
0.73 |
1.02 |
0.76 |
1.10 |
0.76 |
|
14 |
1.21 |
0.83 |
1.19 |
0.84 |
1.26 |
0.85 |
|
17 |
1.36 |
0.91 |
1.35 |
0.92 |
1.41 |
0.93 |
|
20 |
1.50 |
0.98 |
1.49 |
0.98 |
1.54 |
1.01 |
|
23 |
1.64 |
1.05 |
1.63 |
1.04 |
1.67 |
1.08 |
|
26 |
1.76 |
1.12 |
1.76 |
1.10 |
1.79 |
1.14 |
|
29 |
1.88 |
1.18 |
1.89 |
1.15 |
1.90 |
1.20 |
|
32 |
2.00 |
1.23 |
2.01 |
1.20 |
2.01 |
1.26 |
|
35 |
2.11 |
1.29 |
2.12 |
1.25 |
2.11 |
1.31 |
|
38 |
2.22 |
1.34 |
2.24 |
1.29 |
2.21 |
1.37 |
Table 10 shows the values of the allometric indexes for length and width of lapilli of the species and by sex. The value of b=0.661 shows a negative allometric growth index for all the individuals (R2=0.516 and F=370), which means that the lapillus grows more lengthwise than in thickness as the fish grows old. Similar data were obtained for females: b=0.587 and males b=0.773.
|
LL vs. LW |
a |
b |
R2 |
F’ |
n |
|
All |
0.753 |
0.661 |
0.516 |
370 |
347 |
|
Females |
0.783 |
0.587 |
0.342 |
103 |
196 |
|
Males |
0.718 |
0.773 |
0.797 |
326 |
84 |
Relationships between total length of the fish and length and width of the lapillus are observed in table 11. Data represent negative allometric indexes that show that growth of lapillus in length as in width is much smaller than the growth of the fish.
|
Total length (cm) |
|
a |
b |
R2 |
F’ |
n |
|
LL (mm) |
All |
0.244 |
0.607 |
0.736 |
964 |
346 |
|
Females |
0.226 |
0.630 |
0.535 |
225 |
196 |
|
|
Males |
0.287 |
0.561 |
0.577 |
116 |
85 |
|
|
LW (mm) |
All |
0.229 |
0.487 |
0.560 |
439 |
345 |
|
Females |
0.272 |
0.429 |
0.246 |
65 |
196 |
|
|
Males |
0.245 |
0.472 |
0.546 |
101 |
84 |
Identification of the growth rings
No growth rings could be clearly observed on asterisci, because the dorsal area of the otolith is too narrow, which makes it difficult to observe the continuity of the growth marks on the dorsal and ventral areas, as in the case of the asterisci of other members of the Centropomidae family as happens with C. nigrescens. Due to its thickness, growth rings in the lapillus could not be observed.
DISCUSSION
In the asterisci it was not possible to appreciate clearly the growth rings because the dorsal area is too narrow, this makes the continuity of the growth marks between the dorsal and ventral areas difficult to observe. In the case of C. nigrescens, it was possible to observe growth rings in the asterisci [16]. Due to its thickness, growth rings in the lapillus could not be observed.
As in other members of the Centropomidae family, growth rings identification on scales is very unreliable because the formation of rings has a very irregular pattern, also ring formation is not continuous, that is, the growth rings do not cover the entire scale, but consist of fragments of marks such as C. robalito [6] and Centropomus undecimalis [35]. Tovilla-Hernández & Castro-Aguirre [6] reported a maximum length of 32 cm or more. Average length determined by polymodal curve analysis are shown in table 12 (data from Zacapulco, coast of Chiapas, Mexico). Their average sizes considered for each polymodal curve are higher than those found by us, probably because of difference in feeding of a higher fishing pressure of this species and consequently a reduction in the commercial size.
|
Growthrings |
TL (cm) (a) |
TL (cm) (b) |
|
0 |
7.95 |
|
|
1 |
14.36 |
17 |
|
2 |
19.35 |
23 |
|
3 |
22.42 |
25 |
|
4 |
25.49 |
29.5 |
|
5 |
27.54 |
32 |
|
6 |
29.30 |
33.5 |
|
7 |
31.90 |
|
|
8 |
||
|
9 |
34.00 |
CONCLUSION
Growth rings could not be observed in asterisci because the dorsal area is too short. In the case of lapilli, marks were not recognized due to the thickness of the otolith. No statistically significant morphometric differences were observed between the right and left otolith, but between sexes there were differences in the case of the sagittae, asterisci and lapilli.
The growth of the three pairs of otoliths is eccentric to the core; a larger quantity of material is deposited in the dorsal areas and borders, in relation to the borders and ventral areas.
The relationship between total length of the fish and sagitta length showed that this structure is suitable to determine the age of this species.
Nine growth rings were identified on sagittae, nevertheless the time of formation of these rings will have to be evaluated.
RECOMMENDATIONS
The time of growth ring formation has to be evaluated to consider them as annual age groups and conduct studies on the growth of this species, calculating the von Bertalanffy growth constants and compare these results with those obtained by other authors.
Analysis of the reproduction of C. robalito has to be carried out in order to determine the sex proportion of sexes, hermaphroditism, fecundity, and lengths and season at which the phenomena of sexual inversion might take place, as well as reproduction zones for the species.
Also the analysis of feeding, hepatosomatic index, condition factor, relationships of length weight per sex, by season, months and different year are important. These studies will help have a better comprehension of the age and growth phenomena.
Finally, once this information is obtained, an analysis of the fishery should be carried out, to evaluate the captures, indexes and exploitation rates and suggest improvements in the fishery.
ACKNOWLEDGEMENT
REFERENCES
- Muhlia-Melo A, Arvizu-Martínez J, Rodríguez-Romero J, Guerrero-Tortolero D, Gutiérrez-Sánchez FJ, et al. (1995) Sinopsis de información biológica, pesquera y acuacultural acerca de los robalos del género Centropomus en México. Programa de Evaluación de Recursos Naturales del Centro de Inbestigaciones Biológicas del Noroeste, 51.
- Chávez H (1963) Contribución al conocimiento de la biología de los robalos chucumite y constantino (Centropomus spp.) del estado de Veracruz (Pisces: Centropomidae). Ciencia Mex 22: 141-161.
- Allen GR (1998) Peces del Pacífico Oriental Tropical, (2nd edn). Conabio, Agrupación Sierra Madre y Cemex, Texas, USA. Pg no: 327.
- Fischer W, Krupp F, Schneides W, Sommer C, Carpenter KE, et al. (1995) Guía FAO para la identificación de especies para los fines de la pesca: Vertebrados. FAO, Rome, Italy. Pg no: 644-1813.
- SAGARPA (2017) Secretaría de Agricultura, Ganadería, Desarrollo Rural, Pesca y Alimentación. SAGARPA, Mexico City, Mexico.
- Hernandez CH, Aguirre JLC (1981) Algunos aspectos de la biologia del robalo (Centropomus robalito, jord. Y gilb.) en el area lagunar de Zacapulco, Chiapas, México. Simposio Latinoamericano sobre Oceanografía Biológica, Acapulco, Guerrero, México. Pg no: 547-572.
- Gil-López HA, Labastida-Che A, Sarmiento-Náfate S, Villalobos-Toledo J, Oviedo-Piamonte JA, et al. (2010) Evaluación biológica del robalito (Centropomus robalito) en el Sistema Lagunar Mar Muerto, Oaxaca y Chiapas, Méx. V Foro Científico de Pesca Ribereña. Boca del Río, México. Pg no: 71-72.
- Madrid-Vera J, JA Rodríguez-Preciado, R Meraz-Sánchez, V Moreno-Bo-rrego, MA Osuna-Zamora (2012) Estructura de tallas y biomasa de Centropomus robalito capturado como fauna de acompañamiento de camarón en la zona costera marina de Sinaloa y Nayarit, México. VI Foro Científico de Pesca Ribereña. Tuxtla Gutiérrez, Mexico.
- Flores-Ortega JR, González-Sansón G, Aguilar-Betancourt C, Kosonoy-Aceves D, Venegas-Muñoz A, et al. (2015) Hábitos alimentarios de los jóvenes de Centropomus robalito (Centropomidae: Actinopterygii) en la laguna de Barra de Navidad, Jalisco, México. Int J Trop Biol 63: 1071-1081.
- Secor BW, Dean JM, Laban EH (1992) Otolith removal and preparation for microstructural examination. In: Stevenson DK, Campana SE (eds.). Otolith microstructure examination and analysis. Can Spec Publ Fish Aquat Sel 117. Pg no: 19-57.
- Gallardo-Cabello M, Espino-Barr E, Garcia-Boa A, Cabral-Solís EG, Puen-te-Gómez M (2006) Morphologic and morphometric analysis and growth rings identification of otoliths: Sagitta, Asteriscus and lapillus of Caranx caballus (Pisces: Carangidae) in the coast of Colima, Mexico. International Journal of Zoological Research 2: 34-47.
- Gallardo-Cabello M, Espino-Barr E, Nava-Ortega RA, Garcia-Boa A, Cabral-Solís EG, et al. (2011) Analysis of the otoliths of sagitta, asteriscus and lapillus of Pacific sierra Scomberomorus sierra(Pisces: Scombridae) in the coast of Colima México. Journal of Fisheries and Aquatic Science 6: 390-403.
- Gallardo-Cabello M, Espino-Barr E, Cabral-Solís EG, Puente-Gómez M, Garcia-Boa A (2012) Study of the otoliths of stripped mullet Mugil cephalus linnaeus, 1758 in Mexican Central Pacific. Journal of Fisheries and Aquatic Science 7: 346-363.
- Gallardo-Cabello M, Espino-Barr E, Cabral-Solís E, Puente-Gómez M, García Boa A (2014) Morphometric analysis on sagitta, asteriscus and lapillus of shortnose mojarra diapterus brevirostris(teleostei gerreidae) in cuyutlan coastal lagoon, colima, Mexico. Revista de Biología Marina y Oceanografía 49: 209-223.
- Gallardo-Cabello M, Espino-Barr E, Garcia-Boa A, Puente-Gómez M (2016) Analysis of the otoliths sagitta, asteriscus and lapillus of bigeye scad selar crumenophthalmus (teleostei: Carangidae) in Manzanillo bay, Mexican Central Pacific. International Journal of Development Research 6: 9541-9550.
- Gallardo-Cabello M, Espino-Barr E, Puente-GómezM, Garcia-Boa A (2017) Age analysis of Centropomus nigrescens by otoliths sagitta, asteriscus and lapillus in Mexican Central Pacific. International Journal of Development Research 7: 6499-16507.
- Gallardo-Cabello M, Espino-Barr E, Macías-Zamora R, Vidaurri-Sotelo AL (2017) Morphologic and morphometric analysis of the otoliths: Sagitta, asteriscus and lapillus of istiophorus platypterus (perciformes: Istiophoridae) in the Mexican Pacific coast. International Journal of Development Research 7: 16919-16926.
- Espino-Barr E, Gallardo-Cabello M, Garcia-Boa A, Cabral-Solís EG, Puente-Gómez M (2006) Morphologic and morphometric analysis and growth rings identification of otoliths: Sagitta, asteriscus and lapillus of Caranx caninus (Pisces: Carangidae) in the coast of Colima, Mexico. Journal of Fisheries and Aquatic Science 1: 157-170.
- Espino-Barr E, Gallardo-Cabello M, Cabral-Solís EG, Puente-Gómez M, García-Boa A (2013) Otoliths analysis of Mugil curema (Pisces: Mugilidae) in Cuyutlan Lagoon, Mexico. Avances de Invstigación Agropecuaria 17: 35-64.
- Espino-Barr E, Gallardo-Cabello M, Garcia-Boa A, Puente-Gómez M (2015) Growth analysis of Mugil cephalus (Percoidei: Mugilidae) in Mexican Central Pacific. Global Journal of Fisheries and Aquaculture. Science Research Journals 3: 238-246.
- Espino-Barr E, Gallardo-Cabello M, Valdez-Flores JJ, Garcia-Boa A (2018) Morphologic and morphometric analysis of the otoliths: Sagitta, asteriscus and lapillus of Kajikia audax and Makaira mazara (Perciformes: Istiophoridae) in the Mexican Pacific coast. Journal of Aquatic Science and Marine Biology 1: 12-23.
- Daniel WW (1991) Biostatistics: A Base for the Health Sicence’s Analysis. Noriega-Limusa, Mexico. Pg no: 667.
- Jerrold HZ (1996) Biostatistical Analysis, (3rd edn). Prentice Hall, Upper Saddle River, New Jersey, United States. Pg no: 662.
- Lagler KF, Bardach JE, Miller RR (1962) Ichthyology. John Wiley and Sons 2: 506.
- Mugiya Y (1964) Calcification in fish and shell-fish Seasonal occurrence of a pre-albumin fraction in the otolith fluid of some fish corresponding to the period of opaque zone formation in their otoliths. Bull Jap Soc Scient Fish 30: 445-467.
- Mugiya Y (1966) Calcification in fish and shell-fish. A study on paper electophoretic patterns of the acid mucopolysaccharides and Pas-positive materials in the otolith fluid of some fish. Bull Jap Soc Scient Fish 32: 117-129.
- Mugiya Y (1966) Calcification in fish and shell-fish Seasonal change in calcium and magnesium concentration of the otolith fluid in some fish with special reference to the zone formation of their otolith. Bull Jap Soc Scient Fish. 32: 549-557.
- Holst E (1950) Die Arbeitsweise des Statolithen-Apparates bei Fischen. Zeitschrift für vergleichende Physiologie 32: 60-120.
- Lowenstein O (1957) The sense organs, the acusticolateralis system. In: Brown ME (ed.). The physiology of fishes. Academic Press, Massachusetts, USA.
- Degens ET, Deuser WG, Haedrich RL (1969) Molecular structure and composition of fish otoliths. Mar Biol 2: 105-113.
- Hickling CF (1931) The structure of the otolith of the hake. Journal of Cell Science 74: 547-562.
- Sasaki H, Miyata J (1955) Experimentelle Studien über Otolithen. Z Laryngol Rhinol Otol 34: 740-748.
- Carlström D (1963) A crystallographic study of vertebrate otoliths. Biol Bull Mar Biol Lab 125: 441-463.
- Gallardo-Cabello M (1986) Estudio de la ultraestructura del otolito “sagitta” de la brótola phycis blennoides (Brunnich 1768) en el Mediterráneo occidental (Pisces: Gadidae). An Inst Cienc del Mar y Limnol UNAM 13: 197-206.
- Taylor RG, Whittington JA, Grier HJ, Crabtree RE (2000) Age, growth, maturation, and protandric sex reversal in common snook, Centropomus undecimalis, from the east and west coasts of South Florida. Fish Bull 98: 612-624.
Citation: Espino-Barr E, Gallardo-Cabello M, Puente-Gómez M, Garcia-Boa A (2019) Study of the Age of Centropomus robalito by Otoliths Analysis of Sagitta, Asteriscus and Lapillus in Mexican Central Pacific. J Aquac Fisheries 3: 013.
Copyright: © 2019 Elaine Espino Barr, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.

