
An Improved Combination Treatment for Ensuring Safety and Extending Shelf Life of Sweet Corn Kernels
*Corresponding Author(s):
Satyendra GautamFood Technology Division, Bhabha Atomic Research Centre, Mumbai, India
Tel:+91 2225595379,
Fax:+91 2225505151
Email:sgautam@barc.gov.in
Abstract
The shelled sweet corn kernel is prone to microbial and pathogenic contaminations primarily due to post-harvest handling which affect its safety and storage life. As an attempt to address this issue, a method was developed which included NaOCl wash (200 ppm; 5 min), hot water blanching (60°C; 5 min), and gamma irradiation (5 kGy). The treatments resulted in shelf life extension up to 30 days upon storage at 4°C. Beyond this storage, microbial load was found to be quite high with sharp declination in organoleptic rating. Now, the method has been further improved by replacing chlorination with other treatments such as thermosonication and antioxidant (cold ascorbate) dip. Besides, in the improved method required radiation dose was reduced to 2 kGy instead of 5 kGy. Thus, the improved method included thermosonication (34 kHz; 60°C; 7 min), cold ascorbate dip (4°C; 0.5%; 5 min), air drying (2 h), vacuum packaging (40%) and gamma irradiation (2 kGy) which extended the shelf life of sweet corn kernels up to 40 days when stored at 4°C. These samples were found to be free from presumptive coliform and Staphylococcus species and also low in microbial load. The quality attributes in terms of physical, nutritional, sensory and antioxidant properties were found to be well retained during storage. The study provides better combinations to ensure safety and extend shelf life of the shelled sweet corn kernels.
Keywords
INTRODUCTION
The shelled sweet corn kernel is widely used in various ready-to-eat fast food preparations worldwide as salads, steamed corn, and soup [1,2]. The kernels are quite vulnerable to microbial and pathogenic contaminations due to high moisture and sugar contents and hence, cannot be considered safe for raw consumption [3]. Partial cooking/steaming may not completely ensure its safety. A recent study from this laboratory has reported the occurrence of presumptive coliforms in freshly procured shelled sweet corn kernels from the market [4]. The major source of contamination is post-harvest handling such as shelling and further processing which results in its poor storage life. To address these issues, some studies have been performed earlier. These included film -over-wrap in tray and polyolefin stretch films [5]; shrink wrapping, refrigeration, and gamma irradiation (upto 1 kGy) [6]; and packaging in perforated packets, cold (4°C) acclimatization for 5 days followed by storage at -1°C [7]. The shelf life of kernels could not be extended beyond 20 days by these treatments. Some of these treatments even could not seem to be effective in ensuring inactivation of pathogens. The data from the USA (~ 713 produce-related outbreaks between 1990 and 2005) and the UK (~ 88 outbreaks between 1996 and 2006) have reported the involvement of fresh agri-produce in foodborne illnesses [8].
Recently, a combination process was developed in this laboratory. The process involved chlorination (NaOCl; 200 ppm, 5 min), blanching (60°C, 5 min), packaging in Low Density Polyethylene (LDPE) packets and gamma irradiation (5 kGy). The process could extend the shelf life of kernels up to 30 days at 4°C. Although chlorination has been recommended as a sanitization treatment for different food applications by US FDA, its direct application in food has not been approved in European countries such as the Netherlands, Sweden, Germany, and Belgium [9]. The prime reason for this is the safety concerns associated with possible formation of chlorinated compounds such as trihalomethanes, chloramines, haloketones, chloropicrins, and haloacetic acids, suspected to be potential carcinogens [10,11]. Hence, an utmost need was felt to improve the combination process by replacing chlorination with another physical sanitization treatment.
Ultrasonication treatment was explored as an alternative to the chlorination. Ultrasound refers to pressure waves with a frequency of ≥ 20 kHz. Higher-power ultrasound at lower frequencies (20 to 100 kHz), is referred to as “power ultrasound” and has the ability to cause cavitations, which has applications in food processing to inactivate microorganisms [12]. A major advantage of ultrasound is that the sound waves are generally considered safe and non-toxic [13]. In parallel, ultrasonication coupled with blanching (thermosonication) was also explored. As a part of combination treatment, the processed samples were stored at low temperature (4ºC). Ascorbic acid treatment has several beneficial properties such as control of browning and discoloration in food samples due to antioxidant and pH lowering properties [14,15]. In the improved combination, effect of vacuum packaging was also examined as lack of O2 in packages minimizes the radiation induced oxidative changes and storage associated quality deterioration [16]. As a final treatment, gamma irradiation was included in the process however, here the dose was reduced which may help in making the process more cost effective and abide the new food rule governing radiation processing approved by Government of India [17]. Irradiation is a non-thermal food processing technology having wide range of applications and has been approved by many national/international organizations such as World Health Organization (WHO), Food and Agriculture Organization (FAO), International Atomic Energy Agency (IAEA), and codex alimentarius and Food Safety and Standards Authority of India (FSSAI) [18,19].
To sum up, in the current study, a popular sweet corn variety (sugar 75) was subjected to various treatments such as thermosonication, ascorbate dip, vacuum packaging, and gamma irradiation for ensuring safety and extending the shelf life. The treated samples were stored at low temperature (4°C) and periodically examined for microbial, sensory as well as physical, biochemical and antioxidant properties.
MATERIALS AND METHODS
Procurement of sweet corn
Chemicals
Treatment conditions
Microbiological analysis
Analysis of physical qualities
Analysis of biochemical properties and antioxidant capacity
Organoleptic evaluation
Analysis of genotoxic safety of the processed product
Statistical analyses
RESULTS AND DISCUSSION
Microbiological quality of fresh shelled kernels
| Treatments | Storage Period (days) | APC1 | ASC2 | AnSC3 | PC4 | PS5 | YMC6 |
| Fresh (Control) | 0 | 8.6 ± 0.06t | 2.3 ± 0.04 | 3.1 ± 0.02t | 4.5 ± 0.03t | 5.1 ± 0.05t | 6.2 ± 0.09t |
| Ultrasonication At ambient temp (26 ± 2°C; 7 min) |
0 | 6.2 ± 0.07u | ND* | 1.3 ± 0.01u | 4.2 ± 0.04t | 3.6 ± 0.05u | 4.3 ± 0.03u |
| At blanching temp. (60°C; 7 min) | 0 | 2.3 ± 0.02v | ND | ND | ND | ND | ND |
| Irradiation (2 kGy) | 0 | 4.8 ± 0.03w | ND | ND | ND | ND | 3.6 ± 0.04v |
| Ultrasonication (60°C; 7 min) and Irradiation (2 kGy) | 0 | ND | ND | ND | ND | ND | ND |
| Processed# | 0 | ND | ND | ND | ND | ND | ND |
| 35 | 2.1 ± 0.01v | ND | ND | ND | ND | ND | |
| 40 | 3.1 ± 0.02x | ND | ND | ND | ND | ND | |
| 45 | 5.1 ± 0.04y | ND | ND | ND | ND | ND |
Evaluation of different processing methods to achieve hygienization with retained sensory quality of shelled sweet corn kernels
Treatments for microbial decontamination
Thermosonication (60°C) was optimized through a preliminary trial study to achieve maximum microbial inactivation without affecting sensory qualities such as textural and discoloration of samples (data not shown). Ultrasonication treatment at the blanching temperature of 60°C for 7 min was found to reduce APC by 6 log cfu/g and ASC, AnSC, PC, PS, and YMC counts to below detectable level (Table 1). In other study too, thermosonication was found to be more effective than blanching (65°C) alone in reducing microbial load from red bell peppers, strawberries and watercress [30]. Thus, in the current study, thermosonication was preferred for sample processing instead of sonication alone.
Further, combinations of thermosonocation (60°C, 7 min) or gamma radiation (2 kGy) were explored to achieve the best possible level of hygienization (preferably < 10 cfu/g). This will help in extending the shelf life of the processed kernels up to the maximum period and thus reduce the post harvest losses. The combination of thermosonication and gamma radiation reduced the microbial load to below detectable level (Table 1).
Treatments for retention of organoleptic quality:
Effect of ascorbate dip: The thermosonicated samples were dipped in cold ascorbate (4°C, 0.5%) which was found to prevent discoloration during storage and thus helped in retaining its visual appeal (data not shown). The cold treatment has earlier been reported in reducing the over blanching effect of the product [31]. Ascorbate at the concentration > 0.5% was not found to further improve the shelf life and appeal of the product (data not shown). Ascorbate at 0.5% concentration has also been reported earlier for prevention of egg plant discoloration [32].
Effect of vacuum packaging: The above treated (thermosonicated and cold ascorbate dipped) samples were air dried under aseptic condition for 2 h and vacuum (40%) packed to extend the life by reducing radiation induced or storage associated oxidative damage and inhibiting growth of microbes [33]. Vacuum packaging at 40% was found to be optimal for the produce. At further higher level of vacuum (50 or 60%), significant change in texture was observed in the samples (data not shown).
Optimized processing protocol
Cold water (sterile) instead of cold ascorbate (0.5%) dip was not found to be very effective. Similarly, ascorbate (0.5%) dip at ambient temperature was also not found to be effective. These changes were found to be critical as gradual discoloration in these samples observed during storage (data not shown). Organoleptically, these samples were acceptable only up to 25-30 days (data not shown).
Quality evaluation of processed samples during storage
Microbiological quality:
Physico-chemical properties and antioxidant capacity:
| Unprocessed | Processed# | ||||
| Parameters | 0 d | 15 d | 0 d | 40 d | 45 d |
| Moisture (%) | 73 ± 2a | 65 ± 2b | 74 ± 2a | 74 ± 1a | 74 ± 1a |
| Texture (g) | 31 ± 3a | 23 ± 4b | 30 ± 3a | 32 ± 4a | 32 ± 6a |
| Color | |||||
| ‘L’ value | 81.2 ± 3.6a | 83.0 ± 2.1a | 81.8 ± 2.1a | 79.4 ± 1.5a | 80.7 ± 1.4a |
| ‘a’ value | 5.4 ± 1.5a | 4.6 ± 2.3a | 5.0 ± 0.7a | 5.5 ± 1.1a | 3.0 ± 1.1b |
| ‘b’ value | 28.4 ± 6.4a | 28.9 ± 4.7a | 8.5 ± 1.9a | 30.0 ± 2.5a | 28.7 ± 2.6a |
| ‘C’ | 26.8 ± 6.6a | 28.8 ± 5.2a | 28.9 ± 2.0a | 30.4 ± 2.4a | 28.9 ± 2.8a |
| ‘h’ | 79.7 ± 2.2a | 81.1 ± 3.3a | 80.0 ± 1.8a | 80.2 ± 1.5a | 83.2 ± 1.7b |
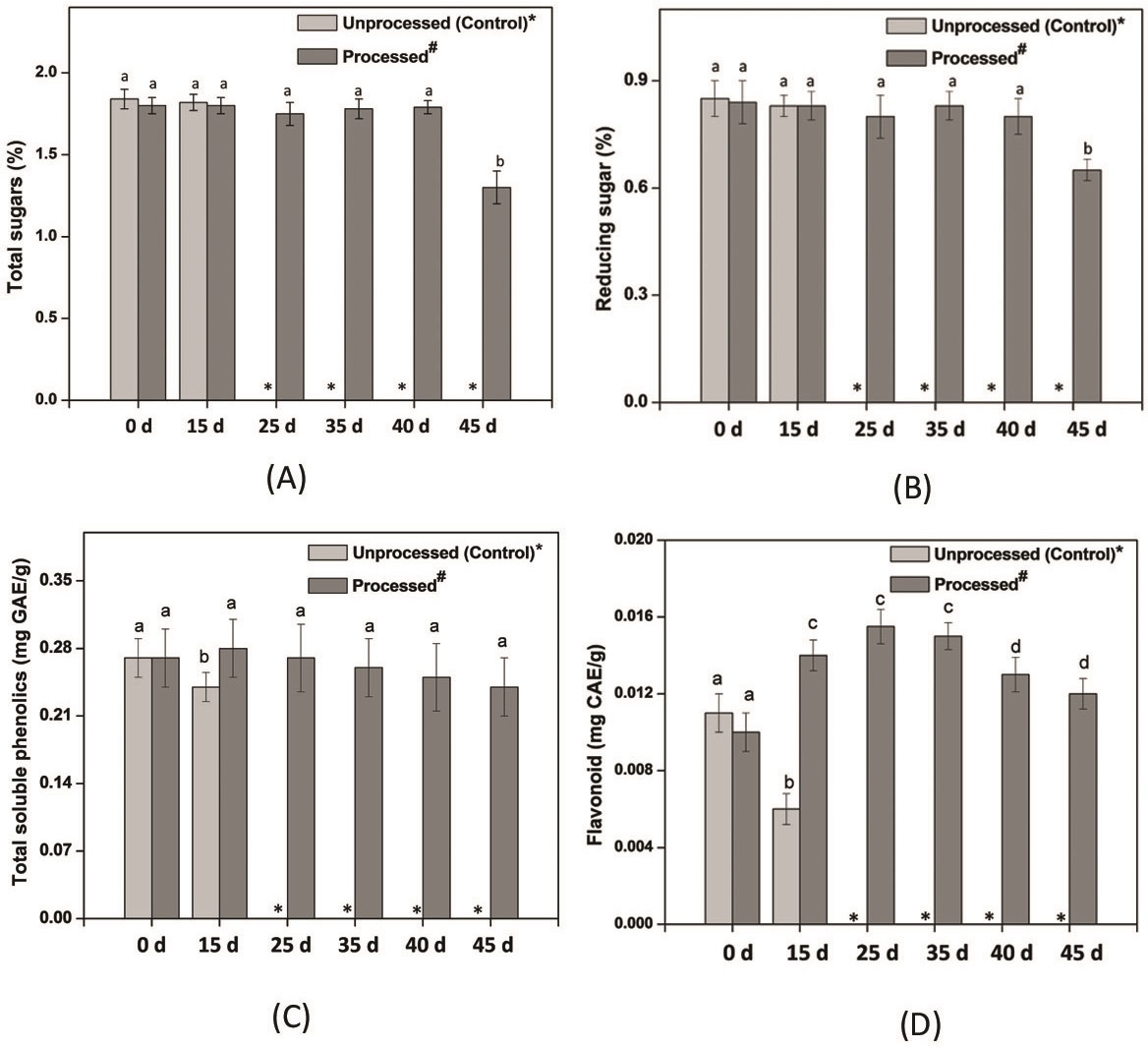 Figure 1: Biochemical properties of Processed# sweet corn kernels during storage.
Figure 1: Biochemical properties of Processed# sweet corn kernels during storage.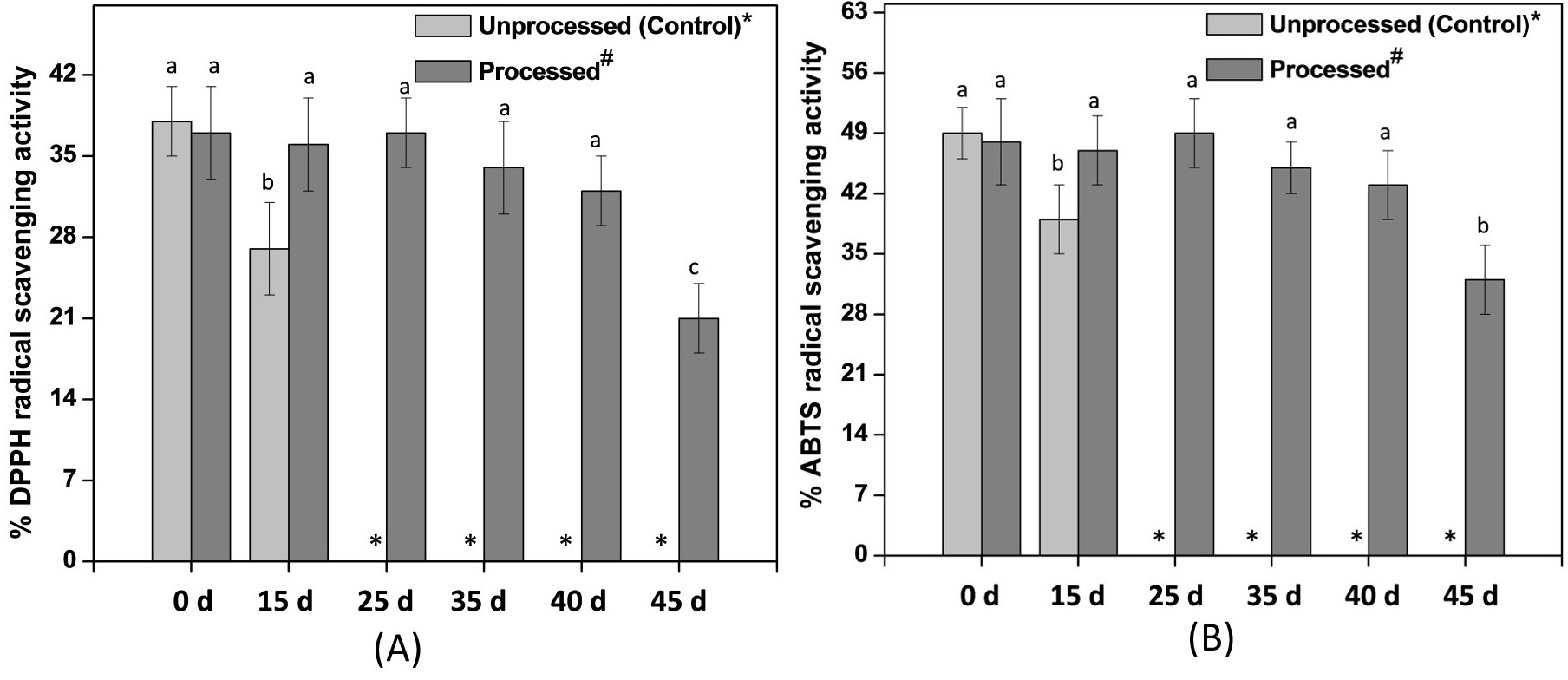
Organoleptic properties:
| Sensory attributes | Unprocessed | Processed# | ||
| 0 d | 2 d | 0 d | 4 d | |
| Appearance | 7.9 ± 1.0a,t | 7.5 ± 1.0a,t | 7.8 ± 0.9a,t | 7.5 ± 1.0a,t |
| Aroma | 7.6 ± 1.2a,t | 4.3 ± 1.1b,u | 7.6 ± 1.1a,t | 7.6 ± 0.9a,t |
| Taste | 7.5 ± 0.9a,t | 5.2 ± 0.7b,u | 7.5 ± 1.2a,t | 7.3 ± 1.1a,t |
| Texture | 7.2 ± 1.1a,t | 6.1 ± 0.6a,t | 7.1 ± 1.2a,t | 7.2 ± 0.8a,t |
| Overall acceptability | 7.5 ± 0.9a,t | 4.6 ± 0.9b,u | 7.4 ± 1.1a,t | 7.2 ± 1.1a,t |
Genotoxic safety assessment
CONCLUSION
A process including thermosonication, cold ascorbate dip, air drying, vacuum packaging in LDPE and gamma radiation (2 kGy) was found to ensure microbiological safety and extend the shelf life of freshly shelled sweet corn kernels up to 40 days at 4°C. Thus, developed method assured better shelf life than our earlier reported combinations where shelf life up to 30 days was achieved. The quality attributes in terms of physical, nutritional, sensory, and antioxidant properties of combination processed kernels were also well retained during storage.
ACKNOWLEDGEMENTS
The authors wish to express thanks to Ajaykumar Yadav and Varsh More for their help during the investigation. Authors declare that there is no conflict of interest. The funding agency is the Government of India.
REFERENCES
- Esena RK, Owusu E (2013) Quality of cooked foods in urban schools in Ghana: Review of food borne diseases and health implications. International Journal of Scientific & Technology Research 2: 267-275.
- López-Martínez LX, Parkin KL, Garcia HS (2012) Effect of processing of corn for production of masa, tortillas and tortilla chips on the scavenging capacity of reactive nitrogen species. Int J Food Sci Technol 47: 1321-1327.
- Suleiman RA, Rosentrater KA, Bern CJ (2013) Effects of deterioration parameters on storage of maize: A review. Journal of Natural Sciences Research 3: 147-165.
- Kumar S, Gautam S, Sharma A (2015) Hurdle technology including chlorination, blanching, packaging and irradiation to ensure safety and extend shelf life of shelled sweet corn kernels. Journal of Food Processing and Preservation 39: 2340-2347.
- Aharoni Y, Copel A, Gil M, Fallik E (1996) Polyolefin stretch films maintain the quality of sweet corn during storage and shelf-life. Postharvest Biology and Technology 7: 171-176.
- Deák T, Heaton EK, Hung YC, Beuchat LR (1987) Extending the shelf life of fresh sweet corn by shrink-wrapping, refrigeration, and irradiation. Journal of Food Science 52: 1625-1631.
- Shao X, Li Y (2011) Quality control of fresh sweet corn in controlled freezing-point storage. African Journal of Biotechnology 10: 14534-14542.
- Goodburn C, Wallace CA (2013) The microbiological efficacy of decontamination methodologies for fresh produce: A review. Food Control 32: 418-427.
- US Food and Drug Administration (2013) Microbiological safety evaluations and recommendations on sprouted seed. US Food and Drug Administration, Maryland, USA.
- Bachelli ML, Amaral RD, Benedetti BC (2014) Alternative sanitization methods for minimally processed lettuce in comparison to sodium hypochlorite. Braz J Microbiol 44: 673-678.
- Ölmez H, Kretzschmar U (2009) Potential alternative disinfection methods for organic fresh-cut industry for minimizing water consumption and environmental impact. LWT-Food Sci Technol 42: 686-693.
- Piyasena P, Mohareb E, McKellar RC (2003) Inactivation of microbes using ultrasound: a review. Int J Food Microbiol 87: 207-216.
- Kentish S, Ashokkumar M (2011) The physical and chemical effects of ultrasound. In: Feng H, Barbosa-Cánovas, Weiss J (eds.). Ultrasound Technologies for Food and Bioprocessing. Springer, London, UK. Pg no: 1-12.
- Garcia E, Barrett DM (2002) Preservative treatments for fresh-cut fruits and vegetables. In: Lamikanra O (ed.). Fresh-cut fruits and vegetables: Science, technology and market. CRC Press, Boca Raton, Florida, USA.
- Cortez-Vega WR, Becerra-Prado AM, Soares JM, Fonseca GG (2008) Effect of L-ascorbic acid and sodium metabisulfite in the inhibition of the enzymatic browning of minimally processed apple. International Journal of Agricultural Research 3: 196-201.
- Zhou GH, Xu XL, Liu Y (2010) Preservation technologies for fresh meat - a review. Meat Sci 86: 119-128.
- Food Safety and Standards Authority of India (2012) Draft Food Safety and Standards (Packaging and Labelling) & (Food Products Standards and Food Additives) (Amendment) Regulations, 2015 related to inclusion of New Atomic Energy (Radiation Processing of Food and Allied Products), Rules, 2012. Food Safety and Standards Authority of India, New Delhi, India.
- Diehl JF (1995) Safety of irradiated foods. Marcel Dekker Inc., New York, USA.
- Roberts PB (2014) Food irradiation is safe: Half a century of studies. Radiation Physics and Chemistry 105: 78-82.
- Kumar S, Mishra BB, Saxena S, Bandyopadhyay N, More V, et al. (2012) Inhibition of pericarp browning and shelf life extension of litchi by combination dip treatment and radiation processing. Food Chemistry 131: 1223-1232.
- US Food and Drug Administration (1988) Bacteriological analytical manual. US Food and Drug Administration, Arlington, Virginia, USA.
- Kumar S, Khade HD, Dhokane VS, Behere AG, Sharma A (2007) Irradiation in combination with higher storage temperatures maintains chip-making quality of potato. J Food Sci 72: 402-406.
- Kumar S, Gautam S, Powar S, Sharma A (2010) Microbial decontamination of medicinally important herbals using gamma radiation and their biochemical characterisation. Food Chemistry 119: 328-335.
- Arnao MB, Cano A, Acosta M (2001) The hydrophilic and lipophilic contribution to total antioxidant activity. Food Chemistry 73: 239-244.
- Hajare SN, Gautam S, Nair AB, Sharma A (2014) Formulation of a nasogastric liquid feed and shelf-life extension using gamma radiation. J Food Prot 77: 1308-1316.
- Kumar S, Gautam S, Sharma A (2013) Antimutagenic and antioxidant properties of plumbagin and other naphthoquinones. Mutat Res 755: 30-41.
- Kumar S, Gautam S, Sharma A (2013) Identification of antimutagenic properties of anthocyanins and other polyphenols from rose (Rosa centifolia) petals and tea. J Food Sci 78: 948-954.
- Jeddi MZ, Yunesian M, Gorji ME, Noori N, Pourmand MR, et al. (2014) Microbial evaluation of fresh, minimally-processed vegetables and bagged sprouts from chain supermarkets. J Health Popul Nutr 32: 391-399.
- Alighourchi H, Barzegar M, Sahari MA, Abbasi S (2014) The effects of sonication and gamma irradiation on the inactivation of Escherichia coli and Saccharomyces cerevisiae in pomegranate juice. Iran J Microbiol 6: 51-58.
- Alexandre EMC, Santos-Pedro DM, Brandão TRS, Silva CLM (2011) Study on thermosonication and ultraviolet radiation processes as an alternative to blanching for some fruits and vegetables. Food and Bioprocess Technology 4: 1012-1019.
- De Corcuera JIR, Cavalieri RP, Powers JR (2004) Blanching of foods. In: Heldman DR (ed.). Encyclopedia of agricultural, food, and biological engineering. Marcel Dekker Inc., New York, USA.
- Barbagallo RN, Chisari M, Patanè C (2012) Use in vivo of natural anti-browning agents against polyphenol oxidase activity in minimally processed eggplant. Chem Eng Trans 27: 1-6.
- Gould GW (1996) Methods for preservation and extension of shelf life. Int J Food Microbiol 33: 51-64.
- Jouki M, Yazdi FT (2014) The effect of gamma irradiation and vacuum packaging upon selected quality traits of refrigerated ostrich meat. Part 2. Colour, texture and lipid oxidation properties. Animal Science Papers and Reports 32: 161-171.
- Mantilla SPS, Santos EB, Vital HC, Mano SB, Freitas MQ, et al. (2011) Microbiology, sensory evaluation and shelf life of irradiated chicken breast fillets stored in air or vacuum. Braz Arch Biol Technol 54: 569-576.
- United States Department of Agriculture (2014) Microbiological standards and guidelines. United States Department of Agriculture, Beltsville, Maryland, USA.
- United Nations Industrial Development Organization (1984) Guidelines for commercial plantation and manufacture of medicinal and aromatic plants. United Nations Industrial Development Organization, Vienna, Austria.
- Shao Y, Luo Y, Chen A, Chu H, Lu C, et al. (2011) Effects of a vacuum infiltration-based method with ascorbic acid on internal browning of plum (Prunus salicina lindell cv. Yuhuang) during cold storage. Journal of Food Processing and Preservation 35: 581-586.
- Luo Y, Wang Q (2012) Bioactive compounds in corn. In: Yu L, Tsao R, Shahida F (eds.). Cereals and pulses: nutraceutical properties and health benefits. John Wiley & Sons, Hoboken, NJ, USA.
- Riad GS, Brecht JK (2001) Fresh-cut sweet corn kernels. Proc Fla State Hort Soc 114: 160-163.
Citation: Kumar S, Gautam S (2016) An Improved Combination Treatment for Ensuring Safety and Extending Shelf Life of Sweet Corn Kernels. J Food Sci Nutr 2: 006.
Copyright: © 2016 Sanjeev Kumar, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.

