
Effect of Yogurt With or Without Probiotic Addition on Body Composition Changes and Immune System in an Obese Model
*Corresponding Author(s):
Carolina Maldonado-GaldeanoCentro De Referencia Para Lactobacilos, Cátedra De Inmunología, Instituto De Microbiologia, Fac Bqca Qca Y Fcia, CERELA-CONICET, Tucumán, Argentina
Fax:+54 03814005600
Email:cmaldo@cerela.org.ar
Abstract
Yogurt is a dairy product made by milk fermented with Lactobacillus bulgaricus and Streptococcus thermophilus. Probiotic bacteria are added to conventional yogurt in order to boost benefits on the consumer health.
In present work we studied in an obese mouse model the effect of the yogurt or probiotic yogurt supplementation to the diet on body weight, in biochemical parameters and in the thymus and intestinal immune system improvement. Adult mice received conventional balanced diet or a high-fat diet supplemented with milk, conventional yogurt or probiotic yogurt over 60 d. The body weight and biochemical parameters in serum (glucose, total HDL-cholesterol and triglycerides) were determined. Small intestine and thymus histology were analyzed. The changes in the gut microbiota, the possible translocation to internal organs and the levels of IgA and cytokines were also investigated. Probiotic yogurt was the most effective supplement for decreasing body weight and normalizing biochemical parameters in serum and in the improvement of small intestine and thymus histology. The gut microbiota showed increased bifidobacteria and recovered the balance of Enterobacteria population in obese mice given probiotic yogurt without translocation to internal organs, showing increases in IgA+ cells and cytokine production in gut. The present study demonstrated the safety and useful of yogurt consumption in obese host, benefiting mainly the innate mucosal immunity, biochemical parameters, intestine and thymus histology. These results allows advance in the research about the influence of functional foods on the thymus cells activity, which play a key role in the immune response.
Keywords
INTRODUCTION
Yogurt is a millenary fermented food with high digestibility, bioavailability of nutrients, it is contains lactic acid bacteria as Lactobacillus bulgaricus and Streptococcus thermophilus, which may affect positively gut microbiota [1]. Méchnikov in 1907 in the book “The prolongation of life” describe the importance of the consumption of fermented product on the microbiota [2]. Yogurt is a complex mixture of proteins (whey protein and casein in the ratio of 20:80), fats (saturated, mono and poly-unsaturated fatty acids) and carbohydrate (lactose), providing high-quality nutrients, which have a wide range of bioactivities [3]. Yogurt consumption has been associated with healthy dietary patterns and lifestyles, better diet quality and healthier metabolic profiles. Recent epidemiological and clinical evidence suggests that yogurt contributes to better metabolic health, including the control of energy balance, body weight and glycemia.
Nowadays the original yogurt can be added with other lactic acid bacteria considered probiotics.
The word “probiotic” originated from Greek meaning “for life.” Probiotics are defined as “live microorganisms, which when administered in adequate amounts confer a health benefit on the host” [4].
Hundreds of different bacteria species are the natural and predominant constituents of intestinal microbiota. Among the numerous intestinal microbes, those that exhibit potential health benefits to the host through modulation of the intestinal microbiota are commonly selected as probiotics. Species belonging to the genera Lactobacillus (L) and Bifidobacterium (B) have been reported to be the beneficial probiotic bacterial strains. The representative species include L. acidophilus, L. casei, L. plantarum, B. lactis, B. longum, and B. bifidum [5].
Health benefits include: controlling gastrointestinal infections, improvement in lactose metabolism, anticarcinogenic and antimutagenic properties, reduction in serum cholesterol, and improvement in inflammatory bowel disease, Helicobacter pylori infections, and immune system stimulation [6].
The mechanism by which the immune system is modulated by probiotic organisms is not entirely known. Probiotic bacteria may counteract the inflammatory process by stabilizing the gut microbial environment and the permeability of the intestinal barrier. One mechanism of action of probiotic microorganisms include promotion of immunologic barrier via improvement of the intestinal secretory immunoglobulin A (S-IgA) and innate responses or on the non-immunologic gut defense barrier by controlling the intestinal permeability, increasing the intercellular adhesion molecules of the epithelium, or influencing the gut microecology [4,7].
At present there is much evidence concerning the role of probiotics on the health, especially Lactic Acid Bacteria (LAB). Probiotics include in fermented food, may exert a beneficial effect on allergic reaction and in lactose intolerance, and have also been attributed other effects, such as the increase of nutrient bioavailability, the decrease of the serum cholesterol concentrations, and the improvement of urogenital health [8].
Some of effects from probiotic fermented milk containing probiotic strain have been attributed to an increase in the innate immune response and others to an increase in the acquired immune response [9]. Food containing probiotic bacteria are also able to stimulate the immunoglobulin A (IgA) immune response.
The immunological properties of probiotic bacteria have been studied previously and showed that certain LAB, such as Lactobacillus casei, Lactobacillus rhamnosus, and Lactobacillus plantarum enhance both systemic and mucosal immunity. In vitro studies have shown that several LAB strains promote the immunopotentiator capacity of cells from the innate immune system, as macrophages [9].
The diseases that impairs the balance between energy intake and caloric expenditure, such as malnutrition and obesity, impairs nutritional status will have a direct effect on the immune system, increasing the risk of infections, inflammatory processes and other associated pathologies that affect the quality of life and may even lead to the death [10].
The malnourished host has an impairment of intestinal barrier functions, indicating that the intestine may become increasingly permeable to the absorption of dietary and other environmental antigens. In severe malnutrition there is bacteria translocation of the normal microbiota. It was well demonstrated that the presence of a number of growth factors and hormones in the milk of various species including human and bovine together with the low proteolityc activity in the gastrointestinal tract, suggest the potential use of these milks in the renutrition process. In the same way in fermented milk, the peptides produced in the fermentation process are functional and can improve the digestive tract, or elsewhere in the body and exert an immunomodulatory effect [11].
The beneficial effect of certain probiotic strains on body weight, metabolic parameters and some immunological parameters evaluated in an obesity animal models, diabetes and hyperlipidemia have been previously described [12].
It was also demonstrated that probiotics can improve the microbiota in obesity and modulate the genes associated with the liver metabolites and the adipose tissue [13-18]. These facts show to probiotic supplementation to the diet as possible alternative to help the immunity in obesity and on its associated disorders.
The aim of the present work was to study the effect of the supplementation to the diet with yogurt or probiotic yogurt on body weight, biochemical parameters and in the improvement of the intestinal immune system and on thymus recovery in an obese mice model.
MATERIALS AND METHODS
Animals and study design
Low-fat milk, yogurt and probiotic yogurt were used as dietary supplement. Yogurt and probiotic yogurt was provide by DANONE SA, it came in two bottles labeled and stored in refrigerator at 4°C. The conventional yogurt contained yogurt starter cultures (L. delbrueckii subsp. bulgaricus 108 CFU/ml and Streptococcus thermophilus 108 CFU/ml) Probiotic yogurt contained the starter bacteria and the probiotic bacteria Bifidobacterium animalis (DN-173 010), added in the same concentration (108 CFU/ml) to ensure that a large number of viable bacteria reach the intestine [19]. The probiotic and the started bacteria used in the yogurt elaboration can resist the adverse condition to gastrointestinal tract and arrive alive to the small intestine, without necessary to be included in a protect cover [20,21]. Each group was subdivided in sub-groups according to dietary supplement administered.
Groups
- Normal Control (NC): The animals were fed ad libitum with conventional food and water, and three sub groups that received conventional diet supplement with: Low Fat Milk (M), Yogurt (Y) or Probiotic Yogurt (PY).
- Obese Control (OC): The animals were fed ad libitum with HFD and water during 60 days. Three subgroups received HFD similar to OC and they were also supplemented with Low-Fat Milk (OM), Yogurt (OY) or Probiotic Yogurt (OPY) during 60 days.
The volume of the each supplement consumed was measured daily. Each animal consumed about 2-3 ml of liquid per day. The bottles containing the supplements were replaced daily to maintain the quality of them.
The mice were maintained in a room with 12-h light/dark cycles at 20±2°C; they were weighted three times per week.
Mice from each group were sacrificed at 30 and 60 days by cervical dislocation. Serum, intestinal fluid, small intestine, large intestine, thymus, liver and spleen were taken for further studies. All animal protocols were preapproved by the Animal Protection Committee CERELA (CRL-BIOT-LI-2010/1A) and all experiments comply with the laws in Argentina.
Determination of body and organ weight
Determination of biochemical parameters
Analysis of bacteria translocation
Analysis of some population of the intestinal microbiota
Sampling of the intestinal fluid and small intestine for histological studies
Determination of IgA+ cells in the small intestine by direct Immunofluorescence
Cells were observed with fluorescent light microscope (Carl Zeiss, Germany). The number of fluorescent cells, in the lamina propria of the small intestine, was counted in 30 fields of vision at 1000x magnification. Results were expressed as the number of positive cells per 10 fields of view.
Determination of cytokines and Secretory IgA (S-IgA) in intestinal fluid
Total S-IgA determination was performed as described by de Moreno de LeBlanc and others [23]: coating with goat anti-mouse IgA affinity-purified antibody (BETHYL Laboratories INC, Montgomery, TX, USA) was used. Kappa IgA purified immunoglobulin (SIGMA, Saint Louis, USA) was used as standard. Detection was performed with Antimouse IgA (α-chain specific) peroxidase conjugated developed in goat (SIGMA, Saint Louis, USA). All reactions were revealed and stopped with sulfuric acid 2 N. Absorbance read at 450 nm. Results for total S-IgA were expressed as concentration (µg/ml).
STATISTICAL ANALYSIS
For all the studies, each group assayed was from three mice. Each experiment was performed three times. All statistical analyses and plotting were carried out using Graph Pad Prism 6.01 software (Graph Pad Software, La Jolla, Calif). The significance of the difference was determined using one-way-ANOVA when more than 2 groups were conducted, whereas T test was conducted to compare two experimental groups. A P value <0.05 was considered as statistically significant.
RESULTS AND DISCUSSION
The worldwide prevalence of obesity increased more than doubled between 1980 and 2014 and for today WHO has declared obesity as global epidemic and took it under control [24]. In 2014, more than 1.9 billion adults older than 18 years (39%) are overweight. Overall, about 13% or 600 million of this adult population (11% of men and 15% of women) was obese. People affected by obesity are more likely to suffer from additional medical conditions, such as cardiovascular diseases (mainly heart disease and stroke), which were the leading cause of death in 2012; diabetes; musculoskeletal disorders (especially osteoarthritis); some cancers (including endometrial, breast, ovarian, prostate, liver, gallbladder, kidney, and colon) [13].
In a present paper we evaluated the useful of yogurt supplementation to obese mice in the body weight.
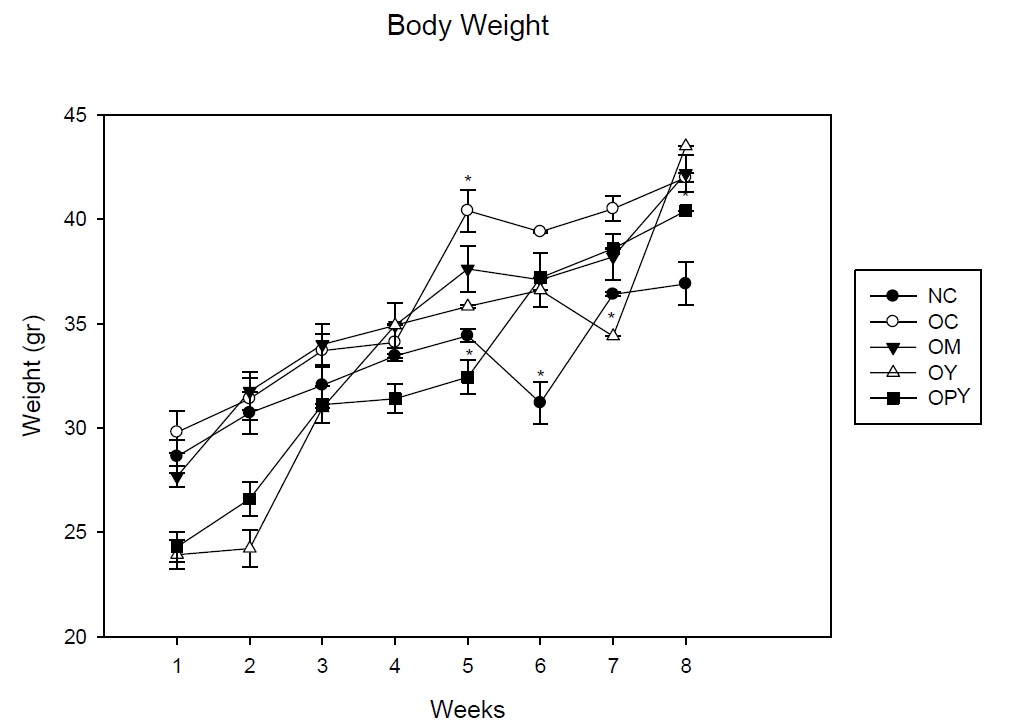
The HFD induced increases in the body weight of mice, showing a peak at 5 weeks, achieving a 30% increase in body weight over the normal control. At the same time, obese mice receiving probiotic yogurt as a supplement showed lower body weight than the other experimental groups even than normal control.
At 8 weeks, body weight of animals that received conventional yogurt was higher than the other experimental groups, while body weight of animals who were fed with probiotic yogurt decreased, being these values lower than the other groups that receiving the high fat diet. The increase in body weight registered of this group of mice was more controlled than the others obese groups (Figure 1).
It should be noted that the measurable effect of probiotic yogurt on this parameter was in the first months of administration (4-5 weeks), when the weight gain in obese control group is about 30% with respect to the non-obese mice, indicating weight regulation, even taking into account that the animals consumed the high-fat diet during all the time experience. At 60 days, where obesity increased over 50% of body weight, only the animals receiving probiotic yogurt have a smaller body weight than obese control mice. We did not found effect with the others supplementary diet.
As regard to the relation of the body weight/organs (liver, spleen and thymus), we observed not significant changes compared to the normal control in the relation body weight/liver or spleen for all the supplements administered. Obese control showed a significant diminution for body weight/spleen relation, the probiotic yogurt normalize values near to the normal control. The HFD increase thymus weight at 60 days, showing a diminution in the relation body weight/thymus. We observed for thymus a market effect with an increase in the weight at 60 days in the group given yogurt or probiotic yogurt (Table 1).
| Liver | Spleen | Thymus | ||||||||||
| 30 days | SD | 60 days | SD | 30 days | SD | 60 days | SD | 30 days | SD | 60 days | SD | |
| NC | 0,06a | 0,0082 | 0,05b | 0,0058 | 0,005a | 0,0005 | 0,006b | 0,00925 | 0,002c | 0,0040 | 0,004b | 0,0030 |
| OC | 0,05a | 0,0087 | 0,05c | 0,0047 | 0,003c | 0,0037 | 0,003d | 0,00048 | 0,003b | 0,00030 | 0,002a | 0,0012 |
| OM | 0,05b | 0,0050 | 0,05d | 0,0017 | 0,004a | 0,0004 | 0,003a | 0,00002 | 0,004d | 0,00086 | 0,002a | 1,00E-04 |
| OY | 0,06d | 0,0009 | 0,06e | 0,0003 | 0,006b | 0,0010 | 0,005a | 0,00068 | 0,003a | 0,00003 | 0,003b | 0,00026 |
| OPY | 0,06e | 0,0004 | 0,05c | 0,0037 | 0,005a | 2,1E-05 | 0,005a | 1,73E-05 | 0,004a | 0,00026 | 0,002d | 0,00058 |
The changes observed in thymus from obese mice were also evident at the level of histology, showing severe alterations in the structure of the thymus with increase in the cellularity, without differentiation between cortical and medullar zone in obese group at 8 weeks. Thymus histology was recovery after yogurt supplementation, being probiotic yogurt more effective, showing a similar structure to the normal control. This effect was observed previously with other probiotic milk [25]. Low fat milk supplementation did not have effect on thymus histology. Figure 2 show representative pictures of the different groups analyzed. The stimulation observed could be due to the production of IL-7 which is involved in the ontogeny and maturation of T lymphocytes [26-28]. These studies confirm that yogurt supplementation not only improves thymus weight as we mentioned before, but also significantly improves the histology of this organ (Figure 2).
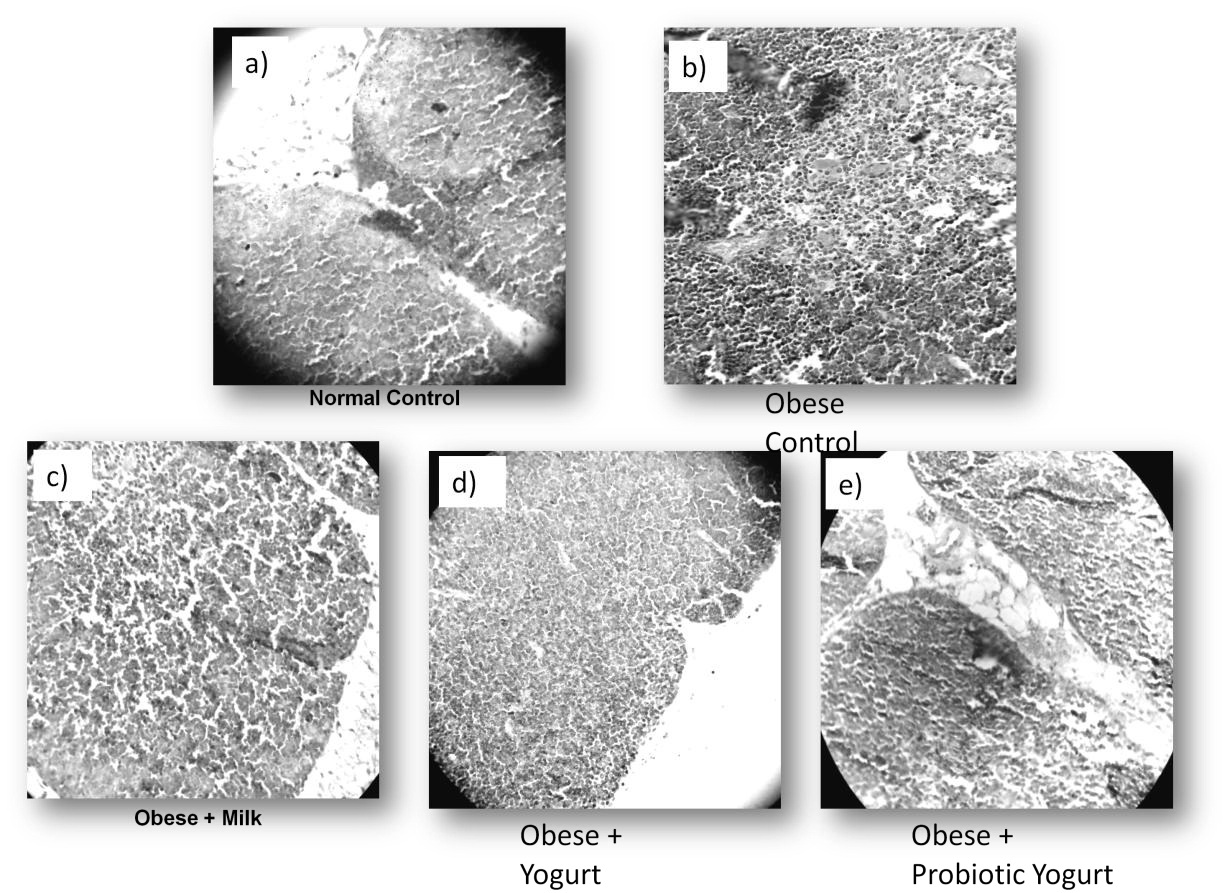
The results obtained with probiotic yogurt over thymus are important considering that, thymus is the organ responsible for the development of self-restricted, self-tolerant, immunocompetent T cells but has no self-renewal properties relying on the continuous replenishment of new T cell progenitors from the bone marrow. Maturation of these cells occurs through a series of proliferation and differentiation stages dependent upon receiving instructions from the specialized thymus microenvironment [29]. Recent studies show that a high fat diet accelerates the changes that occur in the thymus by over time [30]. This fact was evidenced in this work and we observe a positive effect after 30 days of probiotic yogurt administration, with an increase in the thymus weight and cellularity.
Different studies describe that obesity, associated with caloric excess, compromises the mechanisms regulating T-cell generation by inducing premature thymic involution compromising health and life expectancy [31]. However in other study, authors conclude that, the process of thymic aging, associated with the amount of calories ingested, is characterized by reduced production of naive T cells and replacement of lymphostromal thymic zones with adipose tissue [32]. The improvement in thymus´s weight and cellularity was also observed in malnourished animal model supplemented with probiotic fermented milk [25].
In obesity, it is well described that the level of glucose, total cholesterol, HDL, LDL in serum are increased, as a consequence of metabolic syndrome [33-38]. It was also described that some probiotic strain can improve these values near to the non-obese control [39]. In our study, the analysis of the glucose, cholesterol, LDL, HDL and triglycerides values showed increases in the obese control for 30 days and 60 days in relation to the normal control. Both yogurt administrations induced a decrease in the values for the parameters assayed being the effect more evident for probiotic yogurt. However the values did not reach the NC. The group with low fat milk was not effective and showed similar values to the obese control (Table 2). These results indicate the usefulness of the yogurt administration even in process of obesity to ameliorate the biochemical parameters and this fact is closely related with the weight modulation observed after yogurt supplementation (Table 2).
| Groups | Glucose | Total Cholesterol | LDL | HDL | Triglycerides | |||||
| 30 days | 60 days | 30 days | 60 days | 30 days | 60 days | 30 days | 60 days | 30 days | 60 days | |
| NC | 126,5 ± 0,71 | 108,7 ± 8,1 | 113,5 ± 6,9 | 94,7 ± 4,1 | 129,5 ± 2,3 | 87 ± 8,1 | 70 ± 6,9 | 54,7 ± 4,1 | 258 ± 2,8 | 86,5 ± 2,8 |
| OC | 220 ± 19,8 | 169,5 ± 9,2 | 173 ± 8,7 | 184,5 ± 5,8 | 173,4 ± 1,5 | 120,2 ± 6,8 | 86 ± 8,7 | 103,3 ± 5,8 | 274 ± 9,8 | 184,5 ± 0,7 |
| OM | 212,5 ± 14,9 | 154 ± 2,8 | 172 ± 7,7 | 163,7 ± 6 | 99,4 ± 2,2 | 115,9 ± 3,9 | 93,5 ± 7,8 | 88 ± 6 | 149 ± 12,7 | 183 ± 12,73 |
| OY | 208,7 ± 8,1 | 179 ± 4,24 | 132,7 ± 5,5 | 163 ± 8,3 | 156,5 ± 6,8 | 121,8 ± 1,2 | 72,3 ± 5,5 | 91,3 ± 8,3 | 282,5 ± 0,71 | 201 ± 6,6 |
| OP | 188,7 ± 18,5 | 179 ± 7,1 | 148 ± 4,3 | 157 ± 6,4 | 78,8 ± 5,2 | 119,1 ± 3,4 | 85 ± 4,3 | 88,7 ± 6,4 | 132,5 ± 4,5 | 201 ± 2,1 |
We observe that obese group showed a diminution in the Enterobacteria population in relation to the NC. The groups that received yogurt supplementation, with or without probiotic, have a slight increase in this population (at 30 and 60 days) but they did not reach to the normal control values. Milk supplementation induced a significant increase in the Enterobacteria in relation to the all obese groups, for the periods of time assayed (Figure 3a).
For Lactobacilli population we found that three dietary supplement assayed increased this population (Figure 3b).
Total anaerobe populations increases in mice received yogurt supplementation in relation to the obese and normal control in both periods of time assayed (Figure 3c).
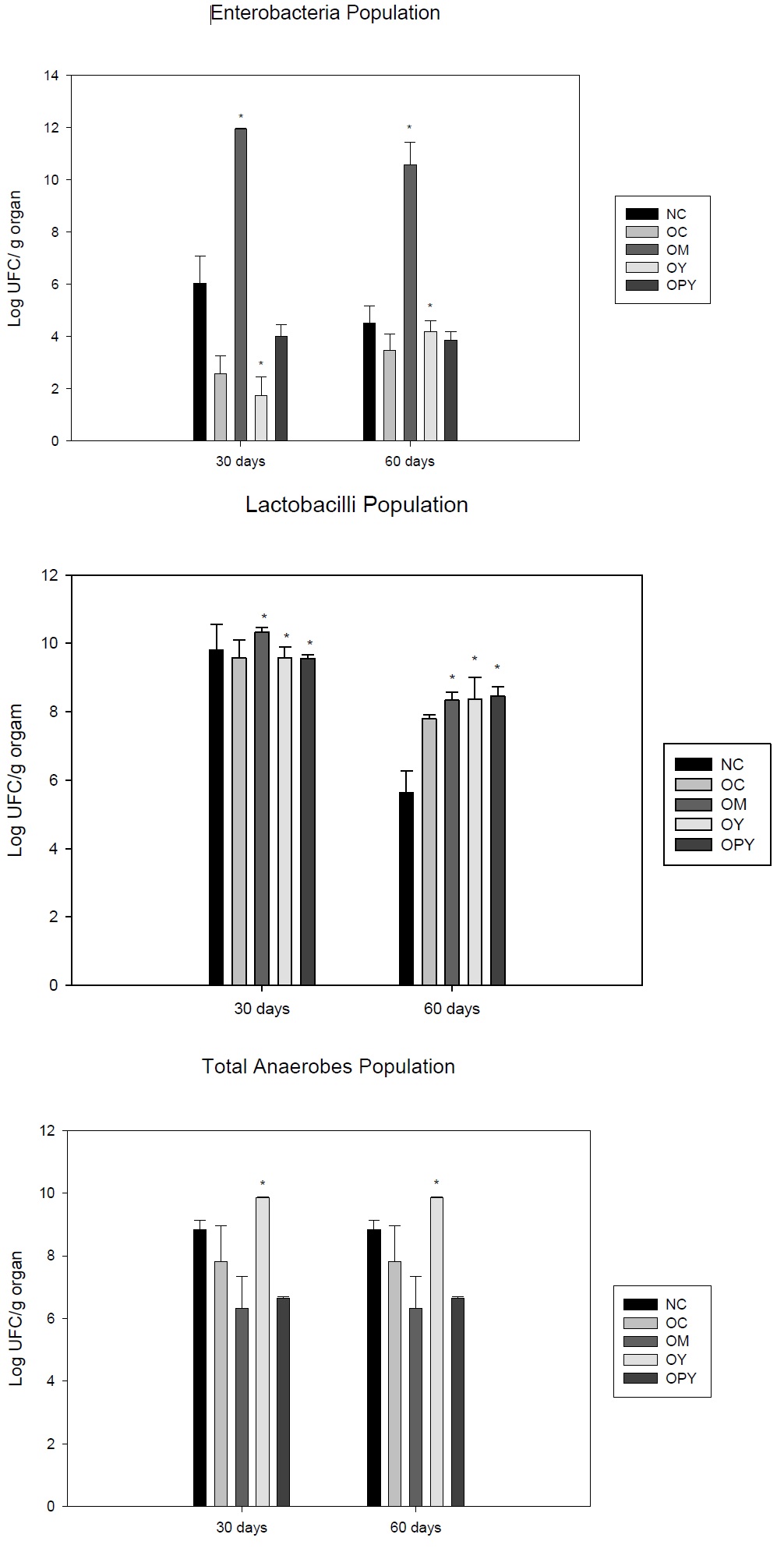
Each mean represents data from nine animals (from three independent experiment). *Significant increase (p < 0.05) compared with the normal control.
For OM and OPY groups we observed a diminution of this population as regard to the obese and control groups.
These results suggest that yogurt administered in obesity could improve the intestinal microbiota principally with the Lactobacilli population increases.
In order to confirm the safety of yogurt supplementation in obesity we performed bacterial translocation assays from the intestine to distant organs as liver and spleen. We analyze two populations, Enterobacteria and Lactobacilli, which are sensitive to translocate when the microbiota is disturbed. At this point it is important to note that; different diet supplementation did not induce bacteria translocation in the NC group. Enterobacteria translocation was determined in liver, only in the group that received milk in both times assayed (more of 200 CFU); no translocation of this population was observed for yogurt administration as well as NC or OC. The OC and milk groups induced also Lactobacilli translocation in the two periods assayed. In the spleen, did not found translocation, for the two populations analyzed in both periods of time, for any supplement assayed. These results demonstrate the safety yogurt administration to obese host, not only on the intestinal microbiota balance but also on the protection the intestinal barrier, contrary to those observed in the milk group and in obese control.
The gut histology is important not only for the nutrient absorption but also for the maintenance of the microbiota balance [43,44]. Studies on small intestine histology were conducted in order to determine whether the probiotic yogurt administration was effective in improving the disturbances on the length of the villi of the small intestine induced by obesity. There was no changes in the small intestine histology for the non obese group that received any of the three supplement assayed, being the histology similar to the normal control. However, the high fat diet induced severe alteration in the histological architecture for obese animals after 60 days; the small intestine showed villi shortened, with an increase in the cellularity of the lamina propria, this fact was improved only after yogurt consumption. The probiotic yogurt was more effective in the recovery of the villi and cellularity, showing a similar histology to the NC. The low fat milk supplementation was not effective in recovery of the small intestine histology. Figure 4 show representative picture of each group analyzed.
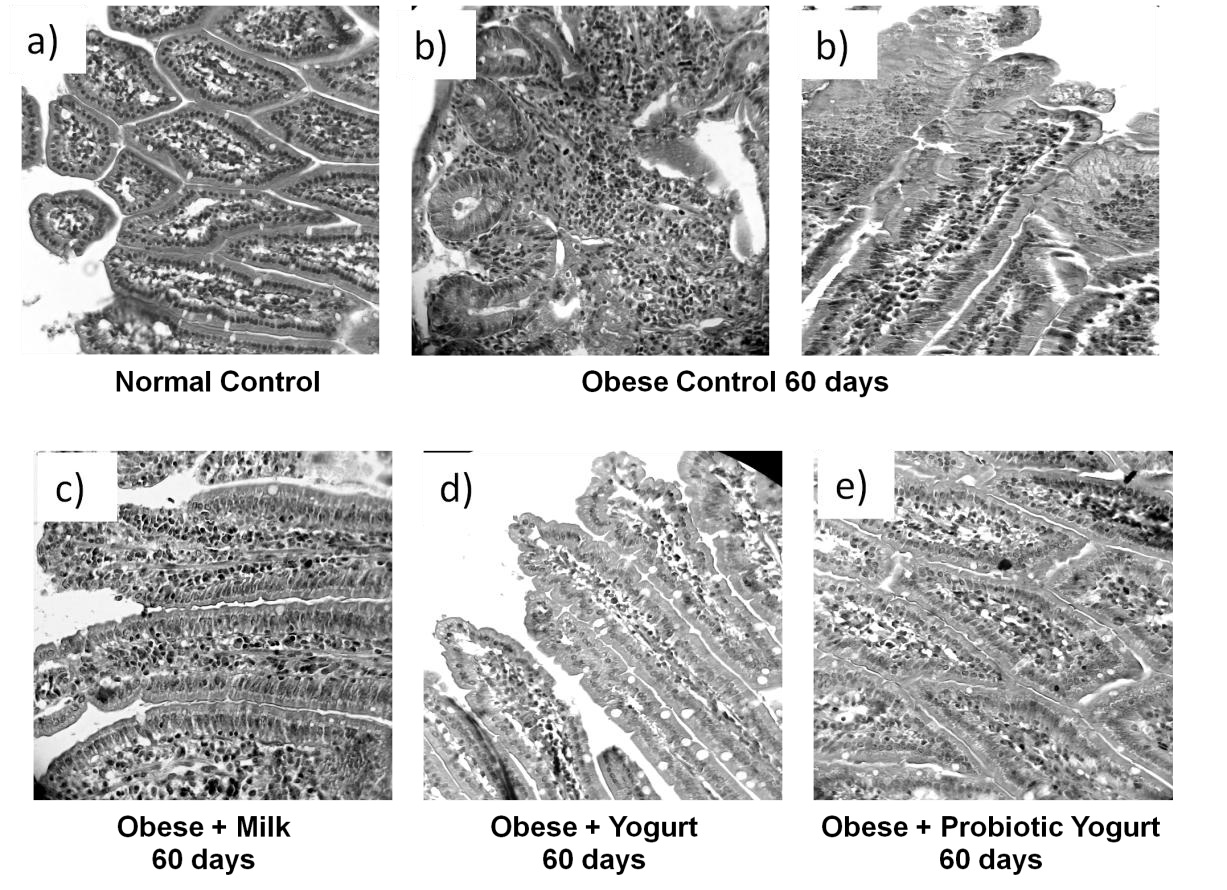
These finding confirm that probiotic administration is able to improve the histology of the small intestine which ensuring a better functioning of the intestinal barrier in the obese host.
The biological role of the Secretory IgA (S-IgA) has been demonstrated [45]. This immunoglobulin is important to maintain the effectiveness of the epithelial barrier. We analyzed the effect of yogurt administration on the IgA+ cells in the lamina propria of the small intestine and the total secretory - IgA. We observed that the values for IgA+ cells at 30 days did not changed in the obese mice as regard to the non-obese group The number of IgA producing cells in the lamina propria of the small intestine for obese and low fat milk groups were similar those of normal control group. After yogurt supplementation these values were increased especially for yogurt containing probiotic strain. For 60 days IgA+ cells increased in all groups with values remarkable for probiotic yogurt (Figure 5a).
For the total S-IgA the values obtained for obese and low fat milk groups, in the two periods of times assayed, were diminished as regard to non-obese group. Yogurt supplementation increased the total S-IgA values after 60 days of administration, respect to the controls (NC and OC) (Figure 5b).
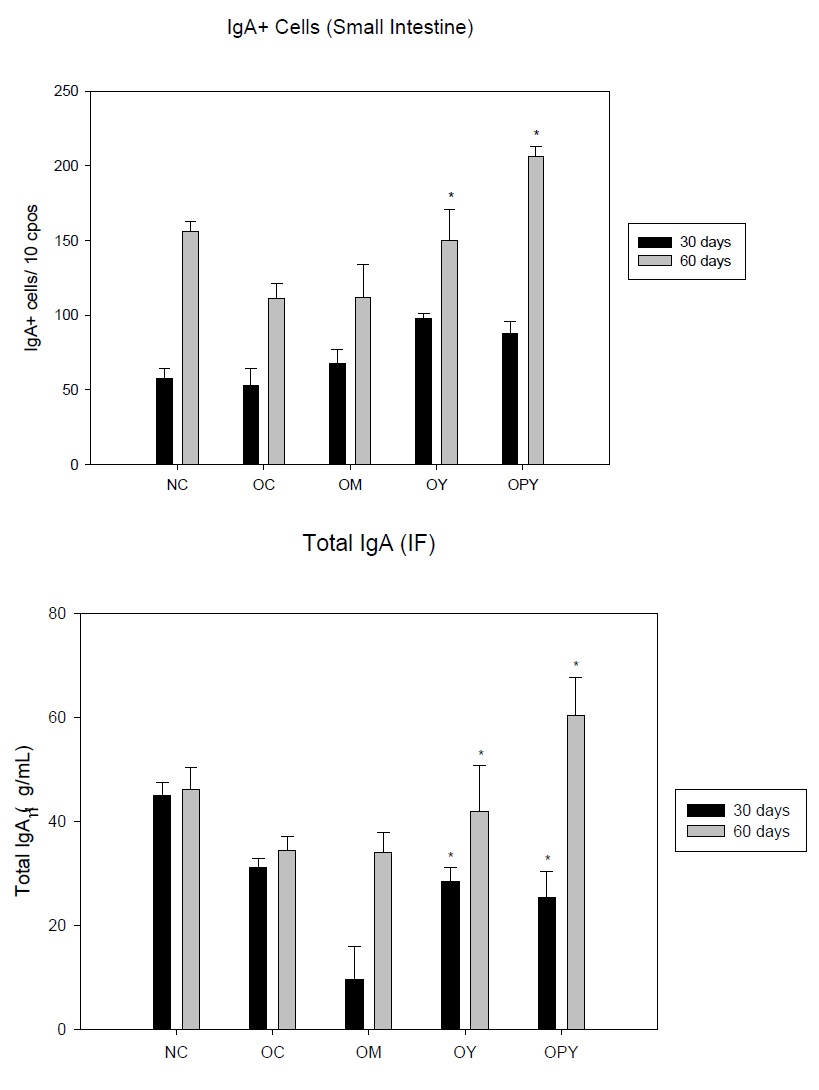
b) Determination of secretory IgA concentrations in small intestinal fluid from mice from different experimental groups: Normal Control (NC), Obese Control (OC), Obese mice given Milk (OM), Obese mice given Yogurt (OY) or Probiotic Yogurt (OPY). Data correspond to the mean ± SD of results of N = 9 animals from three separate experiments. *indicate significantly increases compared with the NC groups (P < 0.05).
We demonstrated that both yogurt assayed specially those containing probiotic strain were effective in old age, increasing the number of IgA cells and the production of S-IgA .The non-fat milk was less effective,as regard the obese control were we found a remarkable decrease in the levels of total IgA-S (Figure 5a and 5b).
It is well known that the Intestinal Epithelial Cell (IEC) play an important role in the signals to activate the immune cells associated to the lamina propria of the intestine. This IEC is activating from the metabolites released by the commensal microbiota [42,47].
The activation of the immune cells associated to the lamina propria and the Intestinal Epithelial Cell (IEC) were evaluated in the intestinal fluid, by determination of cytokines (IL-6, IL-10 and IFNγ) release. These cytokines are the biological messenger to send signals to activate other immune cells from lamina propria.
The IFNγ is produced by the Th1 and IEC and is involved in the activation of cells of innate response as MQ and NK cells, which in turn increase the activation of LT. In addition, IFNγ promotes the differentiation of CD4+ T lymphocytes towards the Th1 group and inhibits the development of Th2 and Th17 lymphocytes. Also stimulates the expression of several different proteins that contribute to increase the expression of MHC-associated antigen and the initiation and amplification of T lymphocyte-dependent immune responses [48]. IL6 is produced by the IEC, macrophages (MQ) and LTh2. This cytokine promote hepatic synthesis of inflammatory mediators in the liver, stimulates the production of neutrophils in the bone marrow, and promotes the differentiation of IL-17. Furthermore, is very important in the differentiation of LB to plasma cell producing Immunoglobulins [49]. IL-6 and IFNγ have a pleiotropic effect, which depends on the levels reached and the microenvironment in which they are produced [50].
For IFNγ we found that OC animals had similar values than the NC in both periods of time assayed (30 or 60 days). The three supplementary diets increase significantly the values in relation to the two controls, for the two periods of time.
There are studies performed in obese mice indicating that the high levels of IFNγ, promote the inflammation in fat tissue [51]. However in the intestinal context, the production of IFNγ is important to maintain the intestinal barrier homeostasis and to orchestrate the innate immune response from the underlying immune cells. These results indicate that yogurt supplementation was able to activate the IEC and immune cells associated to the lamina propria and they are in concordance with previous studies, where this cytokine increase in gut after probiotic administration [52], being a relevant effect in the resolution of a Salmonella thiphimurium infection [53]. Recent studies demonstrate that IFN-γ plays a central role in the modulation of chemokine production and leukocyte recruitment in response to proinflammatory cytokines [54].
IL6 decrease significantly in obese animals respect to normal control. In contrast yogurt or probiotic yogurt administration increased significantly IL-6 at 60 days, with values even higher than those for normal control. Low-fat milk supplementation induced a great increase after 30 days of administration. The values for this IL 6 obtained for both yogurt assayed, are consistent with the increase in the levels of total S-IgA determined.
IL-10 was also significantly decreased in obese mice in both time assayed. Low-fat milk increased this cytokine values at 30 days. The group fed with probiotic yogurt had similar values than normal control. However, the animals given conventional yogurt as supplementation showed an increase at 60 days of the experience. All of these results are expressed in table 3.
| IFNγ | IL-6 | IL-10 | ||||||||||
| 30 days | SD | 60 days | SD | 30 days | SD | 60 days | SD | 30 days | SD | 60 days | SD | |
| NC | 125,6a | 14,8 | 151b | 15.8 | 239,5c | 37,3 | 212,4c | 87,6 | 573,9 | 14,5 | 265,8a | 11,6 |
| OC | 143,7b | 19,9 | 160,3b | 10,5 | 81,4b | 15,1 | 57a | 3,95 | 278,4a | 7,5 | 345,8b | 7,6 |
| OM | 162.lc | 20,2 | 227,lc | 10.6 | 489.2 | 141,2 | 262,5d | 44,5 | 460,9c | 14,9 | 342,3b | 9,7 |
| OY | 239,5c | 12,2 | 322,4f | 12,15 | 340,3f | 32,1 | 302,le | 17,5 | 459,lc | 17,1 | 551,4d | 10,5 |
| OPY | 266,5d | 33 | 305,5e | 18,06 | 297.4d | 19,5 | 374,4f | 18,3 | 251,5a | 16,1 | 321,965b | 10,1 |
The results obtained showed that the increase in the regulatory IL-10 could help to reinforce the epithelial barrier and participated in the IgA+ cells and Total S-IgA increase.
CONCLUSION
We demonstrated that yogurt administration as a dietary supplement in obesity is able to improve some parameters of the intestinal barrier and from the cells of the innate immunity.
The yogurt administration in obese mice did not induce increases in the body weight; being values close to non-obese control. The ratio body/liver and spleen were not altered.
Yogurt supplementation had beneficial effect by increasing thymus weight.
The administration of both yogurts assayed was effective to normalize glucose, HDL, cholesterol, LDL in the serum increased by the metabolic syndrome induced by obesity.
Yogurt improves the intestinal microbiota with an increase in lactobacilli population and decreasing total anaerobes. There are not side effects such as translocation of intestinal microbiota to liver or spleen.
Both yogurts improve the histology of the small intestine especially the group containing probiotic strain and also improve the thymus histology.
Both yogurts promote cytokine production such as IL-6 and INFγ at physiological levels that enhance activation of IEC and the immune cells associated with the lamina propria. These cytokine favor the increase in the IgA+ cells and total IgA. The levels of the IL-10 regulatory cytokine increased, helping to reinforce the epithelial barrier avoiding an inflammatory response.
The present study demonstrated the safety and useful of yogurt consumption in obese host, benefiting mainly the innate mucosal immunity, biochemical parameters, intestine and thymus histology. These results allow advance in the research about the influence of functional foods on the thymus cells activity, involved in maturation and ontogeny of T cells, which play a key role in the immune response.
ACKNOWLEDGEMENT
The present study was financially supported by DANONE, Argentin and Nacional deInvestigaciones Cient?íficas y Técnicas grant, CONICET PIP 0652, CIUNT 26/D442 (Tucumán University).The authors’ contributions are as follows: F.B., C. M. and C. M. G. carried out the microbiological work, animal studies and immunological determination; G. P. and R. W. conceived the study; C. M. G. and G. P. designed the experiments and R. W. provided PFM and participated in the discussion; F. B, C. M. and C. M. G. performed the statistical analysis and prepared the figures. All authors read and approved the final 451 version of the manuscript. The authors declare that they have no conflicts of interest.
REFERENCES
- Codigo Alimentario Argentino.
- Metchnikoff E (1908) The prolongation of life, (1st edn). Putman, New York, USA.
- Panahi S, Fermandez MA, Marette A, Tremblay A (2017) Yogurt, diet quality and lifestyle factors. Eur J Clin Nutr 71: 573-579.
- Shah NP (2007) Functional cultures and health benefits. International Dairy Journal 17: 1262-1277.
- Shi LH, Balakrishnan K, Tiagarajah K, Mohd Ismail NI, Yin OS (2016) Beneficial Properties of Probiotics. Trop Life Sci Res 27: 73-90.
- Ashraf R, Shah NP (2014) Immune System Stimulation by Probiotic Microorganisms. Crit Rev Food Sci and Nutr 54: 938-956.
- Isolauri E, S?tas Y, Kankaanp?? P, Arvilommi H, Salmien S (2001) Probiotics: Effects on Immunity. Am J Clin Nutr 73: 445-505.
- Reid G (2017) Probiotic use in an infectious disease setting. Expert Rev Anti Infect Ther 15: 449-455.
- Galdeano CM, Perdigón G (2006) The Probiotic Bacterium Lactobacillus casei Induces activation of the gut mucosal immune system through innate immunity. Clin Vaccine Immunol 13: 219-226.
- Calatayud GA, Marcos A, Margolles A (2016) Probióticos, prebióticos y salud: evidencia científica. Ergon, Germany. Pg no: 281-285.
- Fuller R, Perdigón G (2000) Probiotics 3: Immunomodulation by the Gut Microflora and Probiotics. Kluwer Academic publishers 40.
- Nuñez IN, Galdeano CM, de LeBlanc Ade M, Perdigón G (2014) Evaluation of immune response, microbiota, and blood markers after probiotic bacteria administration in obese mice induced by a high-fat diet. Nutrition 30: 1423-1432.
- da Silva ST, dos Santos CA, Bressan J (2013) Intestinal microbiota; relevance to obesity and modulation by prebiotics and probiotics. Nutr Hosp 28: 1039-1048.
- Xiong Ma, Jing Hua, Zhiping Li (2008) Probiotics improve High Fat Diet-induced Hepatic Steatosis and Insulin Resistance by Increasing Hepatic NKT Cells. J Hepatol 49: 821-830.
- Hamad EM, Sato M, Uzu K, Yoshida T, Higashi S, et al. (2009) Milk fermented by Lactobacillusgasseri SBT2055 influences adipocyte size via inhibition of dietary fat absorption in Zucker rats. Br J Nutr 101: 716-724.
- Arora T, Singh S, Sharma RK (2013) Probiotics: Interaction with gut microbiome and antiobesity potential. Nutrition 29: 591-596.
- Park DY, Ahn YT, Park SH, Huh CS, Yoo SR, et al. (2013) Supplementation of Lactobacillus curvatus HY7601 and Lactobacillus plantarum KY1032 in dietinduced obese mice is associated with gut microbial changes and reduction in obesity. PLoS One 8: 59470.
- Novotny Núñez I, Maldonado Galdeano C, de Moreno de LeBlanc A, Perdigón G (2015) Lactobacillus casei CRL 431 administration decreases inflammatory cytokines in a diet-induced obese mouse model. Nutrition 31: 1000-1007.
- De Paula JA, Carmuega E, Weill R (2008) Effect of the ingestion of a symbiotic yogurt on the bowel habits of women with functional constipation. Acta Gastroenterol Latinoam 38: 16-25.
- Mokhtari S, Khomeiri M, Jafari SM, Maghsoudlou Y, Ghorbani M (2017) Descriptive analysis of bacterial profile, physicochemical and sensory characteristics of grape juice containing Saccharomyces cerevisiae cell wall-coated probiotic microcapsules during storage. Int J Food Sci Technol 52: 1042-1048.
- Mokhtari S, Jafari SM, Khomeiri M, Maghsoudlou Y, Ghorbani M (2017) The cell wall compound of Saccharomyces cerevisiae as a novel wall material for encapsulation of probiotics. Food Research International 96: 19-26.
- Sainte-Marie G. (1962) A paraffin embedding technique for studies employing immunofluorescence. J Histochem Cytochem 10: 250.
- de Moreno de LeBlanc A, Dogi CA, Galdeano CM, Carmuega E, Weill R, et al. (2008) Effect of the administration of a fermented milk containing Lactobacillus casei DN-114001 on intestinal microbiota and gut associated immune cells of nursing mice and after weaning until immune maturity. BMC Immunol 9: 27.
- WHO (2016) Obesity and overweight. WHO, Geneva, Switzerland.
- Núñez IN, Galdeano CM, Camuerga E, Weill R, de Moreno de LeBlanc A, et al. (2013) Effect of a probiotic fermented milk on the thymus in Balb/c mice under non-severe protein-energy malnutrition. Br J Nutr 110: 500-508.
- Tuulasvaara A, Vanhanen R, Baldauf HM, Puntilla J, Petteri Arstila T (2016) Interleukin-7 promotes human regulatory T cell development at the CD4+CD8+ double-positive thymocyte stage. J Leukoc Biol 100: 491-498.
- Nitta T, Suzuki H (2016) Thymic stromal cell subsets for T cell development. Cell Mol Life Sci 73: 1021-1037.
- Abbas AK, Litchman AH, Pillai S (2015) Desarrollo de linfocitos y reordenamiento del gen del receptor para el antigeno. In: Inmunología Celular y Molecular, (8th edn). Elseiver Saunders, Amsterdam, Netherlands. Pg no: 173.
- Palmer DP (2013) The Effect of age on Thymic function. Front Immunol 4: 316.
- Yang H, Youm H, Vandanmagsar B, Rood J, Kumar KG, et al. (2009) Obesity acelerates thymic aging. Blood 114: 3803-3812.
- Dixit VD (2008) Adipose-immune interactions during obesity and caloric restriction: reciprocal mechanisms regulating immunity and health span. J Leukoc Biol 84: 882-892.
- Yang H, Youm YH, Dixit VD (2009) Inhibition of thymic adipogenesis by caloric restriction is coupled with reduction in age-related thymic involution. J Immunol 183: 3040-3052.
- Makki K, Froguel P, Wolowczuk I (2013) Adipose Tissue in Obesity-Related Inflammation and Insulin Resitance: Cells, Cytokines and Chemokines. ISRN Inflamm.
- Lackey DE, Lazaro RG, Li P, Johnson A, Hernandez-Carretero A, et al. (2016) The role of dietary fat in obesity-induced insulin resistance. Am J Physiol Endocrinol Metab 311: 989-997.
- Kurozumi A, Okada Y, Arao T, Tanaka Y (2016) Excess Visceral Adipose Tissue Worsens the Vascular Endothelial Function in Patients with Type 2 Diabetes Mellitus. Internal Med 55: 3091-3095.
- Navarro-González D, Sánchez-Íñigo L, Fernández-Montero A, Pastrana-Delgado J, Alfredo Martínez J (2016) Are all metabolically healthy individuals with obesity at the same risk of diabetes onset? Obesity (Silver Spring) 24: 2615-2623
- Riccardi G, Giacco R, Rivellese AA (2004) Dietary fat, insulin sensitivity and the metabolic syndrome. Clin Nutr 23: 447-456.
- Jung UJ, Choi MS (2014) Obesity and Its Metabolic Complications: The Role of adipokines and the relationship between obesity, inflammation, insulin resistance, dyslipidemia and nonalcoholic fatty liver disease. Int J Mol Sci 15: 6184-6223.
- Kobyliak N, Conte C, Cammarota G, Haley AP, Styriak I, et al. (2016) Probiotics in prevention and treatment of obesity: a critical view. Nutr and Metab.
- Kübeck R, Bonet-Ripoll C, Hoffmann C, Walker A, Müller VM, et al. (2016) Dietary fat and gut microbiota interactions determine diet-induced obesity in mice. Molecular Metabolism 5: 1162-1174.
- Qian LL, Li HT, Zhang L, Fang QC, Jia WP. (2015) Effect of the Gut Microbiota on Obesity and Its Underlying Mechanisms: an Update. Biomed Environ Sci 28: 839-847.
- Slyepchenko A, Maes M, Jacka FN, Köhler CA, Barichello T, et al. (2016) Gut Microbiota, Bacterial Translocation, and Interactions with Diet: Pathophysiological Links between Major Depressive Disorder and Non-Communicable Medical Comorbidities. Psychother Psychosom 86: 31-46.
- Yamauchi KE, Incharoen T, and Yamauchi K (2010) The relationship between intestinal histology and function as shown by compensatory enlargement of remnant villi after midgut resection in chickens. Anat Rec 293: 2071-2079.
- Semova I, Carten JD, Stombaugh J, Mackey LC, Knight R, et al. (2012) Microbiota regulate intestinal absorption and metabolism of fatty acids in the zebrafish. Cell Host Microbe 12: 277-288.
- Abbas AK, Litchman AH, Pillai S (2015) Inmuidad especializada en las barreras epiteliales y en los tejidos con privilegio inmunitario. In: Inmunología Celular y Molecular, (8th edn). Elseiver Saunders, Amsterdam, Netherlands. Pg no: 297-298.
- Galdeano CM, de Moreno de LeBlanc A, Vinderola G, Bonet ME, Perdigón G. (2007) Proposed Model: mechanisms of immunomodulation induced by probiotic bacteria. Clin Vaccine Immunol 14: 485-492.
- Abbas AK, Litchman AH, Pillai S (2015) Inmuidad especializada en las barreras epiteliales y en los tejidos con privilegio inmunitario. In: Inmunología Celular y Molecular, (8th edn). Elseiver Saunders, Amsterdam, Netherlands. Pg no: 292-295.
- Abbas AK, Litchman AH, Pillai S (2015) Diferenciación y funciones de los linfocitos T efectores CD4+. In: Inmunología Celular y Molecular, (8th edn). Elseiver Saunders, Amsterdam, Netherlands. Pg no: 219-220.
- Abbas AK, Litchman AH, Pillai S (2015) Inmunidad Innata. In: Inmunología Celular y Molecular, (8th edn). Elseiver Saunders, Amsterdam, Netherlands. Pg no: 75.
- Lu Y, Hylander B, Brauner A (1996) Interleukin-10, interferon gamma, interleukin-2, and soluble interleukin-2 receptor alpha detected during peritonitis in the dialysate and serum of patients on continuous ambulatory peritoneal dialysis. Perit Dial Int 16: 607-612.
- Rocha VZ, Folco EJ, Sukhova G, Shimizu K, Gotsman I, et al. (2008) Interferon-gamma, a Th1 cytokine, regulates fat inflammation: a role for adaptive immunity in obesity. Circ Res 103: 467-476.
- Dogi CA, Galdeano CM, Perdigon G (2008) Gut immune stimulation by non pathogenic Gram(+) and Gram(-) bacteria. Comparison with a probiotic strain. Cytokine 41: 223-231.
- Castillo NA, de Moreno de LeBlanc A, M Galdeano C, Perdigon G (2013) Comparative study of the protective capacity against Salmonella infection between probiotic and nonprobiotic Lactobacilli. J Appl Microbiol 114: 861-876.
- Meagher LC, Savill JS, Baker A, Fuller RW, Haslett C (1992) Phagocytosis of apoptotic neutrophils does not induce macrophage release of thromboxane B2. J Leukoc Biol 52: 269-273.
- Abbas AK, Litchman AH, Pillai S (2015) Tolerancia inmunitaria y autoinmunidad. In: Inmunología Celular y Molecular, (8th edn). Elseiver Saunders, Amsterdam, Netherlands. Pg no: 324-496.
Citation: Balcells F, Mariani C, Weill R, Perdigón G, Maldonado-Galdeano C (2017) Effect of Yogurt With or Without Probiotic Addition on Body Composition Changes and Immune System in an Obese Model. J Food Sci Nut 3: 022.
Copyright: © 2017 Florencia Balcells, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.

