
Preliminary Analysis and Biochemical Characterization Related to Health Implications for African Populations in Some Maize Cultivars. A Special Look at the South African Environment
*Corresponding Author(s):
Enrico DoriaCentre Of Sustainable Livelihoods, Vaal University Of Technology, Vanderbijlpark, South Africa
Tel:+27 169509000,
Fax:+27 169509999
Email:enrico.doria@unipv.it
Abstract
After its introduction to Africa, maize was quickly adopted as the cornerstone of local cuisine, especially in sub-Saharan countries. Although nutrient deficiencies constitute a heavy disease burden in the most vulnerable countries, white maize varieties, lacking provitamin A, carotenoids and vitamin A, were widely preferred in Africa. In this work we characterized two black-colored landraces, the Millo Corvo and Spinato cultivars, as well as three non-colored maize genotypes (B73, Argentina and Haiti). We examined the content of carotenoids and anthocyanins for their strong nutritional power, and also the phosphate content, polyphenols and protein levels. The two colored cultivars resulted particularly rich in antioxidant and nutraceuticals factors as carotenoids and anthocyanins pigments, while the white cultivar Argentina resulted particularly interesting for the high and unexpected content of free phosphate. The aim of the project is to cultivate the analyzed maize cultivars, tested to confirm the same nutritional profile observed in Italy, and verify whether they grow productively in South African soil; the subject cultivars will be used for breeding programs and then selected for use as functional food in poorer communities suffering of nutritional deficiencies.
Keywords
INTRODUCTION
Maize (Zea mays) is of paramount importance in the diets of many African populations: of the 22 countries in the world where maize forms the highest percentage of energy in the national diet, 16 are in Africa [1,2]. After its introduction to Africa by new world explorers in the 16th century [3], maize quickly established itself as a main ingredient in the local cuisine because of its relatively high grain yield, low labor requirements and favorable storage characteristics [4]. Lesotho, Malawi and Zambia rank as the world’s top three countries subsisting on maize, surpassing the Mesoamerican countries where the crop originated [3]. The use of the maize in African countries is now comparable to that of rice in Asia [2]. In particular, in South Africa, estimated values calculated from FAO food balance sheets in 2007 showed a maize intake of 288.3g/capita per day, corresponding to 30% of daily energy intake; the same percentage is observed for the daily protein intake. According to the South African Department of Agriculture, Forestry and Fisheries (DAFF), the total amount of commercial maize expected for 2015 is close to 10 million tons, which more or less meets the national demand of around 195kg/capita [5]. African food preparation differs from that of other maize-growing regions of the world in that maize-based dishes are most often boiled or cooked rather than fried or baked [2]. In particular, in rural areas, maize flour serves as the raw material for fermented or boiled beverages and thick porridges, traditionally eaten twice daily; Africans often take pride in this as their own distinctive dish [6,7].
Micronutrient deficiencies constitute a heavy disease burden that is shouldered disproportionately by a highly vulnerable group in the most vulnerable countries in the world: children Under 5 years of age (U5) in Sub-Saharan Africa (SSA) and South Asia. SSA, with 11% of the world’s population, accounts for more than half of the deaths and half of U5 Disability-Adjusted Life Years (DALYs) lost to deficiencies of vitamin A, iron, zinc, and iodine [8,9]. Maize supplies many micronutrients and macronutrients necessary for human nutritional needs; however, it lacks B vitamins and the essential amino acids, Lysine (Lys) and Tryptophan (Trp). White maize varieties, which are widely preferred in much of Africa, lack provitamin A, carotenoids and vitamin A, essential for immunity, growth and eyesight. In addition, some minerals in the maize grain have low bioavailability owing to the presence of a high content of phytic acid [2]. South Africa is one of few countries that meet all of the World Bank’s three priority criteria for urgent nutrition action: (1) stunting/underweight greater than 20 percent; (2) vitamin A deficiency greater than 10 percent or iron-deficiency anemia greater than 10 percent and (3) an emerging overweight problem (Global Alliance for Improved Nutrition). At national level 24.8% and 39.2% of South African women are overweight or obese compared to 20.1% and 10.6% in men respectively, while children generally suffered from both under nutrition (19.2% stunting, 43.6% vitamin A deficiency and for U5 and 10.7% anemia) and over nutrition (prevalence of 16.5% overweight and 7.1% obesity in girls and 11.5% overweight and 4.7% obesity in boys aged 2-14 years) [10,11]. Large-scale fortification is a system used to reduce the burden of malnutrition, and in 2003 the South African government passed legislation requiring all bread (wheat) flour and maize meal to be fortified. Maize biofortification has recently been introduced as a strategy to relieve vitamin A deficiency [12], which can lead to blindness, anemia, weakened resistance to infection and increased risk of death. Moreover, a nutritionally superior maize cultivar, referred to as Quality Protein Maize (QPM), was developed, containing twice the amount of lysine and tryptophan compared with the traditional maize type [2]. There are also several projects for improving the mineral content (mostly Zn and Fe) in crops like maize [13]. In this study we describe some nutritional parameters related to the antioxidant power of certain maize cultivars; in particular, we characterized an ancient colored landrace, the Millo Corvo, cultivated in the Spanish region of Galicia and used to produce different varieties of food: the peculiarity of Millo Corvo is the distinctive dark blue/black coloration of the kernels that imparts a typical blue coloration to the bread cooked using the flour. It is known that maize is able to accumulate pigments in the seeds: carotenoids impart a yellow-orange color and anthocyanins a red, purple, blue and black coloration, which confers antioxidant power, thought to be highly beneficial for human health. Moreover, the carotenoid zeaxanthin and its close relative lutein play a critical role in the prevention of Age-related Macular Degeneration (AMD), the leading cause of blindness [14]. Another parameter examined in this study was the phytic acid content. The myo-inositol 1, 2, 3, 4, 5, 6-hexakisphosphate, commonly called phytic acid, is the primary storage form of phosphorus in the seeds: it represents from 50% to over 80% of total phosphorus in mature seeds and accounts for one to several percent of the dry weight. Phosphorus and mineral cations reserves deposited in the phytate molecule are essential for germination and for the growth and development of seedlings. Moreover, phytic acid, because of its ability to chelate metal cations and, therefore, to reduce their bioavailability in the digestive apparatus, has long been regarded as an anti-nutrient for monogastric animals; on the other hand, phytic acid, by virtue of its ability to chelate iron, is a potent inhibitor of the iron-driven formation of reactive oxygen species [15]. The aim of this preliminary study is to analyze the cited maize cultivars for their nutritional profile in terms of antioxidant activity (especially for the carotenoids and anthocyanins content) and phytic acid content. This work must be considered as part of a multi-step project involving, primarily, the cultivation of these maize cultivars in order to verify whether they grow productively in South African soil; secondly, the subject cultivars will be tested to confirm the same nutritional profile as observed in Italy and then one or more candidates will be selected for breeding programs in poorer communities with nutritional deficiencies related to dietary intakes. In particular, cultivars enriched with antioxidant compounds could play a vital role in the reduction of blindness attributed to traditional maize consumption in SSA. Even the maize cultivar with high percentage of free phosphate and minerals (with relative low amount of phytic acid) will be used to improve the nutritional quality of this staple food.
MATERIALS AND METHODS
Plant material
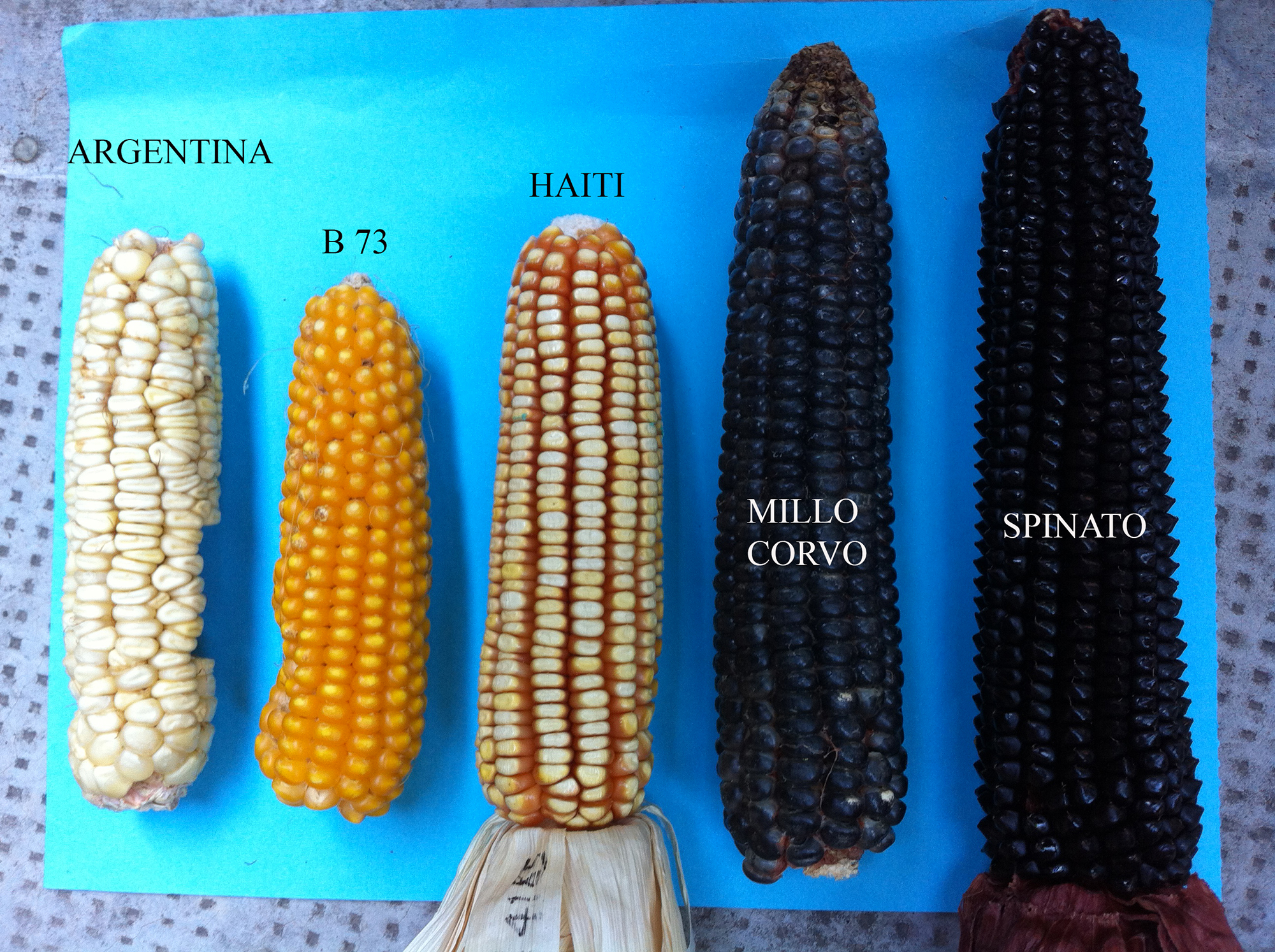 Figure 1: Maize seeds from different cultivars used in this study.
Figure 1: Maize seeds from different cultivars used in this study.Flour samples were obtained using a coffee grinder and the seeds were ground for 3min before being further powdered using liquid nitrogen.
Chemicals and solvents
Protein analysis
DPPH test
Determination of total polyphenolic content
In a test tube, 150µl of the methanol-acetone extract, 2400µl of nanopure water and 150µl of 0.25N Folin-Ciocalteu reagent were combined and then mixed well, using a vortex. The mixture was allowed to react for 3min, after which 300µl of 1N Na2CO3 solution was added and mixed well. The solution was incubated at room temperature for 2h and absorbance was measured at 725nm against a blank. Results were expressed in Gallic Acid Equivalents (GAE; mg/100 g of fresh matter), using a gallic acid (0-0.1mg/ml) standard curve [19].
Extraction and quantification of carotenoids
Carotenoid content was determined in the organo solvent extracts solubilized in hexane through UV-visible spectrophotometry by measuring the absorbance at 450nm (3 replicates/samples). For the purposes of total carotenoid content calculations, the mean absorption coefficient (ε=2,300L m-1mol-1 for hexane) was used.
The HPLC analysis of the carotenoid content was performed according to a modified protocol of Tukaj [20]. The extracted material was dissolved in acetone before being injected in HPLC (Perkin Elmer series 200 equipped with diode array detector UV/Vis and a C18 column, Mediterranean 5µm 25x0.46). The solvents were (A) Methanol: 1M Ammonium Acetate 8:2 and (B) Methanol:Acetone 8:2. The injection volume was 20µl and the flow rate 1ml/min at 450nm of UV absorbance. The gradient for elution was linear from 0 to 100% B in 20min, and, after 5min, 100% of A in 5min. Finally, a linear flow of 100% A for 5min was used to equilibrate the column.
Anthocyanin analysis
Determination of free and phytic phosphate in seeds
Statistical analysis
RESULTS
Protein content
| mg/g protein | |
| B73 | 52.163 ± 0.009 |
| MilloCorvo | 51.786 ± 0.055 |
| Argentina | 50.957 ± 0.011 |
| Spinato | 50.958 ± 0.039 |
| Haiti | 62.038 ± 0.072 |
Table 1: Total protein content spectrophotometrically analyzed by using Bradford reagent. Values are the mean of three replicates.
Antioxidants and polyphenols
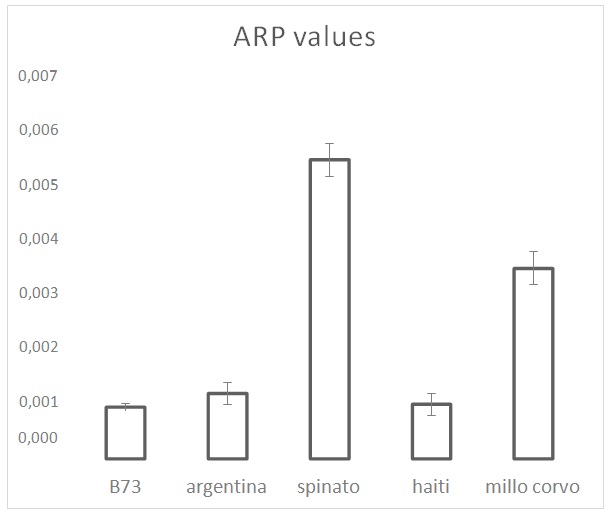 Figure 2: ARP values from the DPPH test on the maize cultivars. ARP values are the reciprocals of flour extract volumes, expressed as µL, required to consume 50% of the initial DPPH amount.
Figure 2: ARP values from the DPPH test on the maize cultivars. ARP values are the reciprocals of flour extract volumes, expressed as µL, required to consume 50% of the initial DPPH amount.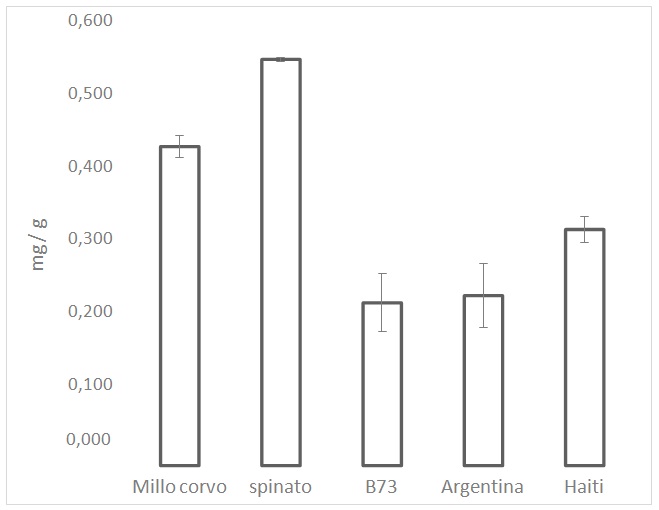 Figure 3: Polyphenols analysis performed by Folin-Ciocalteu. Values are the mean of three replicates.
Figure 3: Polyphenols analysis performed by Folin-Ciocalteu. Values are the mean of three replicates.Carotenoid analysis
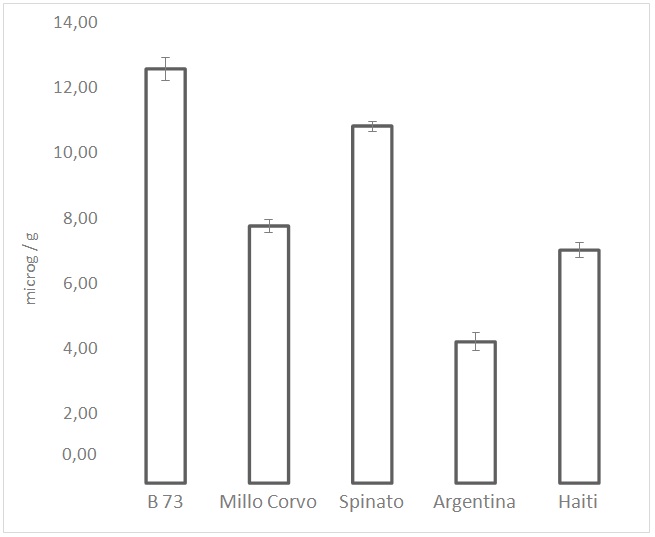 Figure 4: Total content of carotenoids expressed as mg/g. Values are the mean of three replicates.
Figure 4: Total content of carotenoids expressed as mg/g. Values are the mean of three replicates.| µg/g | ||
| Lutein | Zea xanthin | |
| B 73 | 4.902 ± 0.04 | 6.413 ± 0.09 |
| Spinato | 3.824 ± 0.05 | 4.598 ± 0.06 |
| MilloCorvo | 1.711 ± 0.03 | 5.445 ± 0.09 |
| Argentina | 1.583 ± 0.03 | 2.807 ± 0.05 |
| Haiti | 2.712 ± 0.04 | 2.577 ± 0.06 |
Table 2: Results of HPLC analysis of the two main carotenoids present in the examined maize cultivars. Values are the mean of three extraction replicates.
Anthocyanin analysis
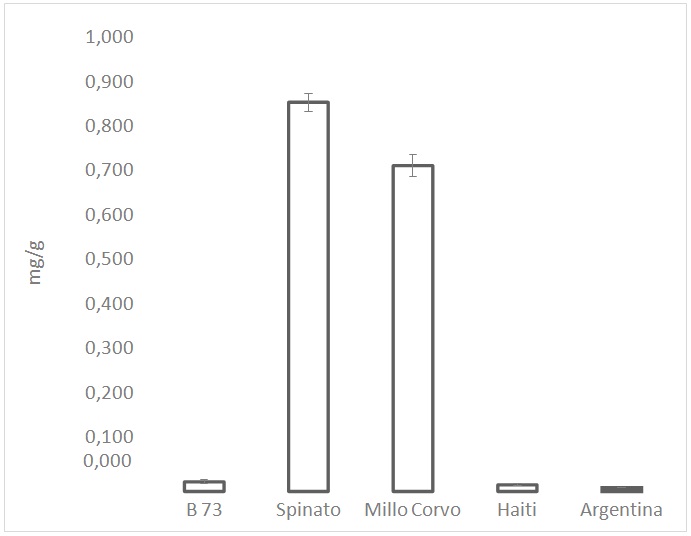 Figure 5: Total content of anthocyanins expressed as cyanidin-3-glucoside equivalent. Values are the mean of three replicates.
Figure 5: Total content of anthocyanins expressed as cyanidin-3-glucoside equivalent. Values are the mean of three replicates.| % | ||
| Anthocyanidin | MilloCorvo | Spinato |
| Cyanidin | 65.90 | 48.50 |
| Pelargonidin | 31.40 | 7.40 |
| Peonidin | 1.90 | 42.10 |
| Delphinidin | 0.75 | 0 |
| Malvinidin | 0 | 1.90 |
Table 3: HPLC analysis of the anthocyanidin content of the two black colored maize cultivars. Results are expressed as percentage of the content of each pigment present in the cultivar.
Free phosphate and phytic acid analysis
| Phytic acid | Free phosphate | |
| Cultivar | mgP/g | mgP/g |
| B 73 | 6.063 ± 0.213 | 0.222 ± 0.050 |
| Spinato | 6.590 ± 0.284 | 0.418 ± 0.050 |
| Argentina | 3.866 ± 0.170 | 1.259 ± 0.080 |
| Haiti | 5.360 ± 0.121 | 0.314 ± 0.040 |
| MilloCorvo | 4.129 ± 0.128 | 0.656 ± 0.040 |
Table 4: Phosphorous level (atomic weight=31), expressed as mg of phosphate per g of seed flour, in the analyzed cultivars. Values are the mean of three replicates.
DISCUSSION
In this study we focused our attention on the positive effects of some compounds present in the dark-colored maize (Spinato & MilloCorvo) which can have an antioxidant effect. In particular, the relevant presence of polyphenols and anthocyanins in the colored maize varieties, compared to the yellow (B73, Haiti) and white maize (Argentina), confer a relevant antioxidant capacity.
The protein content of all 5 maize cultivars, as expected, did not appear very variable; the average of the protein content was around 5%, in line with the values reported in literature, which are between 4 and 10% [24]; FAO corporate document repository (see the link http://www.fao.org/docrep/t0395e/t0395e03.htm). The presence of increasing amounts of pigments and polyphenols in dark varieties does not affect the protein level.
Even not a biological radical, DPPH test provides a good estimation of the total antioxidant activity in the examined maize samples. Results reveal a marked positive influence of the grain color on the measured ARP value. Haiti represents an interesting case in which the lowest ARP value corresponds to a relevant amount of polyphenols (30% higher than in B73 and Argentina) showing the presence, in the other cultivars, of additional factors with antioxidant effects.
Regarding the carotenoids level, the obtained results showed an important variability in the content of pigments with antioxidant activity. In particular the two dark-colored cultivars presented a concentration up to 60% higher than the white coat cultivars. Since the most represented carotenoids in maize are lutein and zea xanthin, the content of each of these pigments was determined by HPLC. As expected, the sum of the two pigments corresponds to almost the total content of carotenoids; lutein and zeaxanthin together represent, in almost all cases, more than 90% of the total content of carotenoids, bearing in mind that the assay for the total carotenoid content is rather rough. Kurilich et al., [25] analyzed several different corn genotypes for the pigment content and found a level of lutein between 0.1 and 20?g/g while the level of zeaxanthin was between 0.01 and 8?g/g.
Kuhnen et al., [26], analyzing a maize variety cultivated in southern Brazil, found a zeaxanthin content of 7?g/g and a lutein content of 3.7?g/g, a result comparable with the one obtained in this study. From HPLC analysis of the maize cultivars, a higher level of zeaxanthin than lutein was found in all the varieties examined except for the Haiti, where the level of the two carotenoids was almost the same. In particular, in the Millo Corvo cultivar, the content of zeaxanthin was threefold higher than the content of lutein, while in the other dark-colored cultivar, Spinato, the zeaxanthin level was found to be only 20% higher than lutein. Comparing these results with the DPPH values, it is possible to notice that the orange-colored B73 cultivar, even presenting the highest carotenoid level (17% higher than Spinato, second highest in content), shows a low ARP value compared with that observed in the two dark maize varieties. Specifically, 5 times lower than that measured in Spinato cv.
Regarding the anthocyanins content, data about the two black-colored cultivars confirmed the high presence of the pigments, even the data regarding Spinato cv could be overestimated due to the presence of phlobaphene in the pericarp layer. Moreover, the obtained results showed as reported also in several publications [27-29], that cyanidin, pelargonidin, and peonidin glycosides are the main anthocyanins present in maize kernels, among which cyanidin 3-glucoside is the most abundant in the dark maize kernels. As a result of these findings, the two coloured examined cultivars therefore should represents an important food in the diet for the prevention of chronic diseases such as cardiovascular disease [30], cancers, respiratory diseases, diabetes and obesity.
Another important studied parameter regards the phosphate content in the examined maize seeds, both free or phytic. Obtained results are in line with those reported by Pilu et al., [23] where the phosphate content of the B73 cultivar was 0.29mg/g and 3.52mg/g for the free P and phytic P respectively. In particular, the most interesting data refer to the amount of free phosphate present in Argentina cv - more than 3 times higher than the average of the other cultivars. The PAP level in the examined cultivars is more homogeneous with respect to the free P level, with only Argentina and MilloCorvo cv presenting a significantly lower level of the PAP. Since this relevant reduction is usually accompanied by an increase of important minerals as iron and magnesium, these cultivars will be taken into account for the nutritional improvement programs. Perhaps the different mineral content of the soil where each cultivar was growing influenced the total amount of phosphate present in the seed.
CONCLUSION
The analyses carried out in this study allowed us to evaluate some nutrient and bioactive content with antioxidant activity in certain maize cultivars. In particular, our attention was focused on some nutritional parameters of two black-colored maize genotypes. MilloCorvo and Spinato; we compared the results obtained for these two cultivars to those obtained for yellow (B73 and Haiti) and white (Argentina) genotypes. The analysis performed showed a significant difference in the total antioxidant power between the black-colored and the other maize cultivars, confirming that the high presence of anthocyanin pigments confers a relevant antioxidant capacity. Regarding the carotenoid analysis, the B73 cv presents the highest level of pigments but the two black cultivars also showed an important level of carotenoids, especially the Spinato cv. It can be concluded. Therefore, that the presence of anthocyanins, together with polyphenols, is chiefly affecting the antioxidant power in the maize seeds examined. Moreover, the different amounts of pigment in all the cultivars did not affect the total protein level, and the same was true for all the seeds analyzed. The black maize genotypes and presumably also the B73 and Argentina cv will therefore be utilized to feed the local South African populations and will be taken into consideration for future breeding programs in order to improve the nutritional quality of the food made from corn.
REFERENCES
- Dowswell CR, Paliwal RL, Cantrell RP (1996) Maize in the third world. Westview Press, Boulder, Colorado, USA. Pg no: 8.
- Nuss ET, Tanumihardjo SA (2011) Quality Protein Maize for Africa: Closing the protein inadequacy gap in vulnerable populations. Adv Nutr 2: 217-224.
- McCann JC (2005) Africa and the world ecology of maize. In: McCann JC (ed.). Maize and Grace: Africa’s encounter with a New World crop, 1500-2000. Harvard University Press, Cambridge, UK.
- Atlin GN, Palacios N, Babu R, Das B, Twumasi-Afriyie S, et al. (2011) Quality protein maize: progress and prospects. In: Janick J (ed.). Plant breeding reviews (vol 34). John Wiley & Sons, Hoboken, NJ, USA.
- National Agricultural Marketing Council (2007) The South African Food Cost Review. Department of Agriculture, National Agricultural Marketing Council. Pretoria, South Africa.
- Jespersen L (2003) Occurrence and taxonomic characteristics of strains of Saccharomyces cerevisiae predominant in African indigenous fermented foods and beverages. FEMS Yeast Res 3: 191-200.
- Department of Health (2000) National Food Consumption Survey: Children aged 1-9 years, South Africa Department of Health. Stellenbosch, South Africa.
- Caulfield LE, Richard SA, Rivera JA, Musgrove P, Black RE (2006) “Stunting, wasting and micronutrient deficiency disorders.” In: Jamison DT, Breman JG, Measham AR, Alleyne G, Claeson M, et al. (eds.). Disease Control Priorities in Developing Countries (2ndedn), World Bank, Washington DC, USA.
- Ezzati JS, Vander Hoorn S, Lopez AD, Danaei G, Rodgers A, et al. (2006) “Comparative quantification of mortality and burden of disease attributable to selected risk factors.” In: Lopez AD, Mathers CD, Ezzati M, Jamison DT, Murray CJL, et al. (eds.). Global Burden of Disease and Risk Factors, World Bank publications, Washington, USA.
- Shisana O, Labadarios R, Rehle T, Simbayi L, Zuma K, et al. (2013) South African National Health and Nutrition Examination Survey (SANHANES-1). HSRC Press, Cape Town, South Africa.
- Faber M, Wenhold F (2007) Water SA. South African Journal of Bioethics and Law 33.
- Gannon B, Kaliwile C, Arscott SA, Schmaelzle S, Chileshe J, et al. (2014) Biofortified orange maize is as efficacious as a vitamin A supplement in Zambian children even in the presence of high liver reserves of vitamin A: a community-based, randomized placebo-controlled trial. Am J Clin Nutr 100: 1541-1550.
- White PJ (2005) Biofortifying crops with essential mineral elements. Calcium. In: Broadley MR, White PJ (eds.). Plant Nutritional Genomics. Blackwell Publishing, Oxford, UK. Pg no: 321.
- Snodderly DM (1995) Evidence for protection against age-related macular degeneration by carotenoids and antioxidant vitamins. Am J Clin Nutr 62: 1448-1461.
- Doria E, Galleschi L, Calucci L, Pinzino C, Pilu R, et al. (2009) Phytic acid prevents oxidative stress in seeds: evidence from a maize (Zea mays L.) low phytic acid mutant. J Exp Bot 60: 967-978.
- Brand-Williams W, Cuvelier ME, Berset C (1995) Use of a free radical method to evaluate antioxidant activity. LWT Food Science and Technology 28: 25-30.
- Doria E, Campion B, Sparvoli F, Tava A, Nielsen E (2012) Anti-nutrient components and metabolites with health implications in seeds of ten common bean (Phaseolus vulgaris L. and Phaseolus lunatus L.) landraces cultivated in southern Italy. Journal of Food Composition and Analysis 26: 72-80.
- Swain T, Hillis WE (1959) The phenolic constituents of Prunus domestica. I.-The quantitative analysis of phenolic constituents. Journal of the Science of Food and Agriculture 10: 63-68.
- Medoua GM, Egal AA, Oldewage-Theron WH (2009) Nutritional value and antioxidant capacity of lunch meals consumed by elderly people of Sharpeville, South Africa. Food Chemistry 115: 260-264.
- Tukaj Z, Matusiak-Mikulin K, Lewandowska J, Szurkowski J (2003) Changes in the pigment patterns and the photosynthetic activity during a light-induced cell cycle of the green alga Scenedesmus armatus. Plant Physiology and Biochemistry 4: 337-344.
- Lago C, Landoni M, Cassani E, Doria E, Nielsen E, et al. (2012) Study and characterization of a novel functional food: purple popcorn. Mol Breeding 31: 575-585.
- Chen PS, Toribara TY, Warner H (1956) Micro-determination of phosphorus. Analytical Chemistry 28: 1756-1758.
- Pilu R, Landoni M, Cassani E, Doria E, Nielsen E (2005) The Maize lpa241 mutation causes a remarkable variability of expression and some pleiotropic effects. Theoretical and Applied Genetics 45: 2096-2105.
- Enysi S, Umoh VJ, Whong CMZ, Abdullahi IO, Alabi O (2014) Chemical and nutritional value of maize and maize products obtained from selected markets in Kaduna State, Nigeria. African Journal of Food Science and Technology 5: 100-104.
- Kurilich A, Juvik J (1999) Quantification of carotenoid and tocopherol antioxidants in Zea mays. J Agric Food Chem 47: 1948-1955.
- Kuhnen S, Lemos PMM, Campestrini LH, Ogliari JB, Dias PF, et al. (2009). Antiangiogenic properties of carotenoids: A potential role of maize as functional food. Journal of Functional Foods 1: 284-290.
- Zili? S, Serpen A, Ak?ll?o?lu G, Gökmen V, Van?etovi? J (2012) Phenolic compounds, carotenoids, anthocyanins, and antioxidant capacity of colored maize (Zea mays L.) kernels. J Agric Food Chem 60: 1224-1231.
- Montilla CE, Hillebrand S, Antezana A, Winterhalter P (2011) Soluble and bound phenolic compounds in different Bolivian purple corn (Zea mays L.) cultivars. J Agric Food Chem 59: 7068-7074.
- Tsuda T (2012) Dietary anthocyanin-rich plants: biochemical basis and recent progress in health benefits studies. Mol Nutr Food Res 56: 159-170.
- Lopez AD, Mathers CD, Ezzati DT, Jamison DT, Murry CJL (2006) Global Burden of Disease and Risk Factors. World Bank, Washington DC, USA.
Citation: Doria E, Daoudou B, Egal AK, Oldewage-Theron WH, Pilu R (2015) Preliminary Analysis and Biochemical Characterization Related to Health Implications for African Populations in Some Maize Cultivars. A Special Look at the South African Environment. J Food Sci Nutr 1: 005.
Copyright: © 2015 Enrico Doria, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.

