
Tomato Fruit Yield and Quality as Affected by Grafting and Shading
*Corresponding Author(s):
Ilic SZFaculty Of Agriculture, University Of Priština-Kosovska Mitrovica, 38219 Lešak, Serbia
Tel:+381 638014966,
Email:zoran.ilic63@gmail.com
Abstract
In the present study, tomato (grafted and nongrafted) was grown in the soil under net-house cover by pearl and red nets (50% shade index) or un-shade condition (open field-control). Commercial tomato cultivars (‘Optima F’1 and ‘Big Beef F1’) were used to determine whether grafting (Maxifort rootstock) could prevent decrease of fruit weight and quality under light stress conditions. Nongrafted ‘Optima F1’ and ‘Big Beef F1’ was used as a control plants. Total yield of grafted plants was higher (51.0%) than non-grafted only in ‘Optima F1’. Shading maintained 30-40% higher marketable yield (reduced the amount of pysiological disorders) than plants from non-shaded conditions in both cultivars. Among the fruit components, lycopene, ascorbic acid, phenols, total sugar and citric acids concentrations and ratio of sugar and acid content were examined. In every case the values of these parameters were lower in the fruits from grafted plants than those from ungrafted ones, meaning that the increase of the yield quantity caused the decrease of main fruit components. The decrease of bioactive compounds had a similar trend and intensity, though some inter-color shade variation was observed. Fruits from shading plants under red nets obtained highest lycopene content in both grafted and non-grafted plants.
Keywords
INTRODUCTION
Tomato (Lycopersicum esculentum Mill.) is one of the most important vegetable species, produced and consumed worldwide, grown in both open-field and protected conditions, in soil or soilless media [1], with regard to both economic and health aspects. The growth, yield and fruit quality of tomato can be influenced by their genetic potential and environmental factors, such as temperature, radiation and grafting [2-6].
High temperatures during the growing season have been reported to be detrimental to growth, reproductive development and yield of tomato. The high market price during the summer period motivates farmers to adopt additional cultural practices to overcome production constraints, such as shading and grafting. Low-cost protected cultivation, such as net houses, has the potential to reduce various biotic and abiotic challenges during summer open field production, creates a microclimate which affects productivity and quality [7]. Photo-selective, coloured, shade nets provide diverse mixtures of natural, unmodified light and scattered spectrally modified light [8]. Spectral modification promotes physiological responses [9], while scattering improves penetration of spectrally modified light into the inner plant canopy [10].
Grafted commercial cultivars (scions) onto selected tolerant rootstocks could be a promising method for producing tomato at suboptimal conditions [11]. Grafting is the union of two or more pieces of living plant tissue [12,13], which are forced to develop vascular connection and grow as a single plant [14]. The root system of grafted plants is stronger and more efficient in uptake of water and nutrients which indirectly improves yield [13,15-17]. In this context, the use of adequate rootstocks through grafting provides an alternative strategy to reduce the losses in production caused by environmental stresses [18], such as the excess of radiation and temperature in the late cropping season [15,19]. Grafting is becoming a common practice in several European countries, such as Spain, Italy, Turkey, Greece and Israel [12].
Regarding the changes in fruit quality by grafting, there are several conflicting reports whether grafting effects are advantageous or disadvantageous [20,21]. A negative effect of tomato grafting was observed regarding fruit yield per plant, number of fruits per plant and total yield [17], but organic acid and lycopene content was significantly higher when the eggplant was used as a rootstock. The overall results showed that tomato grafting on suitable rootstocks elicited positive effects on cultivation performance, but decreased tomato nutritional quality [22]. Obtained similar results with respect to vitamin C reduction because of grafting.
The effect of rootstocks on the amount of TSS was found not statistically significant [23-26], although some researchers have observed that soluble solid content was lower in grafted compared to non-grafted plants [27-29].
According to several authors, lycopene concentration in tomato fruits tends to decrease with grafting [30-33]. The effect of rootstocks on titratable acidity has been found to be significant [25], but in some studies [23,24,27,28], found no effect of grafting on the titratable acidity. Gajc-Wolska et al. [34] and Turhan et al. [28], found decreasing sugar contents in fruits of grafted tomatoes too.
Genetic (cultivar differences) and environmental factors modulate the physiology and metabolism of tomato plants, but it is not clear how technological factors (grafting) combined with abiotic stress (environmental factors-light, temperature) affect tomato’s natural metabolic composition. The objective of our research was to evaluate grafting dependent differences in the response of shades.
MATERIAL AND METHODS
Plant material and cultivation
The cultivar ‘Optima F1’ is characterized by medium early maturing, with round shape and with high quality flesh. The cultivar ‘Big Beef F1’ belong to Solanum lycopersicum L., beef type group, medium maturing with round and oblate shape, respectively and with fine beef quality. Interspecific hybrid ‘Maxifort’ (Solanum lycopersicum L. × Solanum habrochaites S. De Ruiter, Bergshenhoek, The Netherlands), were used as rootstock.
In our experiment, the seeds of scions were sown on 15th March in a seedling tray filled with peat-based substrate. Seeds of the rootstocks were sown two days prior to the sowing of scion seeds. Seedlings were grafted on 28th April. Seedlings were produced in the plastic tunnel with heating. Until the emerging of the tomato plants temperature was maintained at the 25-26°C, and after decreased at 21°C. Seedlings were grafted 25 to 30 days after emerging of the rootstocks and scions, when the stem diameter was 2 mm. Grafting was done by hand using cleft grafting method. During the grafting process it is very important to increase temperature at the 24°C. After the grafting, plants are placed in germination chamber, where the temperature of 20°C and relative humidity of 95% is maintained for 3 days. In next 5 days relative humidity is slowly decreased and temperature was set at 22°C, until the transplanting time. Grafted plants were transplanted in net-house 29th May. The experiment was set as randomized block system with three replications, each replication consists of 30 plants. Plants were transplanted at the distance 80 cm between the rows and 40 cm in the row. This spacing gave the density of 3 plants m-2.
Normal cultural practices for the experiment were followed for irrigation, fertilization and pesticide application. The experiment was terminated on 11.09.2017. The following measurements were recorded: (a) number of plants which survived until the transplanting date; (b) fruit yield (g plant-1) and (c) total number of fruits harvested per plant. Yield measurements were recorded on ripe fruits, which were gently hand-harvested and transported to the laboratory, where they were counted and weighed.
Characteristics of the nets
Light interception by nets
β-Carotene and lycopene content
β-Carotene (mg/100 ml) = 0.216 × A663 - 1.22 × A645 - 0.304 A505 + 0.452 × A453
Lycopene (mg/100 ml) = -0.0458A663 + 0.204A645 + 0.372A505 - 0.0806A453
The results were expressed in µg/g fresh weight.
For determination of total phenol contents, the average sample obtained by mixing the axial cut outs from 9 fruits from each treatment block was used. Extraction was performed from 2 g of homogenized fresh plant material with 40 cm3 of 70% v/v ethanol during 4.5 h at 25°C. The obtained extracts were filtered under vacuum and the solvent was removed by evaporation at 50°C. The extracts were dried in the vacuum dryer at 40°C till constant mass and the content of total extractive matter (TEM, dry extract) was calculated on the basis of dry residue content. TEM were expressed in g/100 g of fresh plant material.
Ascorbic acid content was determined by spectrophotometric methods using potassium permenganate as a chromogenic reagent. Standard calibration curve of ascorbic was established by graphing concentrations versus absorbance of ascorbic standard solutions by taking 10 mL of each of standard solutions and put in a test tube, then 1 mL of KMnO4 solution (100 μg/mL) was added. This solution was let to stand for 5 minutes. The absorbance of this standard solutions were read at 530 nm against blank.
Each of the five of fruit samples were accurately taken as 10.0 mL for each sample, and then transferred into a test tube, and 1.0 mL of KMnO4 (100 μg/mL) was added for each. The contents of each test tube were mixed well and stand for 5 minutes. The prepared solutions were read at 530 nm against blank by spectrophotometer using a suitable concentration for the analysis.
Total Phenol Content (TPC) was determined according to the Folin-Ciocalteu method Singleton et al. [36]. In brief, to 1 mL of extract, 7 mL of water and 0.5 ml of Folin-Ciocalteu reagent diluted with water (1:1; v:v) were added. After 10 minutes of equilibration, 1.5 mL Na2CO3 solution (20%; w/v) was added. Following 30 minutes, the absorbance was recorded at 730 nm (Cintra 303, GBC). The results were calculated from the calibration obtained using gallic acid as the reference standard.
Sugars and organic acids content
For sugar determinations the liquid chromatograph was equipped with Evaporative Light Scattering Detector (ELSD) and Agilent, Zorbax Carbohydrate 4.6 × 250 mm, 5 μm column (Agilent Technologies). Solvent system of acetonitrile and water (75:25 v/v) at a flow-rate of 1.1 mL min-1 with total running time of 12 minutes was used. Injection was performed automatically with injection volume of 10 μL.
For organic acids analysis liquid chromatograph was equipped with Diode Array Detector (DAD) and nucleogel sugar 810 H (MACHEREY-NAGEL) column. 5 mmol H2SO4 was used for elution at a flow-rate of 0.6 mL min-1 at 65°C, and total running time was 25 min. Peaks were recorded at 210 nm. The injected volume of samples and standards was 5 μL and injection was performed automatically.
Standard solutions of sugars (glucose, fructose and sucrose) and organic acids (citric, malic and succinic) were used for development of calibrations. Peaks were identified based on retention time comparisons, and the concentrations were quantified from developed linear regressions.
STATISTICAL METHODS
Significance of effect of different color shade nets on tomato was determined by two way ANOVA followed by Duncan’s multiple range test. Multivariate principal component analysis was used for explanatory data analysis. For all calculations STATISTICA 13 software was used (Dell Inc. (2016). Dell Statistica (data analysis software system), version 13. software.dell.com.).
RESULTS AND DISCUSSION
Light modification by photo-selective nets
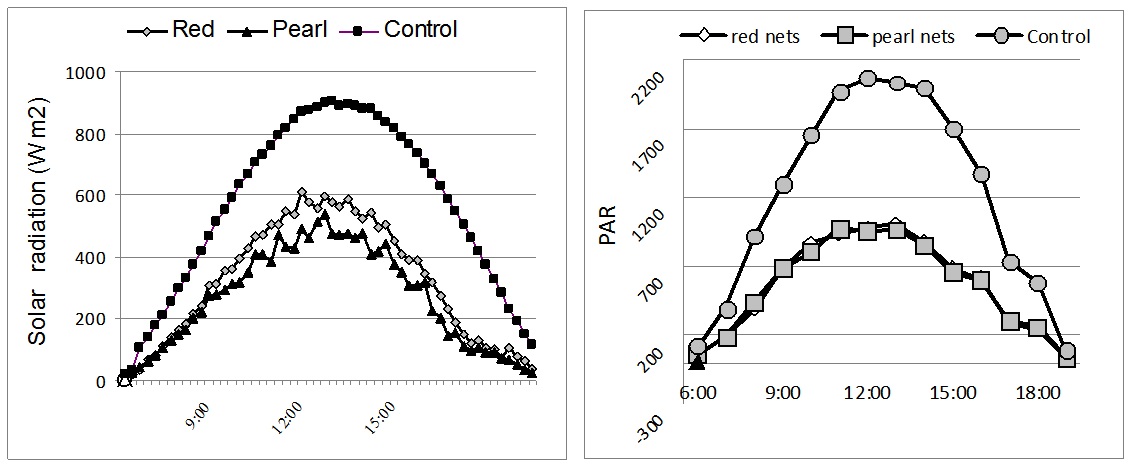 Figure 1: Solar radiation (W m-2) and PAR-Photosynthetically Active Radiation (μmol m-2s-1) measured under different colour nets (average day in July).
Figure 1: Solar radiation (W m-2) and PAR-Photosynthetically Active Radiation (μmol m-2s-1) measured under different colour nets (average day in July).Results from figure 1 show that the reduction of net radiation is mediated by the colour nets. Compared to control, solar radiation was significantly reduced by the 50% shadow intensity. The incoming solar radiation for red and pearl colour-nets was 563 Wm-2 and 469 Wm-2, respectively.
The solar radiation registered on sunny days resulted in high PAR values (maximum 1600 to 2000 µmol m-2s-1), which are common in southern Europe arid conditions. On cloudy days (complete cloud cover), the maximum PAR values ranged from 800 to 900 µmol m-2s-1. The photosynthetically active radiation was halved in comparison to open field under all used shade nets. The maximal level photosynthetically active radiation under the red nets was 1004 µmol s-1m-2, while the maximum intensity of photosynthetically active radiation in the open field reached 2034 µmol s-1m-2 (Figure 1).
Parameters of yield
Shading effect changes total and market yields of tomatoes. Shading maintained 30-40% higher marketable yield (reduced the amount of pysiological disorders) than plants from non-shaded conditions in both cultivars. The greater fruit yield produced from shaded plants may be explained by the assumption that during summer, high temperature increases shedding of tomato flower and reduces fruit set. It is also possible that the increase in tomato yield is due to larger number of branches and flowers per plant. Since the nets are composed of holes, in addition to the translucent photo-selective plastic threads, shade nets actually create mixtures of natural, unmodified light, which is passing through the holes, together with the diffused, spectrally modified light, which is emitted by the photo-selective threads [37].
The reduced total and marketable yields of non-shaded plants were probably due to high heat stress. However, grafted cv. Optima F1 did not significantly affect leaf biomass but increased the total and marketable fruit yield. The difference in marketable fruit yield between grafted and non-grafted plants was 4-16%. A shading of tomatoes may be an option to reduce heat stress conditions and extend the summer season toward September. A possible cause of the decrease of cracked fruit by shading is a decrease in fruit temperature by the shading treatments [38].
Similarly, it was reported that marketable yield increased in tomatoes with rootstock use [23,25,26]. For example, tomato cv. Florida 47 grafted on ‘Beaufort’ and ‘Multifort’ rootstocks, increased the marketable fruit yield by up to 41% [39]. Similarly, the ‘El Cid’ tomato grafted onto interspecific hybrid rootstock ‘Multifort’ obtained a 12.9% increase in fruit yield [40]. Significant decrease of the yield and number of fruits was observed when eggplant rootstock was used [41].
Average fruit weight was similarly in both cultivars. Grafted ‘Optima F1’ un-shaded plants, produce fruits with significant highest weight (222.9 g) than non-grafted plants (173 g). Shading havent effects in increasing fruits weight in grafted ‘Optima F1’ plants, but non-grafted shade plants produce fruits with significantly highest weight than fruits from unshade plants (173 g). Grafted cv. ‘Big Beef F1’ (193 g) obtained significantly highest fruit weight than nongrafted plants. Shading with red and pearl nets has possitive effect in production of highest fruit weight in nongrafted plants in both cultivar. In same time, grafting plants under shading no effect in cv ‘Optima F1’ or decrease fruit weight in cv ‘Big Beef F1’ (Table 1).
|
|
Yield g/Plant |
Total Yield t/ha |
*Marketable Yield % |
Number of Fruit/Plant |
Fruit Weight g |
|
|
Optima F1 |
||||||
|
Grafted |
Control |
5443a |
150.7a |
70b |
24.0a |
222.9a |
|
Red net |
5640a |
156.2a |
89a |
28.6a |
196.9b |
|
|
Pearl net |
5430a |
150.4a |
91a |
25.2a |
214.9a |
|
|
Non grafted |
Control |
3602b |
99.8b |
61b |
19.7b |
173.0c |
|
Red net |
3462b |
95.9b |
85a |
17.3b |
199.5b |
|
|
Pearl net |
3406b |
94.3b |
86a |
17.7b |
192.1b |
|
|
Big Beef F1 |
|
|||||
|
Grafted |
Control |
3275b |
90.7b |
66b |
17.6b |
193.0b |
|
Red net |
4570ab |
126.6ab |
83a |
23.1a |
198.1b |
|
|
Pearl net |
5062a |
140.2a |
84a |
25.0a |
202.5b |
|
|
Non |
Control |
4490ab |
124.4ab |
57b |
26.6a |
168.0c |
|
Red net |
4569ab |
126.6ab |
81a |
22.4a |
203.5b |
|
|
Pearl net |
5007a |
138.7a |
80a |
26.7a |
187.5b |
|
Fruit composition
Fruits of grafted tomato plants from both cultivars have higher water content than fruits from non-grafted plants, resulting in a dilution effect of compounds. In fact, the dry matter content of fruits from ‘Optima’ grafted onto ‘Maxifort’ (5.6%) was lower compared to non-grafted Optima (6.9%) fruits, supporting an inverse relationship between sugar and water content [28]. Statistically significant differences in water content was observed between cultivars and grafting x cultivars. Shading no influence on fruit water content (Figure 2).
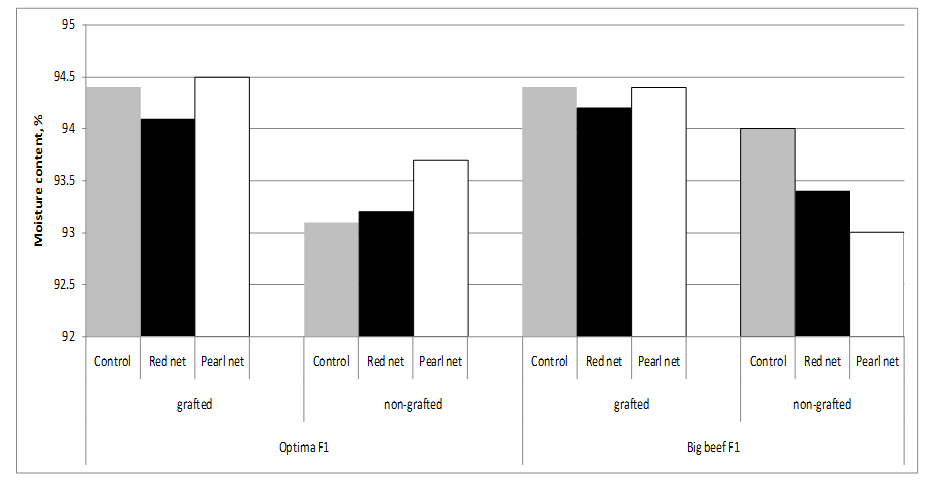 Figure 2: Tomato moisture content as affected by grafting and shading.
Figure 2: Tomato moisture content as affected by grafting and shading.The content of lycopene depends on the tomato cultivar. Red-ripe fruits of most high-lycopene lines showed commercially suitable soluble solids and titratable acidity, in addition to increased levels of lycopene (up to 440 mg/kg fw) and other bioactive phytochemicals (mainly flavonoids and vitamin C) compared to their near isogenic conventional counterparts [49]. In our study, between cultivars in non grafted plants from open field no differences in lycopene content (~210 mg/100g FW). Lycopene content can be influenced by grafting, but it is subject to significant rootstock-scion interaction which indicates that graft combination plays an important role. Thus, lycopene content in grafting cv. Big Beef F1 was significantly lower (158.6 mg/100g FW) than fruits from non grafting plants with tends to decreased with shading condition. Combination shading x grafting characterized by the significant lower lycopene content in both cultivars (Figure 3).
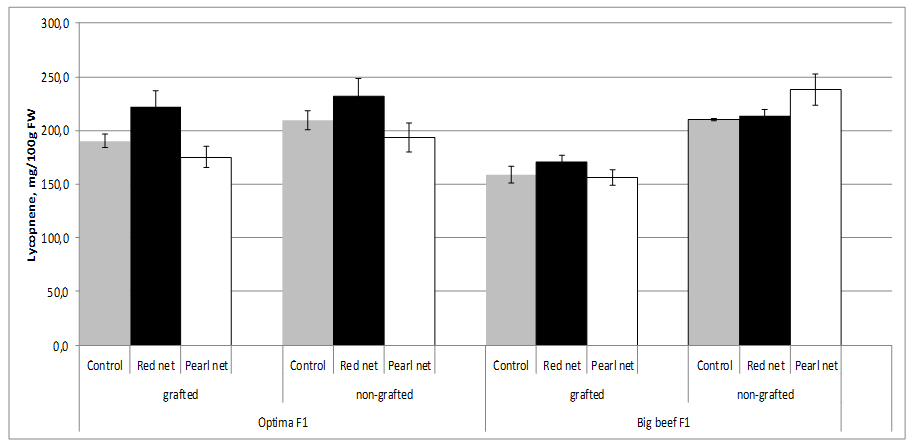 Figure 3: Tomato lycopene content as affected by grafting and shading.
Figure 3: Tomato lycopene content as affected by grafting and shading.Lycopene is the pigment principally responsible for the characteristic deep-red color of ripe tomato fruits and tomato products. According to Brandt et al. [50], significantly higher lycopene content was observed in glasshouse-grown tomato compared to field grown, at different harvesting times. According to Farkas [51], lycopene production is inhibited when environmental temperature is above 32°C. Lycopene content changed significantly during maturation and accumulated mainly in the deep red stage [52].
According to several authors, lycopene concentration in tomato fruits tends to decrease with grafting [31-33], e.g. most out of 15 rootstocks investigated, including ‘Maxifort,’ ‘Beaufort,’ and ‘King Kong,’ decreased the fruit lycopene concentration of tomato scion ‘Jeremy’ and ‘Jack’ [41,42]. Similar results have been reported for tomato scion ‘Cecilia’ grafted onto ‘Beaufort’ and ‘Heman’ [30], for scion ‘Macarena’ grafted onto ‘Maxifort’ [34], as well as for ‘Classy’ grafted onto ‘Brigeor’ [11,45]. Lycopene in tomato fruits decreased in grafting treatments in soilless cultivation [31]. However, their concentrations were increased or unaffected by grafting in soil cultivation due to the fact that plants grown in soilless culture were not under salt stress, unlike those grown in soil [46].
Tomato fruit contains significant amounts of Ascorbic Acid (AA), and these content strongly depend of cultivar origin. Although light is not essential for the synthesis of AA in plants, the amount and intensity of light during the growing season have a definite influence on the amount of AA formed. AA is synthesized from sugars supplied through photosynthesis in plants. Outside fruit exposed to maximum sunlight contain higher amount of vitamin C than inside and shaded fruit on the same plant. In our experiment fruits from non grafting and open field plants cv. Optima F1 has double more AA content (22.5 mg/100g FW) than cv. Big Beef F1 (11.3 mg/100g FW) from same conditions.
In our study we found that AA contents (11.3-22.5 mg/100g FW) depends of cultivar decreased by 21.2-29.3% in the fruits from grafted plants in comparison with the fruit from non-grafted plants. AA content in both cultivars decreased under shade nets exept in non grafting cv. Big Beef F1 were AA content increased in both shade nets. In terms of AA content specifically react to different color nets, thus both cultivars under pearl nets lower contents of AA were recorded. Statistical differences in AA content was observed inside grafting x cultivar combination (Figure 4).
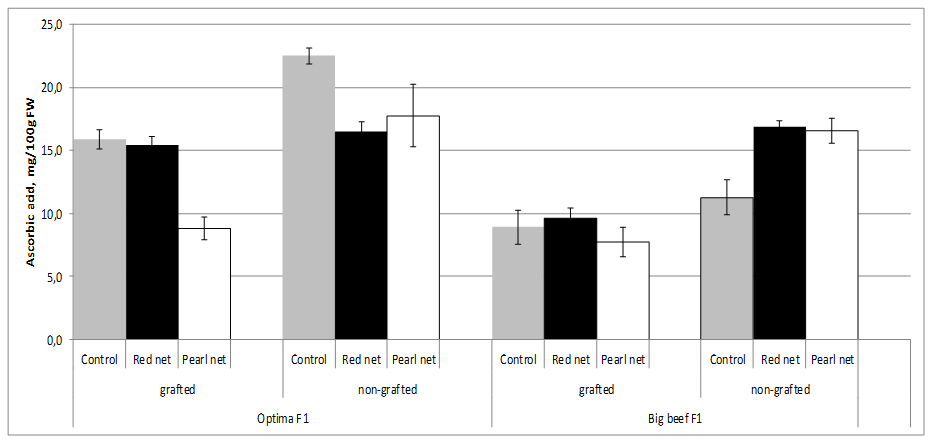 Figure 4: Tomato ascorbic acid content as affected by grafting and shading.
Figure 4: Tomato ascorbic acid content as affected by grafting and shading.Several studies showed that AA content strongly reduced by grafting both in greenhouse and field studies [39,42,53-55]. However, in a different study, Di Gioia et al. [54], found that vitamin C contents decreased by 14-20% in the fruits of plants grafted onto ‘Beaufort’ F1 and ‘Maxifort’ F1, in comparison with the non-grafted treatment. The lower ascorbic acid content could be explained by the higher plant/shoot biomass in grafted plants compared with non-grafted ones or by the fact that grafted plants were initially subjected to stress following the grafting operation. Ascorbic acid is known to control cell differentiation [56], and to promote callus division and growth [57]. The decreased total vitamin C content of the fruits from grafted plants could therefore be a resultant of redistribution or accumulation of vitamin C in other parts of grafted plants [58].
The choice of cultivar as well as grafting and shading as agronomic factors are major contributing factors to the total content of phenolics in tomato. Total phenolics showed the greatest variations between grafted and non-grafted plants in cv. Optima F1.
Under standard growth conditions applied in these experiments, grafting significantly reduced the total phenolics content of fruits only in cv. Optima F1. Total phenol content decreased in grafting plants under shading in both cultivars. It is interesting to note that no significant differences were found in non grafted cv. Big Beef F1 among the shading (Figure 5).
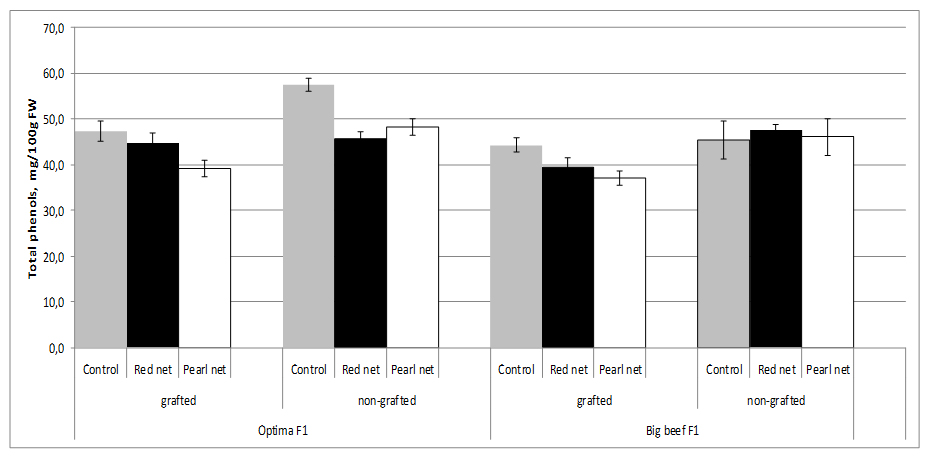
However, total phenolics content changes significantly among the scions-cultivars used in our experiment as well as between cultivars under different shade nets. The results obtained confirm those of Vinkovic-Vrcek et al. [55], who found that grafting significantly reduced the total phenolics content and antioxidant activities of tomato fruits. Changes in light intensity through the utilisation of shade nets were able to change the synthesis of phenolic compounds in plants. Different shade levels, with the resultant changes in plant morphology and physiological characteristics, affected the secondary metabolites such as phenolic compounds in plants. However, different plants had diverse reactions to shade levels, which alter the production of Total Phenolics (TP). Previous studies showed that change in light intensity was able to modify the production and accumulation of TP in lettuce [8].
Sugars, mainly glucose and fructose, account for about half of the dry matter or 65% of the soluble solids of a ripe tomato fruit. The sugar content of fruit fresh weight depending on cultivar and growing method (grafting and shading). The sugar content ranges from 3.52% in grafting unshading cv. Optima F1 to 5.62% in nongrafting plants under red shade nets. Improvement of fruit sweetness related to grafting is rather seldomly reported. Grafting decreased sugar concentrations in both cultivars whereas sugar concentrations were influenced by both cultivar and shading. Shading increased sugar content in fruits from nongrafting plants, thus in cv. Optima F1 the highest sugar content was recorder under red nets (5.62%) and in cv. Big Beef F1 under pearl nets (5.40%), figure 6.
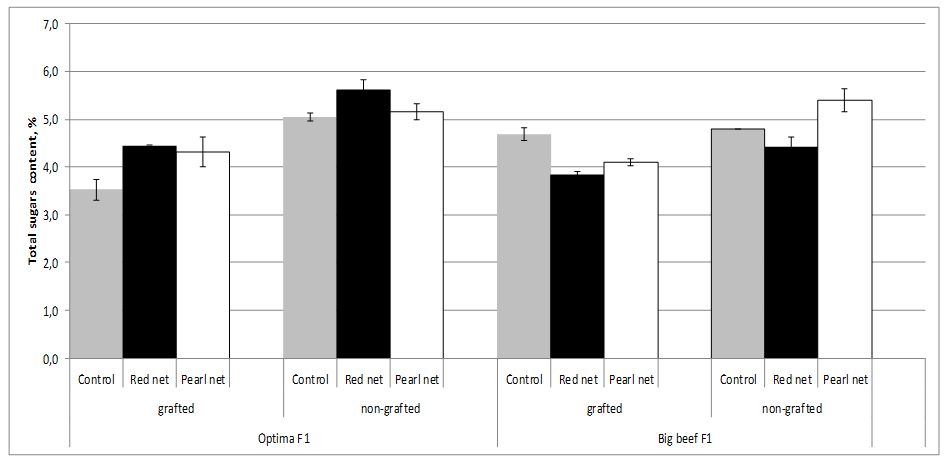 Figure 6:Tomato total sugars content as affected by grafting and shading.
Figure 6:Tomato total sugars content as affected by grafting and shading.In our study, sugar decreased caused by grafting were relatively minor (2.3%) in cv. Bef Beef F1 to very high (30.3%) in cv. Optima F1. For examples, Pogonyi et al. [27], reported a decreased sugar concentration in tomato fruits by up to 25% for tomato plants (Lemance F1) grown in a soil culture and grafted on ‘Beaufort’ compared to ungrafted plants.
The decline in sugars incurred with grafting is reported to account for approximately not more than 16% [59], which does not exceed the range of maximum decline proposed for consumer acceptability [60,61]. In this respect, grafting may be considered a high-input production method, with a prevalent tendency for increasing crop load and potentially suppressing fruit sugar content [20,62].
The reasons for a lower carbohydrate content in grafted tomato may stem indirectly through rootstock effect on scion vigor, timing of flowering, fruit load, yield and, ultimately, fruit maturation, as fruit sugar concentration is highly dependent on fruit maturity at harvest [6,62]. It seems that beside photosynthesis, changes in dry matter content could be responsible for the reduced sugar concentration in grafted plants [11]. Alternatively, water uptake-efficient rootstocks may increase fruit water content even if sufficient assimilates are available, thus, leading to a reduced fruit sugar concentration [11,28].
Total organic acids in tomato fruit are usually in the range of 0.2 to 1.7 g/kg-1 fresh mass, with citric and malic acid being the main components of sourness. The highest Citric Acid (CA) values (1.0%) were found in nongrafted cv. Big Beef F1 tomato plants, whereas the lowest (0.67%) were found in grafted cv. Optima F1 tomato plants. Grafting accounts for an decrease in CA up to 25.6% reported under a range of different shading which indicates a direct rootstock effect (Figure 7).
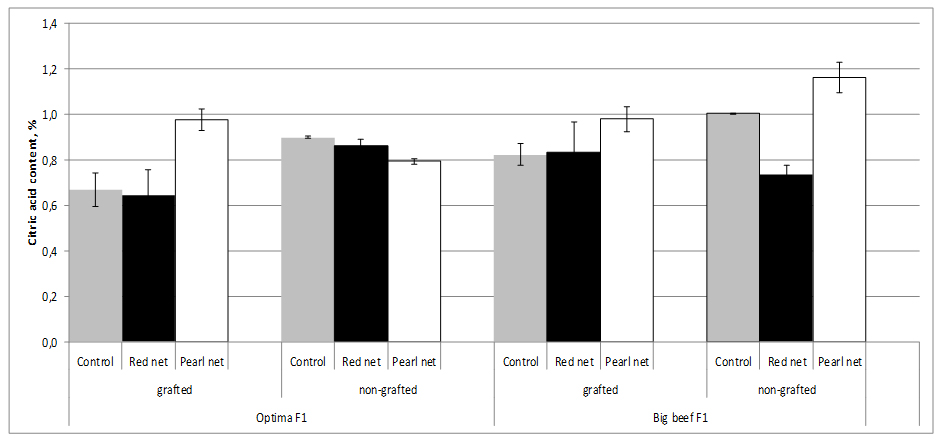 Figure 7:Tomato citic acid content as affected by grafting and shading.
Figure 7:Tomato citic acid content as affected by grafting and shading.Shading, special pearl nets significantly increased CA content in tomato fruits from grafting and nongrafting plants exept in nongrafting cv. Optima F1 plants. The decrease of sugar content was not in relation to the loss of acid content on grafted plants compared with ungrafted ones [27]. Because of the decrease of sugar content was much higher than that of acids, their rates were markedly different for the two shading methods. Grafting may have positive effects on the decreased acidity of the tomato fruit produced. Fernández-Garcia et al. [53], stated that TA values were the most important chemical quality parameters for tomato and were not affected by grafting.
The mechanisms involved in grafting-elicited decrease of fruit CA have not been thoroughly investigated; however, organic acids constitute a direct substrate for respiratory demands and their increased de novosynthesis in developing fruits might be a plausible mechanism for coping with the sugar deficit incurred on the heavy crop load supported by vigorous rootstocks.
Sugars, acids and their interactions are important in relation to sweetness, sourness, and flavour intensity in tomatoes. Much sugar and relatively high concentration of acids are required for the best flavour. High concentration of acids and low sugar content will produce a tart tomato, while high sugar content and low concentration of acids will result in a bland taste. When both sugar and total acid contents are low, the result is a tasteless, insipid tomato. It is supposed the best if the ratio of sugar to acid ranges from 9 to 10 [63].
Sugar to CA ratio in our study rages from 4.6 in cv. Big Beef F1 grafted under red nets to 6.8 in cv. Optima F1 grafted and grown under red nets. This ratio in case of our exploration was lowest because we acidity expressed through citric acid rather than through total acid whose values are slightly higter. Differences in this ratio between cultivars also confirm by Krumbein et al. [11], demonstrated that the flavor compounds (sugars, acids, and aroma volatiles) in tomato fruits grown under shaded condition depends on rootstock-scion combinations. In summary, the quality characteristics of grafted tomato fruits are greatly influenced by rootstock-scion combinations and shading conditions.
CONCLUSION
Grafted plants offer increased yield and consequently higher profits. In tomato plants cv. Optima F1, yield is positively affected by grafting due to the increase in fruit yield, number of fruits/truss and fruit weight. We consider these benefits to be of value to farmers. In same time grafted plants under shading, incresed significantly tomato yield only in cv. Big Beef F1. Although fruit quality values, such as lycopene content, vitamin C content, total sugar and citric acid content were lower in grafted plants, these values were still satisfactory and lied within the adequate ranges. However, lycopene content was slightly increased by shading under red nets. Total phenols remained unchanged depending on the grafting and shading in cv. Big Beef F1, but in cv. Optima F1 total phenols was lower in grafted plants and slight decreased with shading. Grafting decrease citric acid in fruit from both cultivars. In same time, shading increased citric acid only in fruits from grafted plants. Sugar content depend of cultivar and shading condition. Total sugar content is higher in fruits from non grafted and shade plants. Therefore, grafting had no harmful effects on fruit quality, but additional research is needed to determine whether grafting is economically feasible to the producer.
ACKNOWLEDGEMENT
This study is part of the TR-31027 and III46001 projects that were financially supported by the Ministry of Education, Science and Technological Development, Republic of Serbia.
REFERENCES
- Singh H, Kumar P, Chaudhari S, Edelstein M (2017) Tomato grafting: A global perspective. Hortsci 52: 1328-1336.
- Dorais M, Papadopolus AP, Gosselin A (2001) Greenhouse tomato fruit quality: The influence of environmental and cultural factors. Hortic Rev 26: 239-319.
- Dorais M, Ehret DL, Papadopolus AP (2008) Tomato (Solanum lycopersicum) health components: From the seed to the consumer. Phytochem Rev 7: 231-250.
- Dumas Y, Dadomo M, Di Lucca G, Grolier P (2003) Effects of environmental factors and agricultural techniques on antioxidant content of tomatoes. J Sci Food Agric 83: 369-382.
- Krumbein A, Schwarz D, Klaering HP (2006) Effects of environmental factors on carotenoid content in tomato (Lycopersicon esculentum L. Mill.) grown in a greenhouse. J Appl Bot Food Qual 80: 160-164.
- Rouphael Y, Schwarz D, Krumbein A, Colla G (2010) Impact of grafting on product quality of fruit vegetables. Sci Hortic 127: 172-179.
- Ili? ZS, Milenkovi? L, Šuni? L, Fallik E (2015) Effect of coloured shade-nets on plant leaf parameters and tomato fruit quality. J Sci Food Agric 95: 2660-2667.
- Ili? ZS, Milenkovi? L, Šuni? L, Bara? S, Fallik E (2017) Effect of shading by colour nets on yield and fruit quality of sweet pepper. Zemdirbyste-Agric 104: 53-62.
- Li T, Bi G, LeCompte J, Barickman TC, Evans BB (2017) Effect of colored shadecloth on the quality and yield of lettuce and snapdragon. HortTechnol 27: 860-867.
- Stamps RH (2009) Use of colored shade netting in horticulture. HortSci 44: 239-241.
- Krumbein A, Schwarz D (2013) Grafting: A possibility to enhance health-promoting and flavour compounds in tomato fruits of shaded plants? Sci Hortic 149: 97-107.
- Edelstein M, Koren A, Omer S, Cohen R (2015) The history and current status of Cucurbitaceous grafting in Israel. Chron Hortic 55: 10-13.
- Edelstein M, Cohen R, Baumkoler F, Ben-Hur M (2017) Using grafted vegetables to increase tolerance to salt and toxic elements. Israel J Plant Sci 64: 3-20.
- Savvas D, Colla G, Rouphael Y, Schwarz D (2010) Amelioration of heavy metal and nutrient stress in fruit vegetables by grafting. Sci Hortic 127: 156-161.
- Schwarz D, Rouphael Y, Colla G, Venema JH (2010) Grafting as a tool to improve tolerance of vegetables to abiotic stresses: Thermal stress, water stress and organic pollutants. Sci Hortic127: 162-171.
- Moncada A, Miceli A, Vetrano F, Mineo V, Planeta D, et al. (2013) Effect of grafting on yield and quality of eggplant (Solanum melongena L.). Sci Hortic 149: 108-114.
- Miskovic A, Ilic O, Ba?anovi?-Šiši? J, Vujasinovic V, Kuki? B (2016) Effect of eggplant rootstock on yield and quality parameters of grafted tomato. Acta Sci Pol Hort Cultus 15: 149-159.
- Fallik E, Ilic Z (2014) Grafted vegetables-the influence of rootstock and scion on postharvest quality. Folia Hortic 26: 79-90.
- Rivero MR, Ruiz JM, Romero L (2003) Role of grafting in horticultural plants under stress conditions. Food, Agric Environ 1: 70-74.
- Davis AR, Perkins-Veazie P, Hassell R, Levi A, King SR, et al. (2008) Grafting effects on vegetable quality. HortSci 43: 1670-1672.
- Lee JM, Kubota C, Tsao SJ, Biel Z, Echevaria PH, et al. (2010) Current status of vegetable grafting: Diffusion, grafting techniques, automation. Sci Hortic 127: 93-105.
- Nicoletto C, Tosini F, Sambo P (2013) Effect of grafting and ripening conditions on some qualitative traits of 'Cuore di bue' tomato fruits. J Sci Food Agric 93: 1397-1403.
- Gebolo?lu N, Yilmaz E, Çakmak P, Aydin M, Kasap Y (2011) Determining of the yield, quality and nutrient content of tomatoes grafted on different rootstocks in soilless culture. Sci Res Ess 6: 2147-2153.
- Savvas D, Savva A, Ntatsi G, Ropokis A, Karapanos I, et al. (2011) Effects of three commercial rootstocks on mineral nutrition, fruit yield, and quality of salinized tomato. J Plant Nutr Soil Sci 174: 154-162.
- Oztekin GB, Tuzel Y, Tuzel IH (2013) Does mycorrhiza improve salinity tolerance in grafted plants? Sci Hortic 149: 55-60.
- Di Gioia F, Signore A, Serio F, Santamaria P (2013) Grafting improves tomato salinity tolerance through sodium partitioning within the shoot. Hortsci 48: 855-862.
- Pogonyi Á, Pék Z, Helyes L, Lugasi A (2005) Effect of grafting on the tomato’s yield, quality and main fruit components in spring forcing. Acta Aliment 34: 453-462.
- Turhan A, Ozmen N, Serbeci MS, Seniz V (2011) Effects of grafting on different rootstocks on tomato fruit yield and quality. Hortic Sci 38: 142-149.
- Al-Harbi A, Hejazi A, Al-Omran A (2017) Responses of grafted tomato (Solanum lycopersiocon L.) to abiotic stresses in Saudi Arabia. Saudi J Biol Sci 24: 1274-1280.
- Mohammed STM, Humidan M, Boras M, Abdalla OA (2009) Effect of grafting tomato on different rootstocks on growth and productivity under glasshouse conditions. Asian J Agric Res 3: 47-54.
- Helyes L, Lugasi A, Pogonyi Á, Pék Z (2009) Effect of variety and grafting on lycopene content of tomato (Lycopersicon lycopersicum L. Karsten) fruit. Acta Aliment 38: 27-34.
- Brajovi? B, Kastelec D, Šircelj H, Maršic NK (2012) The effect of scion/rootstock combination and ripening stage on the composition of carotenoids and some carpometric characteristics of tomato fruit. Eur J Hortic Sci 77: 261-271.
- Nicoletto C, Tosini F, Sambo P (2013) Effect of grafting on biochemical and nutritional traits of ‘Cuore di bue’ tomatoes harvested at different ripening stages. Acta Agric Scand B 63: 114-122.
- Gajc-Wolska J, Lyszkowska M, Zielony T (2010) The influence of grafting and biostimulators on the yield and fruit quality of greenhouse tomato cv. (Lycopersicon esculentum Mill.) grown in the field. Veg Crops Res Bull 72: 63-70.
- Nagata M, Yamashita I (1992) Simple method for simultaneous determination of chlorophyll and carotenoids in tomato fruit. J Food Sci Technol 39: 925-928.
- Singleton V, Orthofer R, Lamuela-Raventos RM (1999) Analysis of total phenols and other oxidation substrates and antioxidants by means of folin-ciocalteu reagent. Meth Enzymol 299: 152-178.
- Shahak Y, Gal E, Offir Y, Ben-Yakir D (2008) Photoselective shade netting integrated with greenhouse technologies for improved performance of vegetable and ornamental crops. Acta Hortic 797: 75-80.
- Ili? ZS, Milenkovi? L, Stanojevi? L, Cvetkovi? D, Fallik E (2012) Effects of the modification of light intensity by color shade nets on yield and quality of tomato fruits. Sci Hortic 139: 90-95.
- Djidonou D, Simonne AH, Koch KE, Brecht JK, Zhao X (2016) Nutritional quality of field-grown tomato fruit as affected by grafting with interspecific hybrid rootstocks. HortSci 51: 1618-1624.
- Velasco-Alvarado MDJ, Castro-Brindis R, Castillo-Gonzalez AM, Avitia-Garcia E, Sahagún-Castellanos J, et al. (2016) Mineral composition, biomass and fruit yield in grafted tomato (Solanum lycopersicum L.). Interciencia 41: 703-708.
- Miskovic A, Ilin Z, Markovic V (2008) Effect of different rootstock type on quality and yield of tomato fruits. In: Proceedings of the International Symposium on Strategies towards Sustainability of Protected Cultivation in Mild Winter Climate. Acta Hort 807: 619-624.
- Riga P, Benedicto L, Garcia-Flores L, Villano D, Medina S, et al. (2016) Rootstock effect on serotonin and nutritional quality of tomatoes produced under low temperature and light conditions. J Food Compost Anal 46: 59-59.
- Khah EM, Kakava E, Mavromatis A, Chachalis D, Goulas C (2006) Effect of grafting on growth and yield of tomato (Lycopersicon esculentum Mill.) in greenhouse and open-field. J App Hortic 8: 3-7.
- Leonardi C, Giuffrida F (2006) Variation of plant growth and macronutrient uptake in grafted tomatoes and eggplants on three different rootstocks. Eur J Hortic Sci 71: 97-101.
- Schwarz D, Öztekin GB, Tüzel Y, Brückner B, Krumbein A (2013) Rootstocks can enhance tomato growth and quality characteristics at low potassium supply. Sci Hortic 149: 70-79.
- Qaryouti MM, Qawasmi W, Hamdan H, Edwan M (2007) Tomato fruit yield and quality as affected by grafting and growing system. Acta Hortic 741: 199-206.
- Bai Y, Lindhout P (2007) Domestication and breeding of tomatoes: What have we gained and what can we gain in the future? Ann Bot 100: 1085-1094.
- Flores FB, Sanchez-Bel P, Estañ MT, Martinez-Rodriguez MM, Moyano E, et al. (2010) The effectiveness of grafting to improve tomato fruit quality. Sci Hortic 125: 211-217.
- Ilahy R, Siddiqui MW, Tlili I, Montefusco A, Piro G, et al. (2018) When color really matters: Horticultural performance and functional quality of high lycopene tomatoes. Crit Rev Plant Sci 37: 15-53.
- Brandt S, Lugasi A, Barna É, Hóvári J, Pék Z, et al. (2003) Effects of the growing methods and conditions on the lycopene content of tomato fruits. Acta Alim 32: 269-278.
- Farkas J (1994) Paradicsom. (Tomato.) In: Balázs S, Zöldségtermesztök kézikönyve. (Handbook of the vegetable-growers.) Mezögazda Kiadó, Budapest, Hungary. Pg no: 195-225.
- Helyes L, Pék Z (2006) Tomato fruit quality and content depend on stage of maturity. HortSci 41: 1400-1401.
- Fernández-García N, Martínez V, Cerda A, Carvajal M (2004) Fruit quality of grafted tomato plants grown under saline conditions. J Hort Sci Biotechnol 79: 995-1001.
- Di Gioia F, Serio F, Buttaro D, Ayala O, Santamaria P (2010) Influence of rootstock on vegetative growth, fruit yield and quality in ‘Cuore di Bue’, an heirloom tomato. J Hort Sci Biotech 85: 477-482.
- Vinkovic-Vrcek I, Samobor V, Bojic M, Medic-Saric M, Vukobratovic M, et al. (2011) The effect of grafting on the antioxidant properties of tomato (Solanum lycopersicum L.). Spanish J Agric Res 9: 844-851.
- Arrigoni O (1994) Ascorbate system in plant development. J Bioenerg Biomembr 26: 407-419.
- Tabata K, Oba K, Suzuki K, Esaka M (2001) Generation and properties of ascorbic acid-deficient transgenic tobacco cells expressing antisense RNA for L-galactono-1,4-lactonedehydrogenase. Plant J 27: 139-148.
- Wadano A, Azeta M, Itotani S, Kanda A, Iwaki T, et al. (1999) Change of ascorbic acid level after grafting of tomato seedlings. Z Naturforsch 54: 830-833.
- Riga P (2015) Effect of rootstock on growth, fruit production and quality of tomato plants grown under low temperature and light conditions. Hortic Environ Biotech 56: 626-638.
- Kader AA (1999) Fruit maturity, ripening and quality relationships. Acta Hortic 485:203-208.
- Maynard DN, Dunlap AM, Sidoti BJ (2002) Sweetness in diploid and triploid watermelon fruit. Cucurbit Genet Coop Rep 25: 32-35.
- Soteriou GA, Kyriacou MC (2015) Rootstock-mediated effects on watermelon field performance and fruit quality characteristics. In J Veget Sci 21: 344-362.
- Helyes L, Varga G, Pék Z, Dimény J (1999) The simultaneous effect of variety, irrigation and weather on tomato yield. Acta Hort 487: 499-505.
Citation: Milenkovi? L, Mastilovi? J, Kevrešan Z, Jakši? A, Gledi? A, et al. (2018) Tomato Fruit Yield and Quality as Affected by Grafting and Shading. J Food Sci Nut 4: 042.
Copyright: © 2018 Milenkovic L, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.

