A New Effective, User-friendly Bacterial Vaginosis Treatment: A Randomized Multicenter Open-label Parallel-group Two-part Study with a Novel Sustained-release Pessary Containing Oligomeric Lactic Acid
*Corresponding Author(s):
Jeanette RobertssonPharmaceutical Development And Quality, PULS AB, Kullagatan 8, Helsingborg SE-75220, Sweden
Tel:+46 708637419,
Email:jeanette.robertsson@pulsinvest.se
Abstract
Introduction: Bacterial Vaginosis (BV) is an imbalance in the naturally occurring vaginal bacterial flora resulting in lack of vaginal acidity, inhibition of the normal Lactobacilli growth and overgrowth by mixed anaerobic bacterial flora including Gardnerella vaginalis. The prevalence of BV is estimated at approximately 15-30% among fertile women and frequent recurrences several times a year are common. BV has a negative effect on many women’s lives, in particular due to the odorous vaginal discharge. First line treatment with antibiotics is associated with adverse events, high relapse rates, and an emerging risk of bacterial drug resistance development. New antibiotic-free treatments with high efficacy and less frequent administration in combination with high patient acceptance are needed.
The aim of the present study was to investigate a new sustained release treatment, an Oligomeric Lactic Acid (OMLA) pessary in patients with confirmed BV, and to evaluate BV clearance, pH decrease, adverse events, and patient treatment satisfaction.
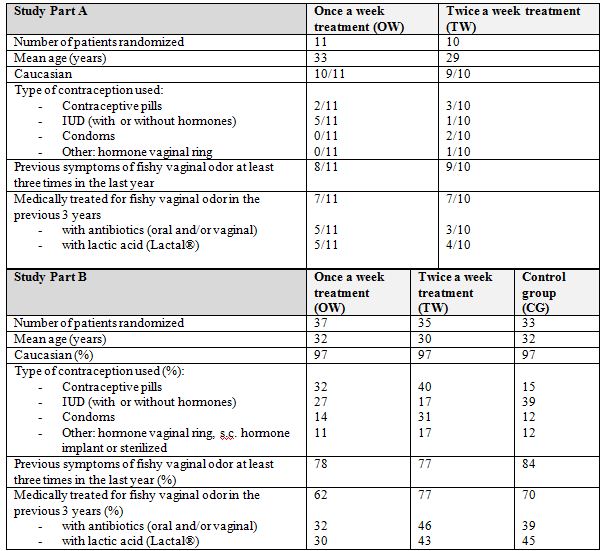
Methods: The study was a randomized parallel-group open-label two-part study at ten gynecological clinics. Part A: A two-week proof-of-concept evaluation, and Part B: A one-week efficacy evaluation. In Parts A and B, the OMLA pessary was administrated either Once (OW) or Twice (TW) a week and in Part B was also compared to an untreated Control Group (CG). Non-pregnant fertile women with confirmed BV could participate. The main outcome measures were Amsel’s criteria to confirm BV, vaginal pH, adverse events, and patient treatment satisfaction.
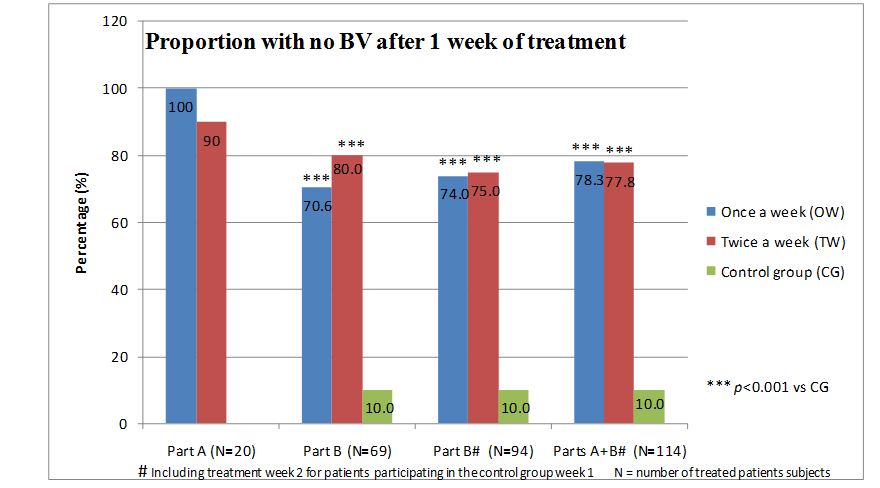
Results: Part A (n=21) showed high safety and treatment efficacy already after one week. In the pooled treatment group, the one week BV clearance ratio was 19/20 (95%). In Part B (n=105) the one week BV clearance ratios were 70.6% in the OW group (p<0.001 vs. CG), 80.0% in the TW group (p<0.001 vs. CG), and 10.0% in the CG. Pooled data from Parts A and B showed a one week BV clearance of 78% in each treatment group. The vaginal pH decreased (p<0.05) in both treatment groups. The results demonstrated high patient treatment satisfaction. Most adverse events were mild and of short duration.
Conclusion: The novel OMLA pessary showed a BV treatment efficacy of 78% after one week of single-dose administration. Along with a good safety profile, user-friendliness, and no risk of bacterial antibiotic resistance development, this represents a significant improvement in BV treatment.
Keywords
ABBREVIATIONS
ADE : Adverse Drug Event
AE : Adverse Event
BV : Bacterial Vaginosis
CG : Control Group
FAS : Full Analysis Set
ICH : International Conference on Harmonization
OMLA : Oligomeric Lactic Acid
OTC : Over the Counter
OW : Once a Week
PP : Per Protocol analysis
SAE : Serious Adverse Event
TW : Twice a Week
INTRODUCTION
Bacterial Vaginosis (BV) is an imbalance in the naturally occurring vaginal bacterial flora resulting in an overgrowth by mixed anaerobic bacterial flora including Gardnerella vaginalis, Prevotella, Bacteriodesspecies, Peptostreptococcus, Mobiluncus, genital Mycoplasma hominis and Ureaplasma species [1-3]. Simultaneously, an increase of the vaginal pH above 4.5 leads to a decline in Lactobacilli species and Lactobacilli-associated antimicrobial activity [4,5].
The most pronounced clinical symptom in BV is the thin white or gray discharge with an offensive or fishy odor caused by an increased concentration of at least seven amines which are presumably produced by bacterial decarboxylases and several volatile and non-volatile organic acid metabolites of anaerobic bacteria [1,2]. Gardnerella vaginalis adherent to squamous epithelial cells produces pathognomonic clue cells, which together with elevated vaginal pH and the odorous discharge constitute the hallmark of BV diagnosis [6]. Diagnosis relies on standardized clinical criteria according to Amsel or on scoring bacterial cell morphotypes on a Gram stained vaginal smear according to Nugent [7-9].
BV is one of the most prevalent vaginal infections in women of reproductive age and affects approximately 15-30% in developed countries [10,11]. The prevalence figures vary greatly between various ethnic groups and geographical areas. Figures can be as high as 50% in certain regions of Africa and China [12].
BV affects many women’s lives in a negative manner, not only due to the odorous vaginal discharge that causes embarrassment and social isolation, but it is also associated with a wide range of medical problems [3,13-15]. In pregnancy, BV is associated with premature labor, premature rupture of membranes, and low birth weight leading to high prenatal mortality. However, it is not known what mechanism(s) causes these adverse pregnancy outcomes [3,16-19]. As BV is found in approximately 16% of pregnant women in industrial countries, it poses a serious problem [3,20-22]. Approximately every second woman, both pregnant and non-pregnant, is asymptomatic, thus making treatment even more cumbersome [6]. Other medical problems include the risk of gynecologic infections following surgery and an increased risk of contracting sexually transmitted diseases, including HIV [14,15,23].
Some women are easily infected and suffer from frequent recurrences several times a year, while others do not seem to be affected at all [13]. A number of sexual and social risk factors for bacterial BV have been suggested, e.g., multiple sexual partners, smoking, douching, and using an intrauterine device, whereas hormonal contraception has been associated with a decreased incidence of BV [24]. However, in a Chinese study of more than 53,000 married women, low sexual activity was identified as a risk factor of BV [25]. To date, there is no scientific evidence showing that bacterial vaginosis is a sexually transmitted disease.
Today, antibiotics such as metronidazole or clindamycin are the most common treatments for BV and, in the US, are recommended by the Center for Disease Control [26]. Recurrences affect approximately half of the women with BV, which means that many women are treated with antibiotics several times a year [13]. The emerging global risk of developing bacterial antibiotic resistance makes BV treatment with antibiotics cumbersome [27]. Apart from relapses, antibiotic treatment often results in side effects, e.g., vaginal Candida vaginitis, which also needs to be treated [28,29]. Furthermore, metronidazole interacts with other drugs and alcohol and must be taken into consideration when administered orally [30]. In vitrostudies have shown that clindamycin and metronidazole inhibit Lactobacillus spp. at concentrations lower than doses topically applied for the treatment of BV [31,32]. Therefore, there is an interest in developing alternative treatments for BV, such as selective antimicrobials, probiotics, and acidification procedures that will inhibit BV pathogenic bacteria without killing healthy Lactobacillus spp [33].
Actively acidifying the vagina with naturally occurring lactic acid and at the same time promoting the growth of indigenous Lactobacillus spp. instead of introducing new species may enhance Lactobacillus spp. colonization and prevent anaerobic overgrowth. Hence, vaginal acidifying might serve as either a therapeutic or a preventive means of BV [7].
Different types of vaginal products containing lactic acid or other weak acids, e.g., ascorbic acid, for treating BV are available on the market and require daily dosing for a minimum treatment cycle of 6-7 days [34,35]. A survey of 2660 women performed in USA, UK and Germany in 2011 clearly showed that a large proportion of those with recurrent BV problems were not satisfied with the treatment alternatives available [13]. The survey displayed pronounced requests for non-smeary Over The Counter (OTC) products that are easy to use and do not require frequent dosing.
The investigational product in this study is being developed by Laccure AB (Sweden) to meet a target product profile of high efficacy after few administrations in combination with high patient acceptance. The product, based on Oligomeric Lactic Acid (OMLA), is intended for the treatment and prevention of BV. When in contact with the vaginal mucus, a mucoadhesive acidic OMLA gel is formed that adheres to the vaginal mucosa; the OMLA is gradually hydrolyzed into lactic acid and released. The OMLA content of one pessary corresponds to 700mg lactic acid, which is adequate for a lasting pH effect for up to approximately one week (Laccure regulatory dossier). The freeze-dried product, buffered to pH 3.5, is designed to release ≥20% after 6 h, ≥35% after 24 h, and ≥45% after 72 h, which mimics the three first doses of lactic acid-based products on the market (Laccure regulatory dossier).
This is the first study using an OMLA pessary on patients with confirmed BV as well as comparing two dosing schedules of the OMLA pessary and focusing on BV clearance, pH decrease, adverse events, and patient treatment satisfaction.
METHODS
Participants
Trial design
During the study planning, a double-blind study design was considered, but had to be abandoned as developing a placebo identical to the OMLA pessary was not possible. Instead, an untreated control group was added in order to study the natural progress of BV.
Study Part A compared the dosage schedules of one administration per week and two administrations per week (on day 1 and day 4 of each week) during a two-week treatment period. In Part B, involving only one week’s treatment to study the immediate treatment effect, clinic visits were performed on day 1 (Visit 1), day 4 (Visit 2) and day 8 (Visit 3). In Part A, there were also visits on day 11 (Visit 4) and day 15 (Visit 5). In Part B, eligible patients were also randomized to a Control Group (CG) not receiving any treatment but still attending the same visits and having the same assessments done as the actively treated groups. Patients in the control group who still had verified BV after the first week of non-treatment were randomized a second time and offered treatment with an OMLA pessary during week 2.
The study was performed according to Good Clinical Practice Consolidated Guidelines (1996) of the International Conference on Harmonization (ICH) E6 (R1) [36]. The study protocol was reviewed and given a favorable recommendation by an Ethics Committee and by the Regulatory Authority. Written informed consent was obtained from all participants. The trial was monitored by an independent contract research organization that also performed the data management and statistics (Norma A/S, Denmark). Two sites were audited at the sponsor’s request.
Objectives and outcome measures
The primary objective of Part B was to investigate the efficacy of the OMLA pessary in patients with BV. The secondary objectives were to evaluate the safety, tolerability, vaginal pH levels, and patient treatment satisfaction of the OMLA pessary in patients with BV.
Detailed objectives and primary outcome measures for Parts A and B are shown in table 1.
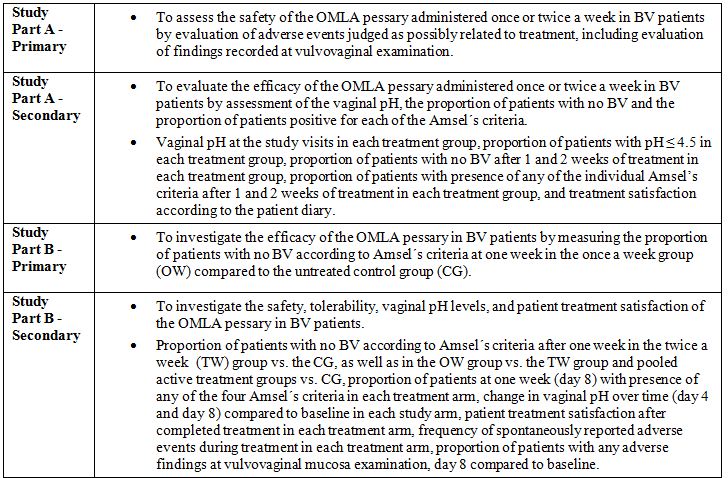
Table 1: Study objectives and outcome measures in Part A and Part B.
Randomization
Sample size and statistical analysis
In Part B, the primary hypothesis, also valid for the sample size estimation, was to evaluate the proportion of treatment successes (no BV at Day 8) according to Amsel’s criteria in the group treated Once a Week (OW) compared with the untreated CG [8]. The sample size estimation was based on estimated treatment success rates of 75% with the OMLA pessary and 40% for the untreated controls. At a significance level of 0.05, a two-sided hypothesis testing and a power of 80%, the patient number needed would be 31 per group. To allow for 5 patients to drop out, it was calculated that 36 patients per group were needed.
The FAS consisted entirely of randomized patients who received at least one dose of the assigned treatment and patients in the randomized CG. All collected data from the treated patients is presented here. No imputations of missing values were performed. A Per Protocol (PP) Analysis set was also defined and analyzed in Part B.
The Wilcoxon signed-rank test was applied for significance testing within groups and the Chi-square test for testing between groups.
Interventions
In Part A, patients were randomized to treatment with the OMLA pessary Once a Week for two weeks (OW), receiving a total of two pessaries (day 1 and day 8) or twice a week for Two Weeks (TW), receiving a total of four pessaries (day 1, day 4, day 8 and day 11).
In Part B, patients were randomized to treatment with one single OMLA pessary for One Week (OW), receiving a total of one pessary (day 1) or treatment with the OMLA pessary Twice a Week for one week (TW), receiving a total of two pessaries (day 1 and day 4) or randomized to the CG without any treatment during the first week. Patients in the CG who still had verified BV after the first week of non-treatment were randomized a second time and offered treatment with the OMLA pessary during week 2, either once or twice a week for one week.
Clinical assessments and collection of study data
For all pH measurements, the pH meter ECPH601PLUS Eutech 6, Scandinovata AB, Bromma, Sweden was used. Study personnel at each site were educated on how and when to calibrate and use the pH meter and the electrode. Vaginal smears for pH and Amsel’s criteria assessments were prepared from mucosal samples in the middle vagina and close to the orifice, respectively. No swabs were to touch the cervix as pH there is close to seven, which would mislead the correct vaginal pH. Of the two pH samples taken, the highest value recorded was used.
Vulvovaginal mucosa examinations were performed pre- and post-treatment by the investigator in order to assess any adverse findings, e.g., vaginal redness and irritation. Treatment compliance was checked by questioning and counting the unused returned pessaries.
In Part A, patients completed a patient diary on a daily basis and registered any Adverse Events (AE). In both parts of the study, treated patients completed a questionnaire on their final visit regarding patient treatment satisfaction. They were asked to answer whether or not they agreed with several treatment related statements, e.g., “I experienced the treatment as user-friendly: easy to insert, not messy to use and comfortable” and “I find it comfortable not to dose more frequently”.
RESULTS
Study part A
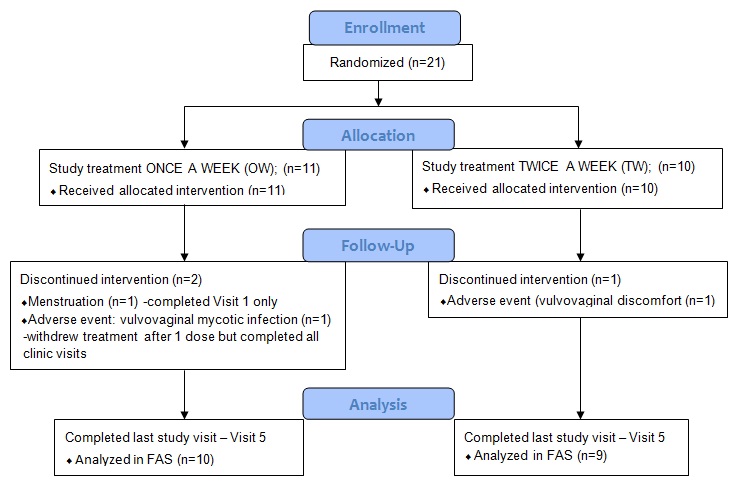
The number of patients with minor protocol deviations was 5 in the OW group and 6 in the TW group. The deviations related to the timing of the study visit windows and the timing of the recommended dosing schedule.
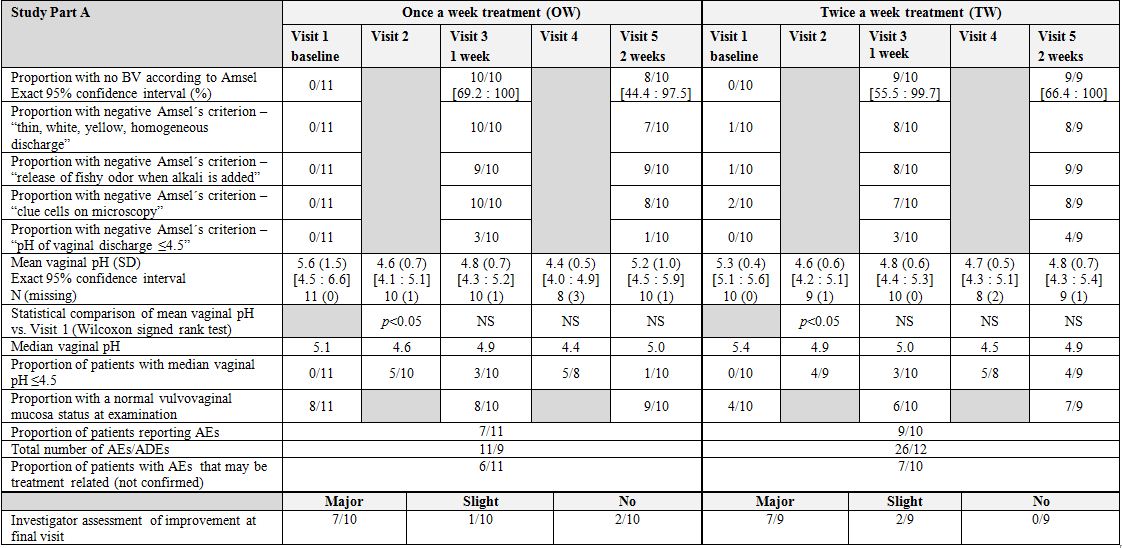
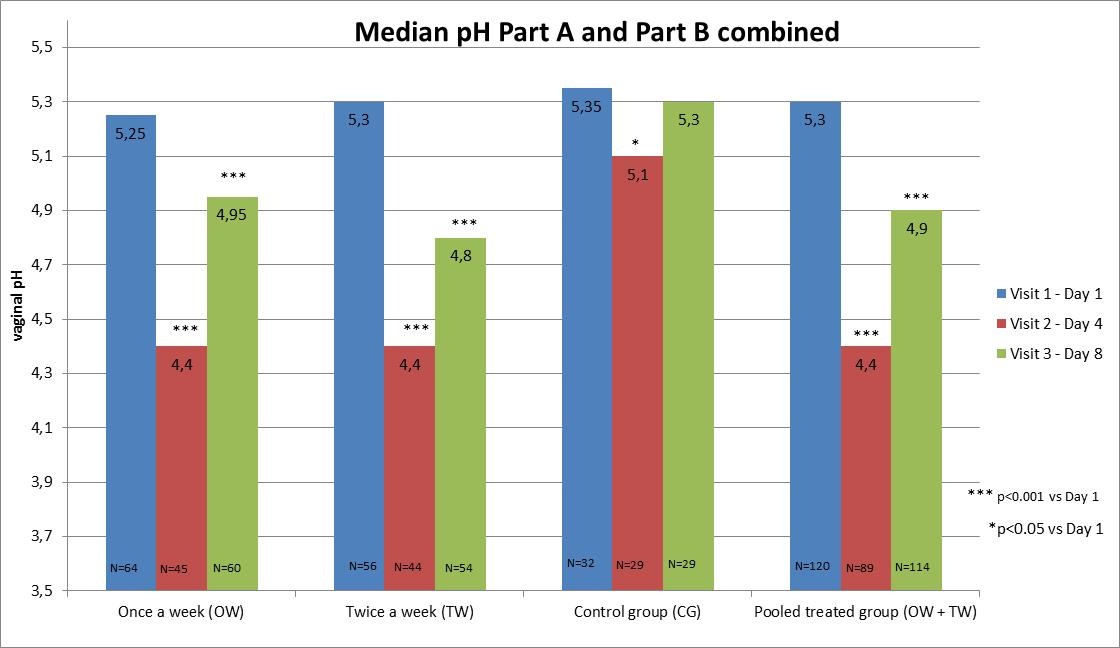
Vaginal pH:The mean absolute vaginal pH was significantly reduced (p<0.05) compared to the baseline in both groups at Visit 2. The proportion of patients with median vaginal pH of ≤4.5 increased from 0 at commencement of the study to 9/19 (47%) at Visit 2 (day 4). Later on in the study, the effect on pH was less pronounced in the OW group. After two weeks, the corresponding proportions were 1/10 (10%) in the OW group and 4/9 (44%) in the TW group.
AEs and vulvovaginal mucosa examination:As expected, no Serious Adverse Events (SAEs) occurred. The majority of events reported were of mild intensity and short duration. Two patients, one patient in each group, were withdrawn from treatment due to an AE considered by the investigator to possibly be treatment related (i.e., an Adverse Drug Event (ADE)). The most common ADEs were vaginal itching and/or vaginal burning experienced by a total of 10 patients. No differences were seen between the groups.
A higher proportion of patients had a normal vulvovaginal mucosa appearance at the end of the study (84%) compared to at inclusion (57%), which shows that the treatment had no visually detectable negative impact on the mucosa.
Patient questionnaire and investigator assessment:The answers to the questionnaire showed that all patients completely or partly agreed that the OMLA pessary treatment was user-friendly, i.e., easy to insert, not messy to use, and comfortable. Furthermore, all patients agreed that it was convenient not to dose more often than once or twice a week.
According to the investigator assessments, 14/19 (74%) of treated patients were considered to have achieved major improvements from the treatment.
Overall results of part A: This study part confirmed not only the efficacy, safety and user-friendliness of the treatment, but also the pH-lowering mechanism of action. However, due to the small number of patients, no conclusions regarding potential differences between the treatment schedules could be drawn. As the OMLA pessary performed as expected, the Proof of Concept was collected.
The results obtained in Part A were used in the final design of study Part B. As both treatment regimens were shown to be highly effective, the decision was made to continue with both regimens in Part B, but for only one week, as this seemed to be adequate time for studying the immediate treatment effects.
Study part B
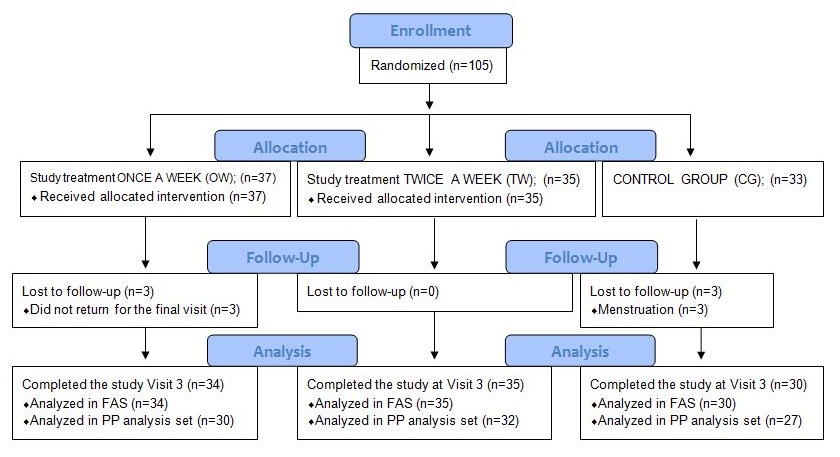
The number of patients with minor protocol deviations was 7 in the OW group, 3 in the TW group and 6 in the CG. These patients were not included in the PP analysis set. The deviations in the three groups related to: missed study Visit 3, menstruation, incorrect number of pessaries, incorrect pH assessment, and incorrect inclusion (post-menopausal).
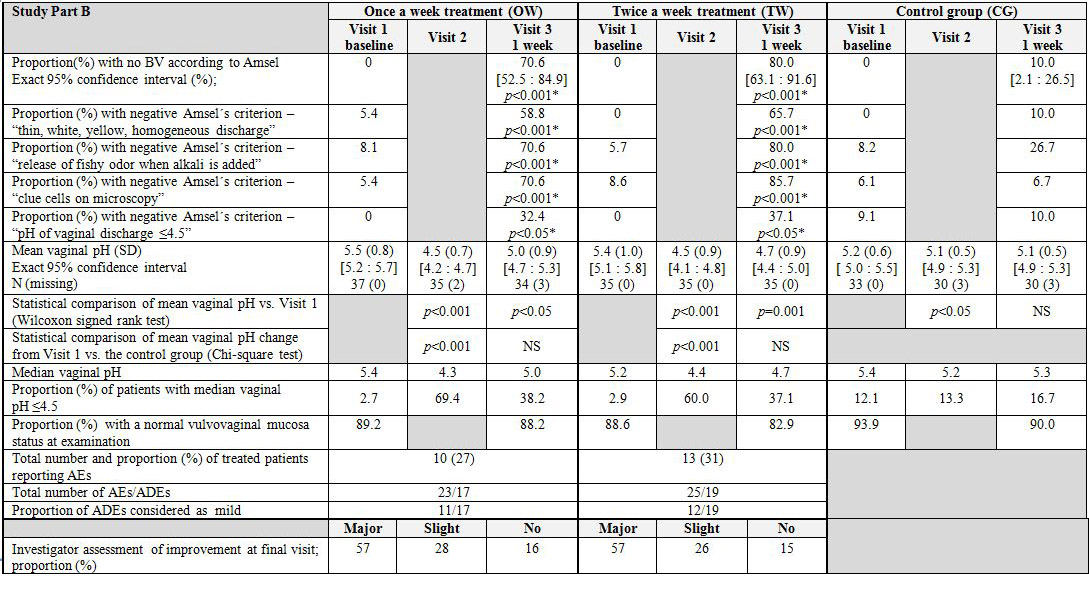
BV according to Amsel’s criteria: A substantial change in terms of no BV was seen in the OW and the TW groups, but not in the CG. The primary comparison of treatment efficacy, the proportion of patients with no BV after one week, was 70.6% in the OW group and 10.0%2) in the CG. This association between treatment and response was highly significant (p<0.001). The proportion of patients with no BV in the TW group was 80.0% (p
The control patients who still had verified BV after the first week of non-treatment were offered treatment with the OMLA pessary during week 2. When the results from the second week were added, the corresponding results for the OW group were 74.0% and 75.0% for the TW group.
For all four Amsel’s criteria, the differences in response rates were statistically significant in favor of each of the treatment groups versus the CG.
Vaginal pH: Compared to baseline, the mean pH value within the OW group was reduced 1.0 unit at day 4 and 0.5 units at day 8 (p
AEs and vulvovaginal mucosa examination: None of the reported AEs or ADEs with a possible relationship to the treatment was deemed serious and no patient withdrew from treatment due to an AE. Approximately 30% of the patients reported at least one AE. The majority (64%) of the ADEs were of mild intensity and normally of short duration. Two patients were diagnosed with a yeast infection.
The most common ADE was vaginal itching reported by 5 patients in the OW group and by 6 patients in the TW group. Other events, reported by more than one subject per group, were vaginal irritation and a genital burning sensation. No clear differences in relation to AEs were seen between the treatment groups.
The majority of the patients had a normal vulvovaginal mucosa status at baseline and at the end of the treatment.
Patient questionnaire and investigator assessment: More than 85% completely agreed with the statement that the OMLA pessary treatment was user-friendly, as it was easy to insert, not messy to use and comfortable. More than 80% completely agreed that it was convenient not have to dose more often. There were no significant differences between the treatment groups in relation to any of the questions. The results verified a high treatment satisfaction and acceptance of the OMLA pessary.
According to the investigator assessments, the clinical outcome of the OMLA pessary treatment was judged as improved (major or slight) compared to baseline for over 83% of the treated patients.
Overall results of part B: Both treatment schedules of the OMLA pessary were shown to be highly effective. The results also confirmed the safety and user-friendliness of the treatment. No clear differences between the treatment schedules were observed.
Consolidated results from parts A and B
DISCUSSION
Cure rates not exceeding 60-70% four weeks after oral or topical antibiotic treatments and relapse rates of approximately 70% in long-term follow-up studies (> 4 weeks after treatment) reported in a review have led to the conclusion that no sound scientific basis yet exists for recommending any particular therapeutic treatment for BV [37].
The dominance of Lactobacilli in healthy vaginal microbiota and its depletion due to BV has given rise to the concept of oral or vaginal use of probiotic Lactobacillus strains for the prevention of BV. However, sufficient evidence for recommending probiotics for the treatment of BV is still lacking [38]. Increase of vaginal pH leads to the decrease of the Lactobacilli-associated antimicrobial activity which may explain the lack of efficacy in patients with established BV [4-5].
These results render the search for new efficacious therapeutic agents for BV even more important.
Main findings
The vaginal pH in healthy women is reported to be 4.0-4.5 [40]. Based on the study results, it can be speculated that women frequently suffering from BV, compared to women never developing BV, have a higher “normal” pH range, which would increase the susceptibility for BV development.
The mechanism of action for the OMLA pessary is its pH-lowering effect, so it may seem somewhat contradictory that among the four Amsel’s criteria, the effect on pH was the least pronounced. However, if the theory presented is correct, the pH limit of 4.5 in Amsel’s criteria should be somewhat higher. There are reports in literature where a pH of 4.7 has been used instead [34,39,41]. In the study by Decena et al., with 30 patients in each of three treatment groups (lactic acid gel, oral metronidazole or lactic acid gel combined with oral metronidazole), there was only a slight pH decrease of 0.4 units and a mean pH of 4.8 in the group with the combined treatment which reported the most pronounced pH effect [39]. Less than 50% of the patients in this group received a pH below 4.7. Despite this moderate pH decrease there were positive effects on the other Amsel’s criteria, which also was the case when patients were treated with lactic acid gel alone.
No alarming events were reported concerning the OMLA pessary in this study. The most frequently reported event was vaginal itching which was mentioned by less than 20% of the participants. Only two patients were reported to have contracted a yeast infection, which is a commonly reported problem after treatment with antibiotics [28,29,38]. The events were generally of short duration and mild intensity and were not a reason for withdrawal from treatment or the study. Furthermore, the vulvovaginal examinations verified that the treatment had no visually detected negative impacts on the mucosa.
Strengths and limitations
Recurrence of symptoms is common among women suffering from BV. With this new OMLA pessary, recurrences are easily and conveniently treated with only one pessary administration. For patients with frequent recurrences, the pessary would be especially appropriate since antibiotics should be restrictively used [27].
Furthermore, the current study focused on the immediate treatment effects of the OMLA pessary with no BV follow-up after completion of treatment. This approach was chosen as there is no generally accepted standard length of follow-up after treatment. Since the follow-up periods differ between studies the results are often not directly comparable. However, as no follow-up observations were done after completion of treatment, it is unclear whether the pessary will provide any sustained relief.
The limitation with a non-blinded study design may have biased the treatment outcomes in a way that AEs from actively treated patients would more easily be interpreted as treatment related compared to those from untreated patients.
According to a recently performed literature review BV should be diagnosed using either clinical (Amsel’s) or laboratory (Gram stain with objective scoring system) criteria [42]. Amsel’s criteria has been presented in section Methods [8]. For Amsel’s criteria the sensitivity and positive predictive value are both 90% [43]. The Nugent Score is a Gram stain scoring system for pap tests to diagnose bacterial Vaginosis [9]. It was first described in 1991 by RP Nugent, whom it is named after [9]. The Nugent score is calculated by assessing for the presence of large Gram positive rods (Lactobacillus morphotypes; decrease in Lactobacillus scored as 0 to 4), small Gram-variable rods (Gardnerella vaginalis morphotypes; scored as 0 to 4), and curved Gram-variable rods (Mobiluncus spp. morphotypes; scored as 0 to 2) and can range from 0 to 10. A score of 7 to 10 is consistent with bacterial Vaginosis [9]. Vaginal Gram stain is reliable and allows for permanent record. Cultures are nonspecific because Gardnerella vaginalis resides in normal vaginal flora as well [43].
Amsel’s criteria and Nugent scoring system are among the most commonly used diagnostic methods of BV. Although Nugent scoring system is considered the gold standard for diagnosing BV, it is time consuming and costly, and its interpretation needs lab equipment and experts. Hence, most physicians are inclined to use simpler clinical criteria that are yet accurate instead [44]. In the current study it was thus decided to use Amsel’s criteria as this method was also considered as logistically easier to use in a multicenter setting.
Interpretation
Favorable effects of vaginal acidification for the prevention of recurrent BV have also been presented in a study of a commercial vaginal gel containing diluted buffered acetic acid. Here, the vaginal gel used was associated with a significant decrease in the number of women with relapses, as well as the recurrence rate [46].
The data reported in the literature support that the OMLA pessary should encompass qualities suitable not only for the acute treatment of BV symptoms but also for a prophylactic use to normalize an abnormal vaginal pH and microflora in order to prevent recurrences. To confirm the restoration of the normal Lactobacilli flora after treatment with the OMLA pessary a new clinical trial in women with BV should be undertaken using the Nugent score. In the same trial, prevention of BV recurrence using one OMLA pessary a month (after completed menstruation) for 4-6 months could also be studied.
CONCLUSION
Citation: Fredstorp M, Jonasson AF, Barth A, Robertsson J (2015) A New Effective, User-friendly Bacterial Vaginosis Treatment: A Randomized Multicenter Open-label Parallel-group Two-part Study with a Novel Sustained-release Pessary Containing Oligomeric Lactic Acid. J Infect Non Infect Dis 1: 006.
Copyright: © 2015 Jeanette Robertsson, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.